Pathway-Specific Therapeutic Modulation of Melanoma: Small-Molecule Inhibition of BRAF–MEK and KIT Signaling in Contemporary Precision Oncology with a Special Focus on Vemurafenib, Trametinib, and Imatinib
Abstract
1. Introduction
2. Cutaneous Melanoma Treatment and Mechanisms of Action
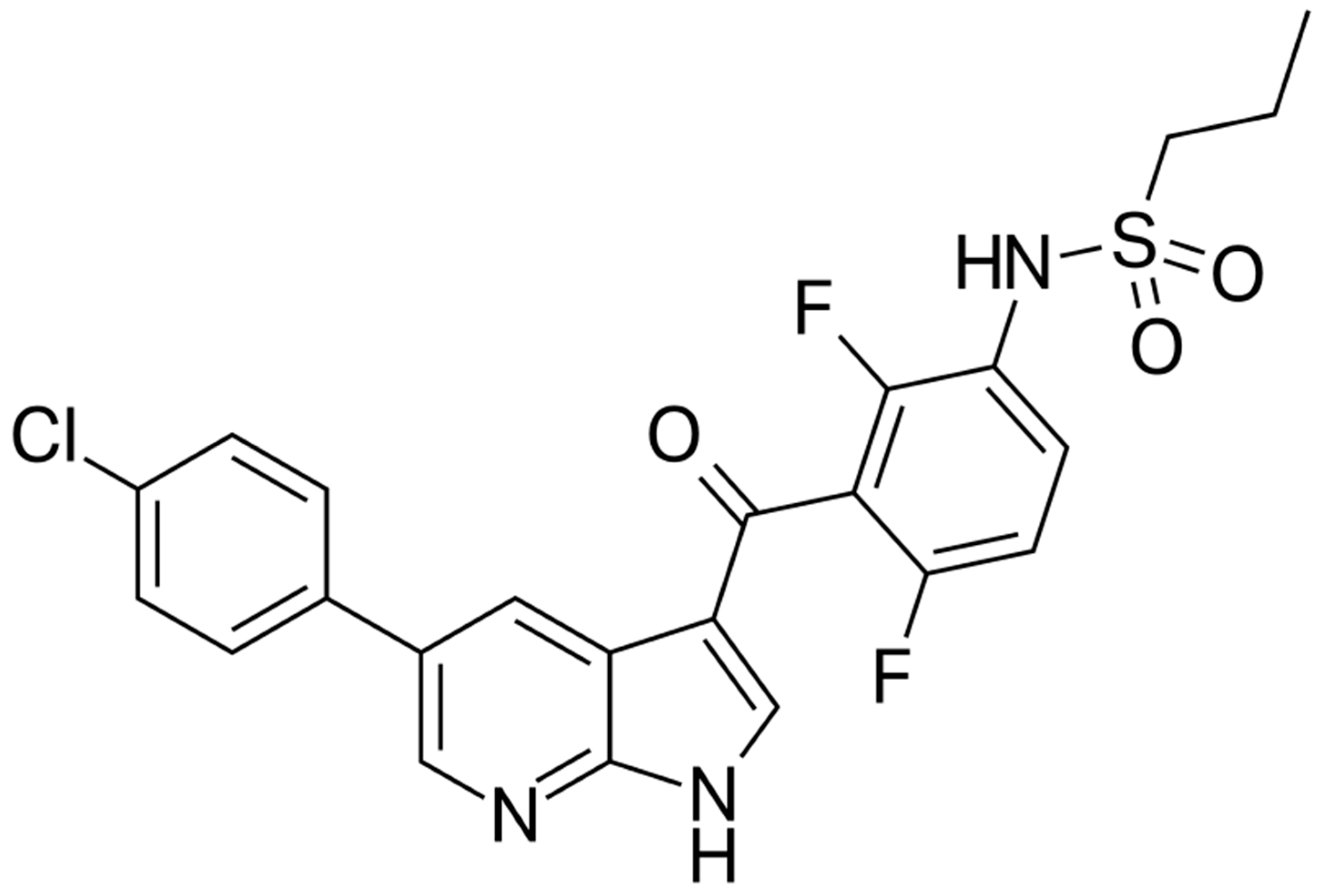
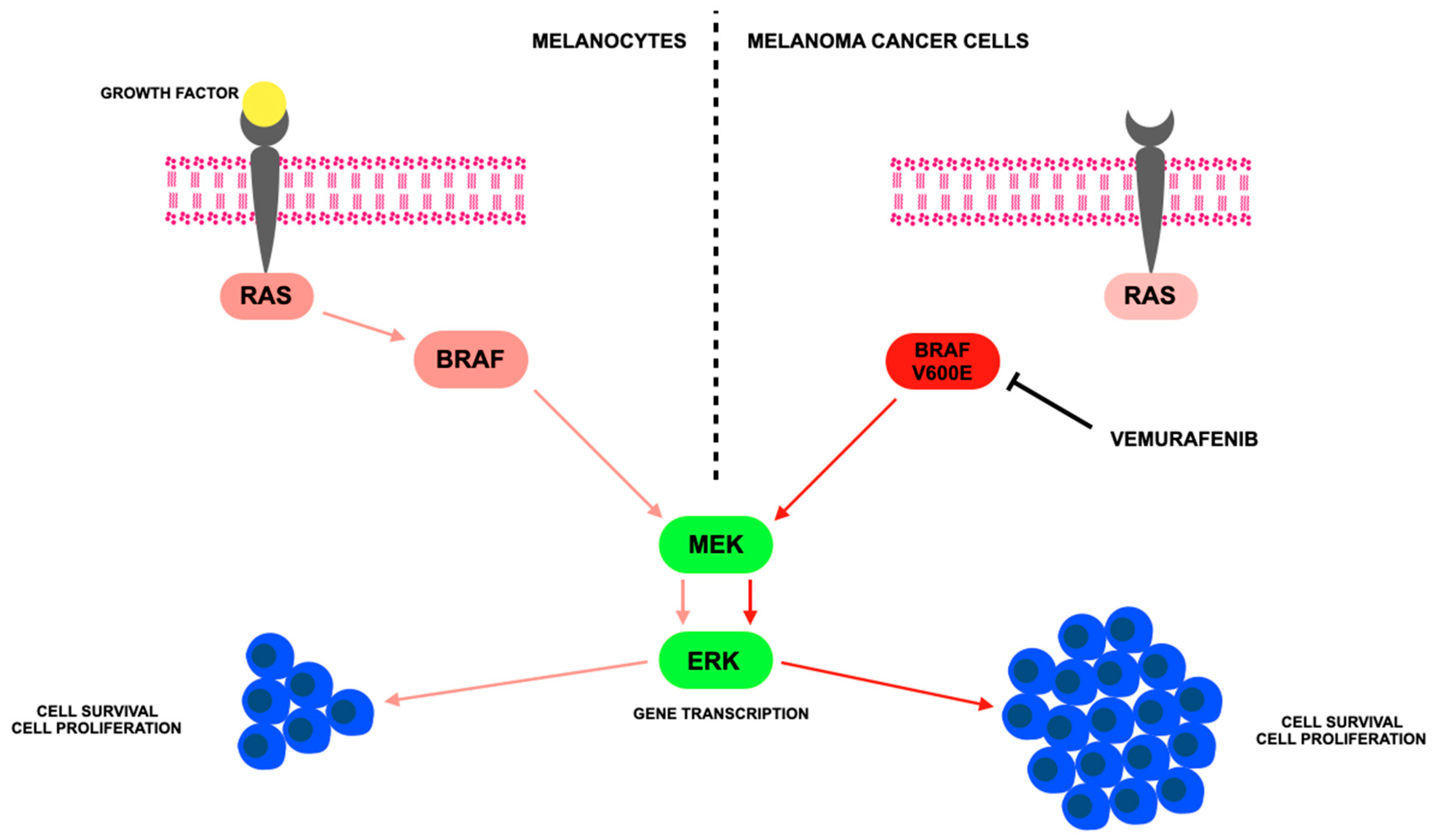
3. Vemurafenib—BRAF Inhibitor
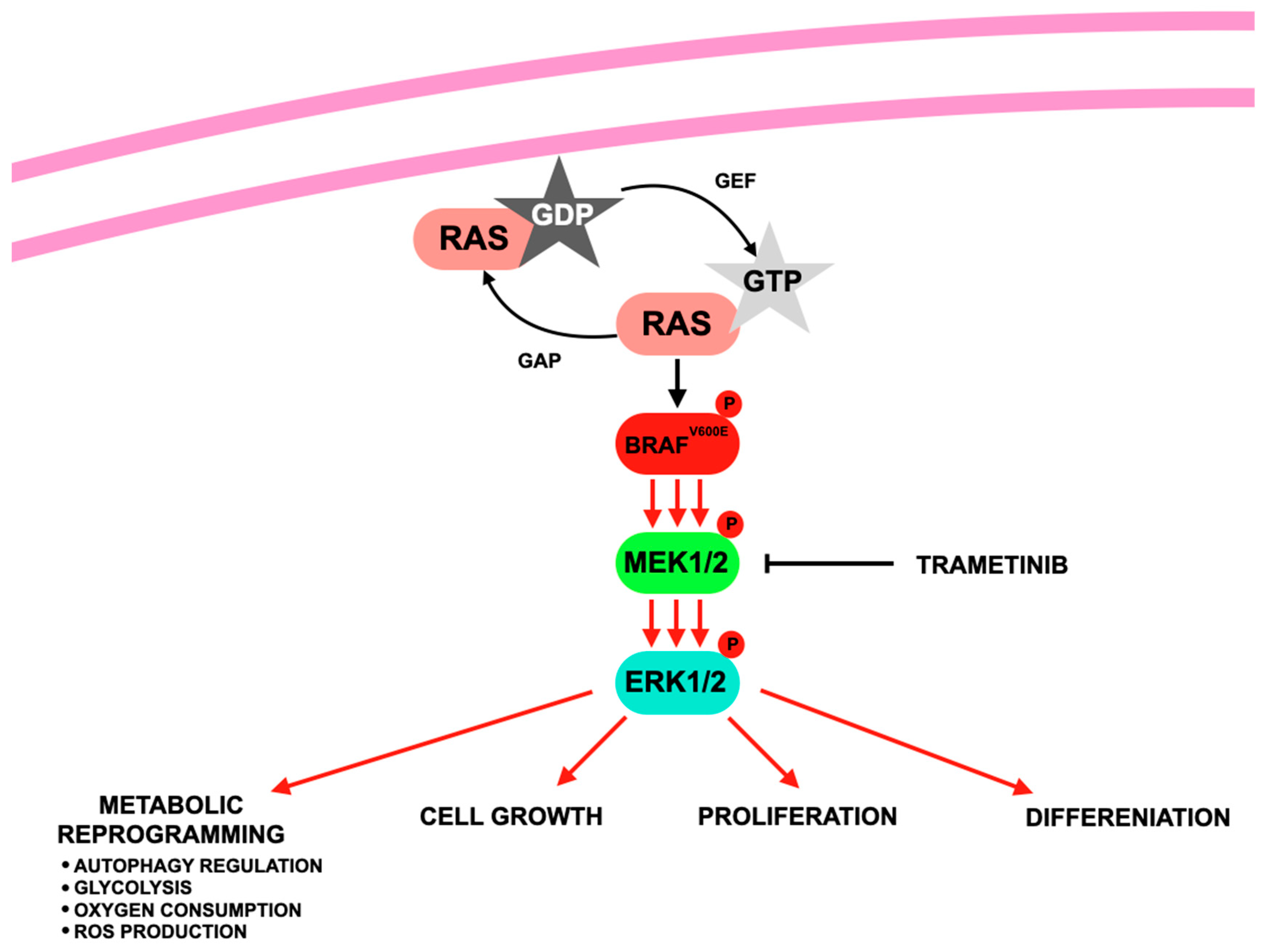
4. Trametinib—MEK Inhibitor
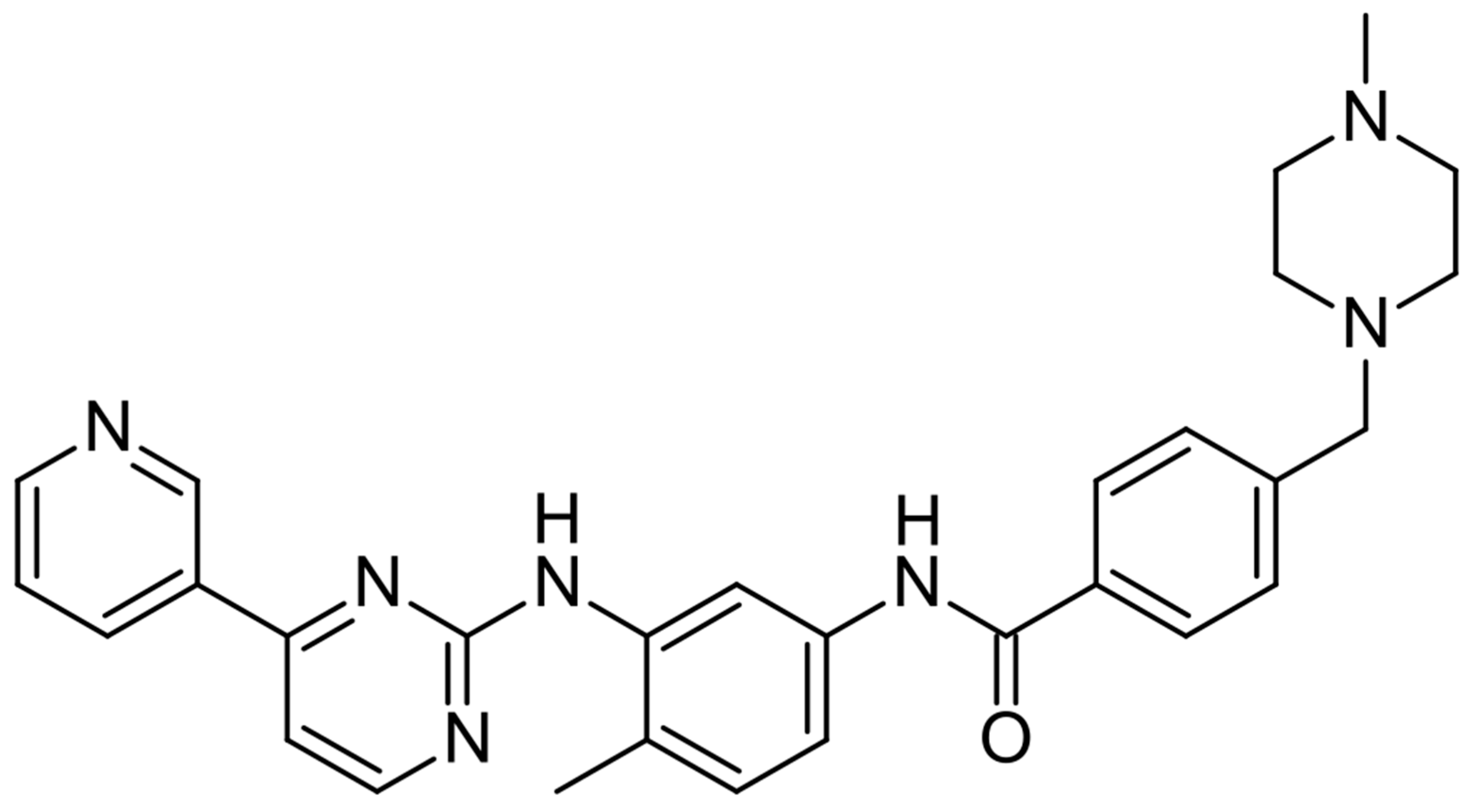
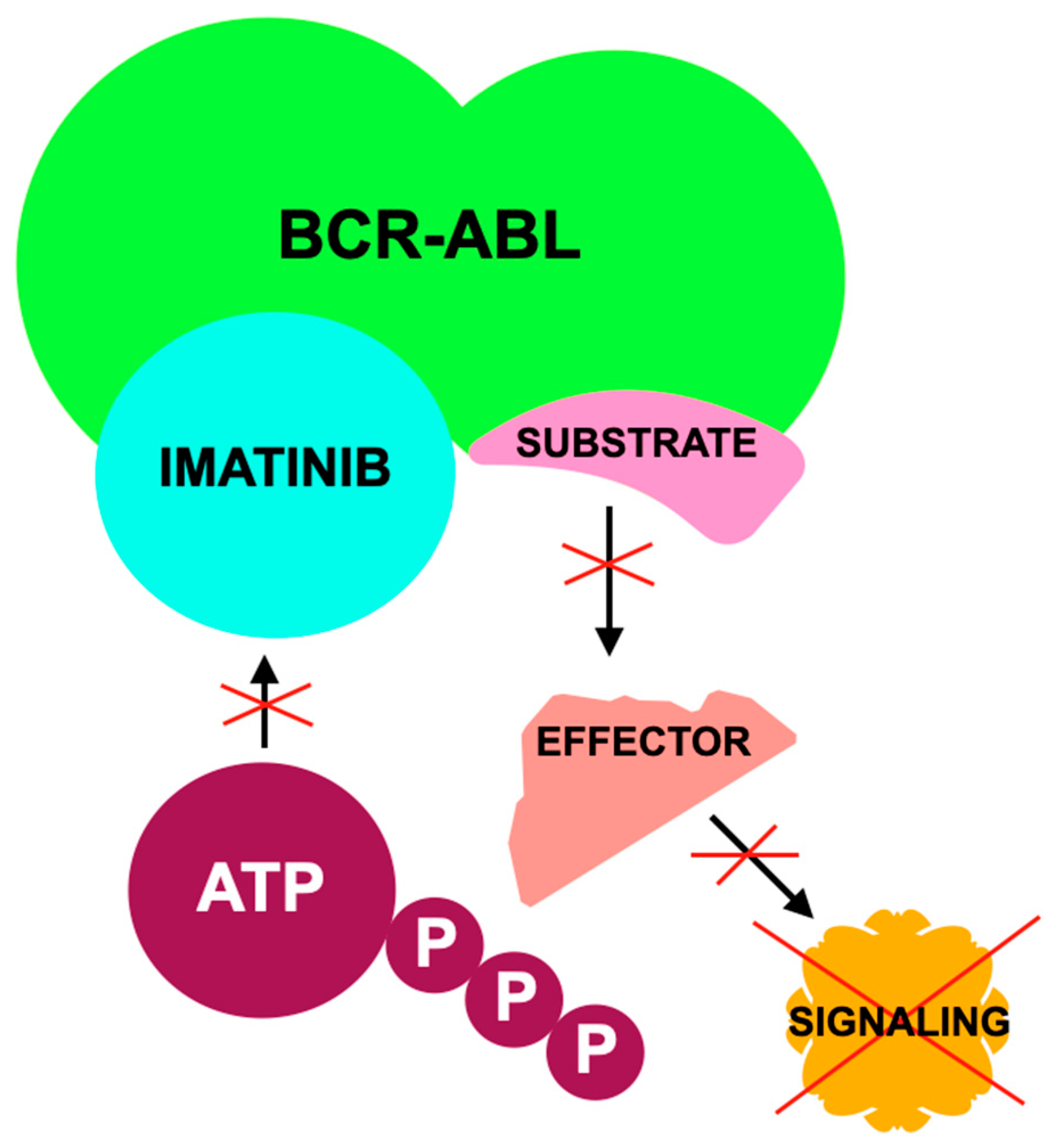
5. Imatinib—C-KIT Inhibitor
6. Comparative Analysis of Vemurafenib, Trametinib, and Imatinib in Cutaneous Melanoma
7. Future Directions and Clinical Relevance
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | Adenosine Diphosphate |
| AEs | Adverse Events |
| AKT | Protein Kinase B |
| ALL | Acute Lymphoblastic Leukemia |
| AR | Androgen Receptor |
| ATP | Adenosine Triphosphate |
| A-RAF | A-Raf Proto-Oncogene, Serine/Threonine Kinase |
| B-RAF | B-Raf Proto-Oncogene, Serine/Threonine Kinase |
| BCR-ABL | Breakpoint Cluster Region-Abelson Fusion Gene |
| BRAF | v-Raf Murine Sarcoma Viral Oncogene Homolog B1 |
| C-RAF | C-Raf Proto-Oncogene, Serine/Threonine Kinase |
| CAR-T | Chimeric Antigen Receptor T-cell |
| CCND1 | Cyclin D1 |
| CDK4/6 | Cyclin-Dependent Kinases 4 and 6 |
| CML | Chronic Myeloid Leukemia |
| CTLA-4 | Cytotoxic T-Lymphocyte–Associated Protein 4 |
| DFSP | Dermatofibrosarcoma Protuberans |
| ECG | Electrocardiogram |
| ERK | Extracellular Signal-Regulated Kinase |
| FISH | Fluorescence In Situ Hybridization |
| GAP | GTPase-Activating Protein |
| GDP | Guanosine Diphosphate |
| GEF | Guanine Nucleotide Exchange Factor |
| GIST | Gastrointestinal Stromal Tumor |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| GTP | Guanosine Triphosphate |
| HCQ | Hydroxychloroquine |
| HES | Hypereosinophilic Syndrome |
| HLA | Human Leukocyte Antigen |
| HSV-1 | Herpes Simplex Virus Type 1 |
| ICI | Immune Checkpoint Inhibitor |
| IFN-γ | Interferon Gamma |
| ILD | Interstitial lung disease |
| JAK | Janus Kinase |
| JAK/STAT | Janus Kinase/Signal Transducer and Activator of Transcription |
| KIT | Stem Cell Factor Receptor/Receptor Tyrosine Kinase |
| LAG-3 | Lymphocyte-Activation Gene 3 |
| LDH | Lactate Dehydrogenase |
| MAP2K1/2 | Mitogen-Activated Protein Kinase Kinase 1 and 2 |
| MAPK | Mitogen-Activated Protein Kinase |
| MEK | Mitogen-Activated Protein Kinase Kinase |
| MHC | Major Histocompatibility Complex |
| mTOR | Mechanistic Target of Rapamycin |
| NGS | Next-Generation Sequencing |
| NK cell | Natural Killer Cell |
| NRAS | Neuroblastoma RAS Viral Oncogene Homolog |
| ORR | Objective response rate |
| OS | Overall survival |
| P | Phosphate |
| PCR | Polymerase Chain Reaction |
| PD-1 | Programmed Cell Death Protein 1 |
| PDGF | Platelet-Derived Growth Factor |
| PDGFRA | Platelet-Derived Growth Factor Receptor Alpha |
| PDGFR | Platelet-Derived Growth Factor Receptor |
| PD-L1 | Programmed Death-Ligand 1 |
| PFS | Progression-free survival |
| Ph+ | Philadelphia Chromosome–Positive |
| PI3K | Phosphoinositide 3-Kinase |
| QT | QT Interval |
| RAF | Rapidly Accelerated Fibrosarcoma |
| RAS | Rat Sarcoma Virus Oncogene |
| RT | Radiotherapy |
| RTK | Receptor Tyrosine Kinase |
| SCC | Squamous cell carcinoma. |
| SCF | Stem Cell Factor |
| SLNB | Sentinel Lymph Node Biopsy |
| STAT | Signal Transducer and Activator of Transcription |
| TERT | Telomerase Reverse Transcriptase |
| TIL | Tumor-Infiltrating Lymphocyte |
| TKI | Tyrosine Kinase Inhibitor |
| TMB | Tumor Mutational Burden |
| Treg | Regulatory T Cell |
| TYP1 | Tyrosinase-Related Protein 1 |
| V600E/K | Valine to Glutamic Acid/Lysine Substitution at Position 600 |
| WT | Wild Type |
References
- Caraban, B.M.; Aschie, M.; Deacu, M.; Cozaru, G.C.; Pundiche, M.B.; Orasanu, C.I.; Voda, R.I. A Narrative Review of Current Knowledge on Cutaneous Melanoma. Clin. Pract. 2024, 14, 214–241. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, H.; Li, C. Signal Pathways of Melanoma and Targeted Therapy. Signal Transduct. Target. Ther. 2021, 6, 424. [Google Scholar] [CrossRef]
- Alsadiq, S.; Kartolo, A.; McWhirter, E.; Hopman, W.; Baetz, T. Efficacy and Safety of Adjuvant Systemic Therapies in Trial Non-Eligible Resected Stages III and IV Melanoma Patients. Melanoma Manag. 2025, 12, 2461963. [Google Scholar] [CrossRef]
- Eddy, K.; Chen, S. Overcoming Immune Evasion in Melanoma. Int. J. Mol. Sci. 2020, 21, 8984. [Google Scholar] [CrossRef]
- Tawbi, H.; Nimmagadda, N. Targeted Therapy in Melanoma. Biologics 2009, 3, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Cerchia, C.; Lavecchia, A. Small Molecule Drugs and Targeted Therapy for Melanoma: Current Strategies and Future Directions. Curr. Med. Chem. 2017, 24, 2312–2344. [Google Scholar] [CrossRef]
- Kwong, A.; Sanlorenzo, M.; Rappersberger, K.; Vujic, I. Update on Advanced Melanoma Treatments: Small Molecule Targeted Therapy, Immunotherapy, and Future Combination Therapies. Wien. Med. Wochenschr. 2019, 169, 314–322. [Google Scholar] [CrossRef]
- Sun, J.; Carr, M.J.; Khushalani, N.I. Principles of Targeted Therapy for Melanoma. Surg. Clin. N. Am. 2020, 100, 175–188. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Menzies, A.M.; Rizos, H. Mechanisms and strategies to overcome resistance to molecularly targeted therapy for melanoma. Cancer 2017, 123, 2118–2129. [Google Scholar] [CrossRef]
- LoRusso, P.M.; Schalper, K.; Sosman, J. Targeted Therapy and Immunotherapy: Emerging Biomarkers in Metastatic Melanoma. Pigment Cell Melanoma Res. 2020, 33, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Robert, C.; Hersey, P.; Nathan, P.; Garbe, C.; Milhem, M.; Demidov, L.V.; Hassel, J.C.; Rutkowski, P.; Mohr, P.; et al. Improved Survival with MEK Inhibition in BRAF-Mutated Melanoma. N. Engl. J. Med. 2012, 367, 107–114. [Google Scholar] [CrossRef]
- Long, G.V.; Flaherty, K.T.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; et al. Dabrafenib plus Trametinib versus Dabrafenib Monotherapy in Patients with Metastatic BRAF V600E/K-Mutant Melanoma: Long-Term Survival and Safety Analysis of a Phase 3 Study. Ann. Oncol. 2017, 28, 1631–1639. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Nazarian, R.; Shi, H.; Wang, Q.; Kong, X.; Koya, R.C.; Lee, H.; Chen, Z.; Lee, M.K.; Attar, N.; Sazegar, H.; et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 2010, 468, 973–977. [Google Scholar] [CrossRef]
- Villanueva, J.; Vultur, A.; Herlyn, M. Resistance to BRAF inhibitors: Unraveling mechanisms and future treatment options. Cancer Res. 2011, 71, 7137–7140. [Google Scholar] [CrossRef]
- Lito, P.; Pratilas, C.A.; Joseph, E.W.; Tadi, M.; Halilovic, E.; Zubrowski, M.; Huang, A.; Wong, W.L.; Callahan, M.K.; Merghoub, T.; et al. Relief of Profound Feedback Inhibition of Mitogenic Signaling by RAF Inhibitors Attenuates Their Activity in BRAFV600E Melanomas. Cancer Cell 2012, 22, 668–682. [Google Scholar] [CrossRef]
- Yu, Q.; Xie, J.; Li, J.; Lu, Y.; Liao, L. Clinical Outcomes of BRAF plus MEK Inhibition in Melanoma: A Meta-Analysis and Systematic Review. Cancer Med. 2019, 8, 5414–5424. [Google Scholar] [CrossRef]
- Larkin, J.; Ascierto, P.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandalá, M.; Demidov, L.V.; Stroyakovskiy, D.; Thomas, L.; et al. Combined vemurafenib and cobimetinib in BRAF-mutant melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef]
- Ascierto, P.A.; McArthur, G.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Di Giacomo, A.M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Cobimetinib Combined with Vemurafenib in Advanced BRAF(V600)-Mutant Melanoma (coBRIM): Updated Efficacy Results from a Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2016, 17, 1248–1260. [Google Scholar] [CrossRef]
- Long, G.V.; Eroglu, Z.; Infante, J.; Patel, S.; Daud, A.; Johnson, D.B.; Gonzalez, R.; Kefford, R.; Hamid, O.; Schuchter, L.; et al. Long-Term Outcomes in Patients with BRAF V600-Mutant Metastatic Melanoma Who Received Dabrafenib Combined with Trametinib. J. Clin. Oncol. 2018, 36, 667–673. [Google Scholar] [CrossRef]
- Sabbah, M.; Najem, A.; Krayem, M.; Awada, A.; Journe, F.; Ghanem, G.E. RTK Inhibitors in Melanoma: From Bench to Bedside. Cancers 2021, 13, 1685. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Corless, C.L.; Giobbie-Hurder, A.; Fletcher, J.A.; Zhu, M.; Marino-Enriquez, A.; Friedlander, P.; Gonzalez, R.; Weber, J.S.; Gajewski, T.F.; et al. Imatinib for Melanomas Harboring Mutationally Activated or Amplified KIT Arising on Mucosal, Acral, and Chronically Sun-Damaged Skin. J. Clin. Oncol. 2013, 31, 3182–3190. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Antonescu, C.R.; Wolchok, J.D.; Chapman, P.B.; Roman, R.A.; Teitcher, J.; Panageas, K.S.; Busam, K.J.; Chmielowski, B.; Lutzky, J.; et al. KIT as a Therapeutic Target in Metastatic Melanoma. JAMA 2011, 305, 2327–2334. [Google Scholar] [CrossRef]
- Jung, S.; Armstrong, E.; Wei, A.Z.; Ye, F.; Lee, A.; Carlino, M.S.; Sullivan, R.J.; Carvajal, R.D.; Shoushtari, A.N.; Johnson, D.B. Clinical and Genomic Correlates of Imatinib Response in Melanomas with KIT Alterations. Br. J. Cancer 2022, 127, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Joshi, U.M.; Hundal, J.; Mata, J.R.; Schollenberger, M.D.; Warrier, G.; Luke, J.J.; Lipson, E.J.; Funchain, P. Beyond Checkpoint Inhibition: Keeping Therapeutic Options Open. Am. Soc. Clin. Oncol. Educ. Book 2025, 45, e473856. [Google Scholar] [CrossRef] [PubMed]
- Hirai, I.; Tanese, K.; Fukuda, K.; Fusumae, T.; Nakamura, Y.; Sato, Y.; Amagai, M.; Funakoshi, T. Imatinib Mesylate in Combination with Pembrolizumab in Patients with Advanced KIT-Mutant Melanoma Following Progression on Standard Therapy: A Phase I/II Trial and Study Protocol. Medicine 2021, 100, e27832. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Cohen, J.V.; Tarantino, G.; Lian, C.G.; Liu, D.; Haq, R.; Hodi, F.S.; Lawrence, D.P.; Giobbie-Hurder, A.; Knoerzer, D.; et al. A Phase II Study of ERK Inhibition by Ulixertinib (BVD-523) in Metastatic Uveal Melanoma. Cancer Res. Commun. 2024, 4, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Najem, A.; Krayem, M.; Perdrix, A.; Kerger, J.; Awada, A.; Journe, F.; Ghanem, G. New Drug Combination Strategies in Melanoma: Current Status and Future Directions. Anticancer Res. 2017, 37, 5941–5953. [Google Scholar] [CrossRef]
- Rager, T.; Eckburg, A.; Patel, M.; Qiu, R.; Gantiwala, S.; Dovalovsky, K.; Fan, K.; Lam, K.; Roesler, C.; Rastogi, A.; et al. Treatment of Metastatic Melanoma with a Combination of Immunotherapies and Molecularly Targeted Therapies. Cancers 2022, 14, 3779. [Google Scholar] [CrossRef]
- Devlin, O.; Oladipo, O. Sequencing of Targeted Treatment and Immunotherapy in Advanced BRAF-Mutant Melanoma. J. Clin. Oncol. 2023, 41, 2295–2296. [Google Scholar] [CrossRef] [PubMed]
- Elkabets, M.; Vora, S.; Juric, D.; Morse, N.; Mino-Kenudson, M.; Muranen, T.; Tao, J.; Campos, A.D.; Rodon, J.; Ibrahim, Y.H.; et al. mTORC1 inhibition is required for sensitivity to PI3K inhibitors in PIK3CA-mutant breast cancer. Sci. Transl. Med. 2013, 5, 196ra99. [Google Scholar] [CrossRef]
- Okimoto, R.A.; Breitenbuecher, F.; Olivas, V.R.; Wu, W.; Gini, B.; Hofree, M.; Asthana, S.; Hrustanovic, G.; Flanagan, J.; Tulpule, A.; et al. Inactivation of Capicua Drives Cancer Metastasis. Nat. Genet. 2017, 49, 87–96. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Tumor immunoevasion through loss of interferon gamma pathway components. Nat. Rev. Clin. Oncol. 2019, 16, 442–456. [Google Scholar]
- Spranger, S.; Gajewski, T.F. Impact of oncogenic pathways on evasion of antitumor immune responses. Nat. Rev. Cancer 2018, 18, 139–147. [Google Scholar] [CrossRef]
- Ribas, A.; Lawrence, D.; Atkinson, V.; Agarwal, S.; Miller, W.H., Jr.; Carlino, M.S.; Fisher, R.; Long, G.V.; Hodi, F.S.; Tsoi, J.; et al. Combined BRAF and MEK Inhibition with PD-1 Blockade Immunotherapy in BRAF-Mutant Melanoma. Nat. Med. 2019, 25, 936–940. [Google Scholar] [CrossRef]
- Hu-Lieskovan, S.; Mok, S.; Homet Moreno, B.; Tsoi, J.; Robert, L.; Goedert, L.; Pinheiro, E.M.; Koya, R.C.; Graeber, T.G.; Comin-Anduix, B.; et al. Improved Antitumor Activity of Immunotherapy with BRAF and MEK Inhibitors in BRAF(V600E) Melanoma. Sci. Transl. Med. 2015, 7, 279ra41. [Google Scholar] [CrossRef] [PubMed]
- Frederick, D.T.; Piris, A.; Cogdill, A.P.; Cooper, Z.A.; Lezcano, C.; Ferrone, C.R.; Mitra, D.; Boni, A.; Newton, L.; Liu, C.; et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment. Clin. Cancer Res. 2013, 19, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Cooper, Z.A.; Juneja, V.R.; Sage, P.T.; Frederick, D.T.; Piris, A.; Mitra, D.; Lo, J.A.; Hodi, F.S.; Freeman, G.J.; Bosenberg, M.W. Response to BRAF Inhibition in Melanoma Is Enhanced When Combined with Immune Checkpoint Blockade. Cancer Immunol. Res. 2014, 2, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Lebbé, C.; Atkinson, V.; Mandalà, M.; Nathan, P.D.; Arance, A.; Richtig, E.; Yamazaki, N.; Robert, C.; Schadendorf, D.; et al. Combined PD-1, BRAF and MEK Inhibition in Advanced BRAF-Mutant Melanoma: Safety Run-In and Biomarker Cohorts of COMBI-i. Nat. Med. 2020, 26, 1557–1563. [Google Scholar] [CrossRef]
- Richmond, C.S.; Vallatharasu, Y.; Deviley, J.A.; Vos, C.R.; Parsons, B.M.; Kenny, P.A. Sequential Treatment Failures in Response to BRAF/MEK and Immune Checkpoint Inhibitors Mediated by MAP2K2 and B2M Mutations in Melanoma. Exp. Mol. Pathol. 2019, 110, 104260. [Google Scholar] [CrossRef]
- Stege, H.; Haist, M.; Schultheis, M.; Fleischer, M.I.; Mohr, P.; Meier, F.; Schadendorf, D.; Ugurel, S.; Livingstone, E.; Zimmer, L.; et al. Discontinuation of BRAF/MEK-directed targeted therapy after complete remission of metastatic melanoma—A retrospective multicenter ADOReg study. Cancers 2021, 13, 2312. [Google Scholar] [CrossRef]
- Arozarena, I.; Wellbrock, C. Overcoming Resistance to BRAF Inhibitors. Ann. Transl. Med. 2017, 5, 387. [Google Scholar] [CrossRef]
- Morris, E.J.; Jha, S.; Restaino, C.R.; Dayananth, P.; Zhu, H.; Cooper, A.; Carr, D.; Deng, Y.; Jin, W.; Black, S.; et al. Discovery of a Novel ERK Inhibitor with Activity in Models of Acquired Resistance to BRAF and MEK Inhibitors. Cancer Discov. 2013, 3, 742–750. [Google Scholar] [CrossRef]
- Hung, C.; Nguyen, T.T.T.; Poulikakos, P.I.; Polsky, D. Recent Developments in Targeting the Cell Cycle in Melanoma. Cancers 2025, 17, 1291. [Google Scholar] [CrossRef]
- Lelliott, E.J.; Sheppard, K.E.; McArthur, G.A. Harnessing the Immunotherapeutic Potential of CDK4/6 Inhibitors in Melanoma: Is Timing Everything? NPJ Precis. Oncol. 2022, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Garutti, M.; Targato, G.; Buriolla, S.; Palmero, L.; Minisini, A.M.; Puglisi, F. CDK4/6 Inhibitors in Melanoma: A Comprehensive Review. Cells 2021, 10, 1334. [Google Scholar] [CrossRef]
- Posch, C.; Vujic, I.; Monshi, B.; Sanlorenzo, M.; Weihsengruber, F.; Rappersberger, K.; Ortiz-Urda, S. Searching for the Chokehold of NRAS Mutant Melanoma. J. Investig. Dermatol. 2016, 136, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Alobaidi, R.; Islam, N.; Olkey, T.; Haribabu, Y.; Shamo, M.; Sykora, P.; Simbulan-Rosenthal, C.M.; Rosenthal, D.S. Combinational Inhibition of MEK and AKT Synergistically Induces Melanoma Stem Cell Apoptosis and Blocks NRAS Tumor Growth. Cells 2025, 14, 248. [Google Scholar] [CrossRef]
- Kado, S.; Komine, M. Recent Advances in Molecular Research and Treatment for Melanoma in Asian Populations. Int. J. Mol. Sci. 2025, 26, 5370. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef] [PubMed]
- Vergara, I.A.; Wilmott, J.S.; Long, G.V.; Scolyer, R.A. Genetic drivers of non-cutaneous melanomas: Challenges and opportunities in a heterogeneous landscape. Exp. Dermatol. 2022, 31, 13–30. [Google Scholar] [CrossRef]
- Norain, A.; Dadachova, E. Targeted Radionuclide Therapy of Melanoma. Semin. Nucl. Med. 2016, 46, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Namikawa, K.; Yamazaki, N. Targeted Therapy and Immunotherapy for Melanoma in Japan. Curr. Treat. Options Oncol. 2019, 20, 7. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Lens, M.; Cocorocchio, E. Combined BRAF-Targeted Therapy with Immunotherapy in BRAF-Mutated Advanced Melanoma Patients. Curr. Oncol. Rep. 2021, 23, 138. [Google Scholar] [CrossRef]
- McKenna, S.; García-Gutiérrez, L. Resistance to Targeted Therapy and RASSF1A Loss in Melanoma: What Are We Missing? Int. J. Mol. Sci. 2021, 22, 5115. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, Y.; Cao, L.; Wang, J.; Liang, H. Research Hotspots and Trends of Immunotherapy and Melanoma: A Bibliometric Analysis during 2014–2024. Hum. Vaccines Immunother. 2025, 21, 2464379. [Google Scholar] [CrossRef]
- Noman, M.Z.; Szpakowska, M.; Xiao, M.; Gao, R.; Van Moer, K.; Kumar, A.; Ollert, M.; Berchem, G.; Chevigné, A.; Janji, B. Targeting the Atypical Chemokine Receptor 2 (Ackr2) Improves the Benefit of Anti-PD-1 Immunotherapy in Melanoma Mouse Model. Oncoimmunology 2025, 14, 2494426. [Google Scholar] [CrossRef]
- Paul, S.; Kaya, M.; Johnsson, O.; Grauers Wiktorin, H.; Törnell, A.; Arabpour, M.; Hellstrand, K.; Martner, A. Targeting Murine Metastatic Cancers with Cholera Toxin A1-Adjuvanted Peptide Vaccines. Hum. Vaccines Immunother. 2025, 21, 2455240. [Google Scholar] [CrossRef]
- Shang, Y.; Zhao, H. Research Progress of Chinese Medicinal Monomers in the Process of Melanoma Occurrence. Pharm. Biol. 2025, 63, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Frey, G.; Wang, J.; Liu, H.; Xing, C.; Chen, J.; Boyle, W.J.; Short, J.M. Preclinical Development of Ozuriftamab Vedotin (BA3021), a Novel ROR2-Specific Conditionally Active Biologic Antibody-Drug Conjugate. MAbs 2025, 17, 2490078. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Si, L.; Kong, Y.; Flaherty, K.T.; Xu, X.; Zhu, Y.; Shi, X.; Yu, X.; Xiao, L.; Ma, X.; et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-KIT mutation or amplification. J. Clin. Oncol. 2011, 29, 2904–2909. [Google Scholar] [CrossRef]
- Beadling, C.; Jacobson-Dunlop, E.; Hodi, F.S.; Le, C.; Warrick, A.; Patterson, J.; Town, A.; Duvic, M.; Haluska, F.G. KIT gene mutations and copy number in melanoma subtypes. Clin. Cancer Res. 2008, 14, 6821–6828. [Google Scholar] [CrossRef]
- Grimaldi, A.M.; Simeone, E.; Festino, L.; Vanella, V.; Palla, M.; Ascierto, P.A. Novel mechanisms and therapeutic approaches in melanoma: Targeting the MAPK pathway. Discov. Med. 2015, 19, 455–461. [Google Scholar]
- Orlandella, F.M.; Arcone, R.; Luciano, N.; Salvatore, G.; Motti, M.L. Novel Biological Strategies for Melanoma Therapy: A Focus on lncRNAs and Their Targeting. Cancers 2025, 17, 1273. [Google Scholar] [CrossRef] [PubMed]
- Menzies, A.M.; Haydu, L.E.; Carlino, M.S.; Azer, M.W.; Carr, P.J.; Kefford, R.F.; Long, G.V. Inter- and intra-patient heterogeneity of response and progression to targeted therapy in metastatic melanoma. PLoS ONE 2014, 9, e85004. [Google Scholar] [CrossRef]
- Wang, X.; Ma, S.; Zhu, S.; Zhu, L.; Guo, W. Advances in Immunotherapy and Targeted Therapy of Malignant Melanoma. Biomedicines 2025, 13, 225. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune Checkpoint Inhibitors in Melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Robert, C.; Gastman, B.; Gogas, H.; Rutkowski, P.; Long, G.V.; Chaney, M.F.; Joshi, H.; Lin, Y.L.; Snyder, W.; Chesney, J.A. Open-Label, Phase II Study of Talimogene Laherparepvec plus Pembrolizumab for the Treatment of Advanced Melanoma That Progressed on Prior Anti-PD-1 Therapy: MASTERKEY-115. Eur. J. Cancer 2024, 207, 114120. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; Brochez, L.; Del Marmol, V.; et al. European Consensus-Based Interdisciplinary Guideline for Melanoma. Part 1: Diagnostics—Update 2024. Eur. J. Cancer 2025, 215, 115152. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, L.; Lancellotta, V.; Fionda, B.; Mangoni, M.; Casà, C.; Di Stefani, A.; Pagliara, M.M.; D’Aviero, A.; Schinzari, G.; Chiesa, S.; et al. Immunotherapy and Radiotherapy in Melanoma: A Multidisciplinary Comprehensive Review. Hum. Vaccines Immunother. 2022, 18, 1903827. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Hauschild, A.; Santinami, M.; Kirkwood, J.M.; Atkinson, V.; Mandala, M.; Merelli, B.; Sileni, V.C.; Nyakas, M.; Haydon, A.; et al. Final Results for Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. N. Engl. J. Med. 2024, 391, 1709–1720. [Google Scholar] [CrossRef]
- Sharma, A.; Shah, S.R.; Illum, H.; Dowell, J. Vemurafenib: Targeted Inhibition of Mutated BRAF for Treatment of Advanced Melanoma and Its Potential in Other Malignancies. Drugs 2012, 72, 2207–2222. [Google Scholar] [CrossRef] [PubMed]
- Shaw, H.M.; Nathan, P.D. Vemurafenib in Melanoma. Expert Rev. Anticancer Ther. 2013, 13, 513–522. [Google Scholar] [CrossRef]
- Bollag, G.; Tsai, J.; Zhang, J.; Zhang, C.; Ibrahim, P.; Nolop, K.; Hirth, P. Vemurafenib: The First Drug Approved for BRAF-Mutant Cancer. Nat. Rev. Drug Discov. 2012, 11, 873–886. [Google Scholar] [CrossRef]
- Tsai, J.; Lee, J.T.; Wang, W.; Zhang, J.; Cho, H.; Mamo, S.; Bremer, R.; Gillette, S.; Kong, J.; Haass, N.K.; et al. Discovery of a Selective Inhibitor of Oncogenic B-Raf Kinase with Potent Antimelanoma Activity. Proc. Natl. Acad. Sci. USA 2008, 105, 3041–3046. [Google Scholar] [CrossRef]
- Heidorn, S.J.; Milagre, C.; Whittaker, S.; Nourry, A.; Niculescu-Duvaz, D.; Dhomen, N.; Marais, R. Kinase-Dead BRAF and Oncogenic RAS Cooperate to Drive Tumor Progression through CRAF. Cell 2010, 140, 209–221. [Google Scholar] [CrossRef]
- Daugaard, N.D.; Tholstrup, R.; Tornby, J.R.; Bendixen, S.M.; Larsen, F.T.; De Zio, D.; Barnkob, M.B.; Ravnskjaer, K.; Brewer, J.R. Characterization of Human Melanoma Skin Cancer Models: A Step towards Model-Based Melanoma Research. Acta Biomater. 2025, 191, 308–324. [Google Scholar] [CrossRef]
- Wanchoo, R.; Jhaveri, K.D.; Deray, G.; Launay-Vacher, V. Renal Effects of BRAF Inhibitors: A Systematic Review by the Cancer and the Kidney International Network. Clin. Kidney J. 2016, 9, 245–251. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Yasothan, U.; Kirkpatrick, P. Vemurafenib. Nat. Rev. Drug Discov. 2011, 10, 811–812. [Google Scholar] [CrossRef]
- Sosman, J.A.; Kim, K.B.; Schuchter, L.; Gonzalez, R.; Pavlick, A.C.; Weber, J.S.; McArthur, G.A.; Hutson, T.E.; Moschos, S.J.; Flaherty, K.T.; et al. Survival in BRAF V600–Mutant Advanced Melanoma Treated with Vemurafenib. N. Engl. J. Med. 2012, 366, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Haroche, J.; Cohen-Aubart, F.; Emile, J.F.; Arnaud, L.; Maksud, P.; Charlotte, F.; Cluzel, P.; Aouba, A.; Hermine, O.; Amoura, Z. Dramatic Efficacy of Vemurafenib in Both Multisystemic and Refractory Erdheim–Chester Disease and Langerhans Cell Histiocytosis Harboring the BRAF V600E Mutation. Blood 2013, 121, 1495–1500. [Google Scholar] [CrossRef]
- Diamond, E.L.; Subbiah, V.; Lockhart, A.C.; Blay, J.Y.; Puzanov, I.; Chau, I.; Wolf, J.; Raje, N.; Van de Velde, H.; Thappa, R.; et al. Vemurafenib for BRAF V600–Mutant Erdheim–Chester Disease and Langerhans Cell Histiocytosis: Analysis of Data from the Histology-Independent, Phase 2 VE-BASKET Study. JAMA Oncol. 2018, 4, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Anaya, Y.A.; Bracho, R.P.; Chauhan, S.C.; Tripathi, M.K.; Bandyopadhyay, D. Small Molecule B-RAF Inhibitors as Anti-Cancer Therapeutics: Advances in Discovery, Development, and Mechanistic Insights. Int. J. Mol. Sci. 2025, 26, 2676. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, A.; Grob, J.J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H.; Kaempgen, E.; et al. Dabrafenib in BRAF-Mutated Metastatic Melanoma: A Multicentre, Open-Label, Phase 3 Randomised Controlled Trial. Lancet 2012, 380, 358–365. [Google Scholar] [CrossRef]
- McArthur, G.A.; Chapman, P.B.; Robert, C.; Larkin, J.; Haanen, J.B.; Dummer, R.; Ascierto, P.A.; Garbe, C.; Testori, A.; Maio, M.; et al. Safety and Efficacy of Vemurafenib in BRAF(V600E) and BRAF(V600K) Mutation-Positive Melanoma (BRIM-3): Extended Follow-Up of a Phase 3, Randomised, Open-Label Study. Lancet Oncol. 2014, 15, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; De Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J.; et al. Dabrafenib and Trametinib versus Dabrafenib and Placebo for Val600 BRAF-Mutant Melanoma: A Multicentre, Double-Blind, Phase 3 Randomised Controlled Trial. Lancet 2015, 386, 444–451. [Google Scholar] [CrossRef]
- Poulikakos, P.I.; Persaud, Y.; Janakiraman, M.; Kong, X.; Ng, C.; Moriceau, G.; Shi, H.; Atefi, M.; Titz, B.; Gabay, M.T.; et al. RAF Inhibitor Resistance Is Mediated by Dimerization of Aberrantly Spliced BRAF(V600E). Nature 2011, 480, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Hugo, W.; Kong, X.; Hong, A.; Koya, R.C.; Moriceau, G.; Chodon, T.; Guo, R.; Johnson, D.B.; Dahlman, K.B.; et al. Acquired Resistance and Clonal Evolution in Melanoma during BRAF Inhibitor Therapy. Cancer Discov. 2014, 4, 80–93. [Google Scholar] [CrossRef]
- Straussman, R.; Morikawa, T.; Shee, K.; Barzily-Rokni, M.; Qian, Z.R.; Du, J.; Davis, A.; Mongare, M.M.; Gould, J.; Frederick, D.T.; et al. Tumour Micro-Environment Elicits Innate Resistance to RAF Inhibitors through HGF Secretion. Nature 2012, 487, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Titz, B.; Lomova, A.; Le, A.; Hugo, W.; Ten Hoeve, J.; Shi, H.; Moriceau, G.; Song, C.; Hong, A.; Johnson, D.B.; et al. JUN Dependency in Distinct Early and Late BRAF Inhibitor Resistance States of Melanoma. Cell Discov. 2016, 2, 16028. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Duvic, M.; Hauschild, A.; Prieto, V.G.; Robert, C.; Schadendorf, D.; Kim, C.C.; McCormack, C.J.; Myskowski, P.L.; Spleiss, O.; et al. Analysis of Dermatologic Events in Vemurafenib-Treated Patients with Melanoma. Oncologist 2013, 18, 314–322. [Google Scholar] [CrossRef]
- Anforth, R.M.; Blumetti, T.C.; Kefford, R.F.; Sharma, R.; Scolyer, R.A.; Kossard, S.; Long, G.V.; Fernandez-Peñas, P. Cutaneous Manifestations of Dabrafenib (GSK2118436): A Selective Inhibitor of Mutant BRAF in Patients with Metastatic Melanoma. Br. J. Dermatol. 2012, 167, 1153–1160. [Google Scholar] [CrossRef]
- Jordan, E.J.; Kelly, C.M. Vemurafenib for the Treatment of Melanoma. Expert Opin. Pharmacother. 2012, 13, 2533–2543. [Google Scholar] [CrossRef]
- Poduje, S.; Brozić, J.M.; Prkačin, I.; Delaš Aždajić, M.; Goren, A. Vemurafenib and Cobimetinib-Induced Toxic Epidermal Necrolysis in a Patient with Metastatic Melanoma. Dermatol. Ther. 2020, 33, e13174. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Viros, A.; Milagre, C.; Trunzer, K.; Bollag, G.; Spleiss, O.; Reis-Filho, J.S.; Kong, X.; Koya, R.C.; Flaherty, K.T.; et al. RAS Mutations in Cutaneous Squamous-Cell Carcinomas in Patients Treated with BRAF Inhibitors. N. Engl. J. Med. 2012, 366, 207–215. [Google Scholar] [CrossRef]
- Oberholzer, P.A.; Kee, D.; Dziunycz, P.; Sucker, A.; Kamsukom, N.; Jones, R.; Prena, J.; Huber, C.; Goldinger, S.M.; Dornbierer, J.; et al. RAS Mutations Are Associated with the Development of Cutaneous Squamous Cell Tumors in Patients Treated with RAF Inhibitors. J. Clin. Oncol. 2012, 30, 316–321. [Google Scholar] [CrossRef]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef]
- Wu, J.; Ding, Q.; Zhang, Q.; Chen, Q.; Wen, X.; Ding, Y.; Li, J.; Chen, Z.; Zhang, T.; Wang, J.; et al. Addition of Anti-PD-1 Immunotherapy to BRAF Inhibitor-Based Targeted Therapy Improves Real-World Survival and Delays Brain Metastases in Patients with BRAFV600-Mutant Advanced Melanoma: A Multicenter Cohort Study. MedComm 2025, 6, e70102. [Google Scholar] [CrossRef]
- Gutzmer, R.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Garbe, C.; de la Cruz-Merino, L.; Schachter, J.; et al. Atezolizumab, Vemurafenib, and Cobimetinib as First-Line Treatment for Unresectable Advanced BRAF(V600) Mutation-Positive Melanoma (IMspire150): Primary Analysis of the Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2020, 395, 1835–1844. [Google Scholar] [CrossRef]
- Dugan, M.M.; Perez, M.C.; Karapetyan, L.; Zager, J.S. Combination Atezolizumab, Cobimetinib, and Vemurafenib as a Treatment Option in BRAF V600 Mutation-Positive Melanoma: Patient Selection and Perspectives. Cancer Manag. Res. 2024, 16, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, E.; Gogas, H.J.; Kandolf, L.; Meier, F.; Eigentler, T.K.; Ziemer, M.; Terheyden, P.; Gesierich, A.; Herbst, R.A.; Kähler, K.C.; et al. Early Switch from Run-In with Targeted to Immunotherapy in Advanced BRAFV600-Positive Melanoma: Final Results of the Randomised Phase II ImmunoCobiVem Trial. ESMO Open 2025, 10, 105053. [Google Scholar] [CrossRef] [PubMed]
- Block, M.S.; Nelson, G.D.; Chen, J.; Johnson, S.; Yang, L.; Flotte, T.J.; Grewal, E.P.; McWilliams, R.R.; Kottschade, L.A.; Domingo-Musibay, E.; et al. Neoadjuvant Cobimetinib and Atezolizumab with or without Vemurafenib for Stage III Melanoma: Outcomes and the Impact of the Microbiome from the NeoACTIVATE Trial. J. Immunother. Cancer 2025, 13, e011706. [Google Scholar] [CrossRef]
- Boz Er, A.B.; Sheldrake, H.M.; Sutherland, M. Overcoming Vemurafenib Resistance in Metastatic Melanoma: Targeting Integrins to Improve Treatment Efficacy. Int. J. Mol. Sci. 2024, 25, 7946. [Google Scholar] [CrossRef]
- Galluzzi, L.; Humeau, J.; Buqué, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with Chemotherapy in the Era of Immune Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Ruscetti, M.; Dadashian, E.L.; Guo, W.; Quach, B.; Mulholland, D.J.; Park, J.W.; Tran, L.M.; Kobayashi, N.; Bianchi-Frias, D.; Xing, Y.; et al. HDAC Inhibition Impedes Epithelial-Mesenchymal Plasticity and Suppresses Metastatic, Castration-Resistant Prostate Cancer. Oncogene 2016, 35, 3781–3795. [Google Scholar] [CrossRef]
- Acquaviva, J.; Smith, D.L.; Jimenez, J.P.; Zhang, C.; Sequeira, M.; He, S.; Sang, J.; Bates, R.C.; Proia, D.A. Overcoming Acquired BRAF Inhibitor Resistance in Melanoma via Targeted Inhibition of HSP90 with Ganetespib. Mol. Cancer Ther. 2014, 13, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Biersack, B.; Nitzsche, B.; Höpfner, M. Histone Deacetylases in the Regulation of Cell Death and Survival Mechanisms in Resistant BRAF-Mutant Cancers. Cancer Drug Resist. 2025, 8, 6. [Google Scholar] [CrossRef]
- Das Thakur, M.; Salangsang, F.; Landman, A.S.; Sellers, W.R.; Pryer, N.K.; Levesque, M.P.; Dummer, R.; McMahon, M. Modelling Vemurafenib Resistance in Melanoma Reveals a Strategy to Forestall Drug Resistance. Nature 2013, 494, 251–255. [Google Scholar] [CrossRef]
- Wang, M.; Yu, F.; Zhang, Y. Present and Future of Cancer Nano-Immunotherapy: Opportunities, Obstacles and Challenges. Mol. Cancer 2025, 24, 26. [Google Scholar] [CrossRef] [PubMed]
- Shebrain, A.; Idris, O.A.; Jawad, A.; Zhang, T.; Xing, Y. Advancements and Challenges in Personalized Therapy for BRAF-Mutant Melanoma: A Comprehensive Review. J. Clin. Med. 2024, 13, 5409. [Google Scholar] [CrossRef]
- Holderfield, M.; Deuker, M.M.; McCormick, F.; McMahon, M. Targeting RAF Kinases for Cancer Therapy: BRAF-Mutated Melanoma and Beyond. Nat. Rev. Cancer 2014, 14, 455–467. [Google Scholar] [CrossRef]
- Karoulia, Z.; Gavathiotis, E.; Poulikakos, P.I. New Perspectives for Targeting RAF Kinase in Human Cancer. Nat. Rev. Cancer 2017, 17, 676–691. [Google Scholar] [CrossRef]
- Kim, G.; McKee, A.E.; Ning, Y.M.; Hazarika, M.; Theoret, M.; Johnson, J.R.; Xu, Q.C.; Tang, S.; Sridhara, R.; Jiang, X.; et al. FDA Approval Summary: Vemurafenib for Treatment of Unresectable or Metastatic Melanoma with the BRAFV600E Mutation. Clin. Cancer Res. 2014, 20, 4994–5000. [Google Scholar] [CrossRef]
- Carlino, M.S.; Fung, C.; Shahheydari, H.; Todd, J.R.; Boyd, S.C.; Irvine, M.; Nagrial, A.; Wilmott, J.S.; Kefford, R.F.; Long, G.V.; et al. Preexisting MEK1P124 Mutations Diminish Response to BRAF Inhibitors in Melanoma. Clin. Cancer Res. 2015, 21, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Prado-Souza, L.F.L.D.; Ferraz, L.S.; Citrangulo, T.T., Jr.; Ribeiro, C.A.J.; Amaral, D.T.D.; Arruda, D.C.; Oliveira, É.A.; Chammas, R.; Maria-Engler, S.S.; Rodrigues, T. Exploiting Paradoxical Activation of Oncogenic MAPK Signaling by Targeting Mitochondria to Sensitize NRAS Mutant-Melanoma to Vemurafenib. Int. J. Mol. Sci. 2025, 26, 2675. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Usuki, S.; Nishizawa, M.; Tanaka, N.; Suhara, Y.; Yajima, I. Acyclic Retinoid Overcomes Vemurafenib Resistance in Melanoma Cells via Dual Inhibition of MAPK and PI3K/AKT/mTOR Pathways. Anticancer Res. 2025, 45, 2265–2278. [Google Scholar] [CrossRef]
- van der Hiel, B.; de Wit-van der Veen, B.J.; van den Eertwegh, A.J.M.; Vogel, W.V.; Stokkel, M.P.M.; Lopez-Yurda, M.; Boellaard, R.; Kapiteijn, E.W.; Hospers, G.A.P.; Aarts, M.J.B.; et al. Metabolic Parameters on Baseline and Early [18F]FDG PET/CT as a Predictive Biomarker for Resistance to BRAF/MEK Inhibition in Advanced Cutaneous BRAFV600-Mutated Melanoma. EJNMMI Res. 2025, 15, 60. [Google Scholar] [CrossRef]
- Foda, B.M.; Baker, A.E.; Joachimiak, Ł.; Mazur, M.; Neubig, R.R. Mechanistic Insights into Rho/MRTF Inhibition-Induced Apoptotic Events and Prevention of Drug Resistance in Melanoma: Implications for the Involvement of Pirin. Front. Pharmacol. 2025, 16, 1505000. [Google Scholar] [CrossRef]
- Kim, J.; Brunetti, B.; Kumar, A.; Mangla, A.; Honda, K.; Yoshida, A. Inhibition of Glutaminase Elicits Senolysis in Therapy-Induced Senescent Melanoma Cells. Cell Death Dis. 2024, 15, 902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, Z.; Wang, H.; Li, L.; Dong, L.; Ding, L.; Li, Q.; Zhu, L.; Zhang, T.; Zhu, Y.; et al. CTHRC1 Is Associated with BRAF(V600E) Mutation and Correlates with Prognosis, Immune Cell Infiltration, and Drug Resistance in Colon Cancer, Thyroid Cancer, and Melanoma. Biomol. Biomed. 2024, 25, 42–61. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Chen, D. Interfering Nuclear Protein Laminb1 Induces DNA Damage and Reduces Vemurafenib Resistance in Melanoma Cells In Vitro. Cancers 2024, 16, 4060. [Google Scholar] [CrossRef]
- Shalem, O.; Sanjana, N.E.; Hartenian, E.; Shi, X.; Scott, D.A.; Mikkelson, T.; Heckl, D.; Ebert, B.L.; Root, D.E.; Doench, J.G.; et al. Genome-Scale CRISPR-Cas9 Knockout Screening in Human Cells. Science 2014, 343, 84–87. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, F.; Chen, T.; Wang, H.; Wang, W.; Jin, H.; Li, C.; Guo, X.; Liu, Y.; Zhang, Y.; et al. Cholesterol Neutralized Vemurafenib Treatment by Promoting Melanoma Stem-Like Cells via Its Metabolite 27-Hydroxycholesterol. Cell. Mol. Life Sci. 2024, 81, 226. [Google Scholar] [CrossRef]
- Park, B.S.; Jeon, H.; Kim, Y.; Kwon, H.; Choi, G.E.; Chi, S.G.; Park, H.M.; Lee, H.; Kim, T. Polyamine and EIF5A Hypusination Downstream of c-Myc Confers Targeted Therapy Resistance in BRAF Mutant Melanoma. Mol. Cancer 2024, 23, 136. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Infante, J.R.; Daud, A.; Gonzalez, R.; Kefford, R.F.; Sosman, J.; Hamid, O.; Schuchter, L.; Cebon, J.; Ibrahim, N.; et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. 2012, 367, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Torres, N.M.; Tao, A.; Gao, Y.; Luo, L.; Li, Q.; de Stanchina, E.; Abdel-Wahab, O.; Solit, D.B.; Poulikakos, P.I.; et al. BRAF mutants evade ERK-dependent feedback by different mechanisms that determine their sensitivity to pharmacologic inhibition. Cancer Cell 2015, 28, 370–383. [Google Scholar] [CrossRef]
- Owen, K.L.; Brockwell, N.K.; Parker, B.S. JAK-STAT Signaling: A Double-Edged Sword of Immune Regulation and Cancer Progression. Cancers 2019, 11, 2002. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Drug Approval Package Mekinist (Trametinib). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204114Orig1s000TOC.cfm (accessed on 24 June 2025).
- Foth, M.; McMahon, M. Autophagy Inhibition in BRAF-Driven Cancers. Cancers 2021, 13, 3498. [Google Scholar] [CrossRef]
- Hoffner, B.; Benchich, K. Trametinib: A Targeted Therapy in Metastatic Melanoma. J. Adv. Pract. Oncol. 2018, 9, 741–745. [Google Scholar]
- Sullivan, R.J.; Flaherty, K.T. Resistance to BRAF-targeted therapy in melanoma. Eur. J. Cancer 2013, 49, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Wagle, N.; Emery, C.; Berger, M.F.; Davis, M.J.; Sawyer, A.; Pochanard, P.; Kehoe, S.M.; Johannessen, C.M.; MacConaill, L.E.; Hahn, W.C.; et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J. Clin. Oncol. 2011, 29, 3085–3096. [Google Scholar] [CrossRef] [PubMed]
- Khunger, A.; Khunger, M.; Velcheti, V. Dabrafenib in Combination with Trametinib in the Treatment of Patients with BRAF V600-Positive Advanced or Metastatic Non-Small Cell Lung Cancer: Clinical Evidence and Experience. Ther. Adv. Respir. Dis. 2018, 12, 1753466618767611. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, P.; Fava, P.; Tonella, L.; Rubatto, M.; Ribero, S.; Fierro, M.T. Treatment of Advanced Metastatic Melanoma. Dermatol. Pract. Concept 2021, 11 (Suppl. S1), e2021164S. [Google Scholar] [CrossRef]
- Gouda, M.A.; Subbiah, V. Expanding the Benefit: Dabrafenib/Trametinib as Tissue-Agnostic Therapy for BRAF V600E-Positive Adult and Pediatric Solid Tumors. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e404770. [Google Scholar] [CrossRef]
- Mubarak, O.; Middleton, G.W. The Impact of the Immunological Context on Outcomes of Solid Cancer Patients Treated with Genotype-Matched Targeted Therapies: A Comprehensive Review. Ann. Oncol. 2025, 36, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shen, F. Metastatic Pancreatic Cancer with Activating BRAF V600E Mutations: A Case Report. World J. Clin. Cases 2025, 13, 101665. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, C.; Cai, Y. Primary Rectal Malignant Melanoma with Schistosomiasis. Intractable Rare Dis. Res. 2025, 14, 145–147. [Google Scholar] [CrossRef]
- Weber, J.; Haque, W.; Markovic, S.N.; Salama, A.K.S.; Mehmi, I.; Sullivan, R.J.; Najjar, Y.G.; van Akkooi, A.C.J.; Menzies, A.M.; Long, G.V.; et al. Relapse-Free Survival with Adjuvant Dabrafenib/Trametinib Therapy after Relapse on a Prior Adjuvant CPI in BRAF V600-Mutated Stage III/IV Melanoma. Oncologist 2025, 30, oyae289. [Google Scholar] [CrossRef]
- Gorry, C.; McCullagh, L.; O’Donnell, H.; Barrett, S.; Schmitz, S.; Barry, M.; Curtin, K.; Beausang, E.; Barry, R.; Coyne, I. Neoadjuvant Treatment for Stage III and IV Cutaneous Melanoma. Cochrane Database Syst. Rev. 2023, 1, CD012974. [Google Scholar] [CrossRef]
- Keser, M.; Atmaca, H. Let-7a MicroRNA Modulates Caspase-3-Dependent Apoptosis in Melanoma Cells Treated with Dabrafenib and Trametinib Combination. Ir. J. Med. Sci. 2025, 194, 797–805. [Google Scholar] [CrossRef]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef]
- Davies, M.A.; Saiag, P.; Robert, C.; Grob, J.J.; Flaherty, K.T.; Arance, A.M.; Demidov, L.V.; Garbe, C.; Chiarion-Sileni, V.; Rutkowski, P.; et al. Dabrafenib plus trametinib in patients with BRAF-mutant melanoma brain metastases (COMBI-MB): A multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Wilmott, J.S.; Tawbi, H.; Engh, J.A.; Amankulor, N.M.; Shivalingam, B.; Banerjee, H.; Vergara, I.A.; Lee, H.; Johansson, P.A.; Ferguson, P.M.; et al. Clinical Features Associated with Outcomes and Biomarker Analysis of Dabrafenib plus Trametinib Treatment in Patients with BRAF-Mutant Melanoma Brain Metastases. Clin. Cancer Res. 2023, 29, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.D.; Xu, Y.; Ren, Z.W.; Li, L.Q.; Zhang, L.; Li, Y.; Lou, L.S.; Yao, W.T.; Liu, Z.; Li, X.A.; et al. Is One Year Enough? Extended Adjuvant Dabrafenib Plus Trametinib for Chinese Patients with Resected Stage III Melanoma. J. Dermatol. 2025, 52, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Chiaravalli, S.; Indini, A.; Del Vecchio, M.; Casanova, M.; Massimino, M.; Bergamaschi, L.; Ferrari, A. Dabrafenib and Trametinib for the Treatment of Pediatric and Adolescent Melanoma: Single Center Experience Data from Italian Compassionate Use. Pediatr. Blood Cancer 2025, 72, e31644. [Google Scholar] [CrossRef] [PubMed]
- Pélouard, F.; Chedru-Legros, V.; Nganoa, C.; Dompmartin, A.; L’Orphelin, J.M. Optimizing Targeted Therapy for Metastatic Melanoma: A Combination of Encorafenib and Trametinib beyond Standard Protocols. Dermatol. Rep. 2025. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Shao, W.; Gao, J.; Xiang, M.; Wang, Y.; Liu, M.; Zhang, W.; Liang, X. MEK Inhibitors for the Treatment of Immunotherapy-Resistant, AGK-BRAF Fusion Advanced Acral Melanoma: A Case Report and Literature Review. J. Cancer Res. Clin. Oncol. 2025, 151, 133. [Google Scholar] [CrossRef]
- Wiesweg, M.; Alaffas, A.; Rasokat, A.; Saalfeld, F.C.; Rost, M.; Assmann, C.; Herster, F.; Hilbrandt, M.; Griesinger, F.; Kron, A.; et al. Treatment Sequences in BRAF-V600-Mutated NSCLC: First-Line Targeted Therapy Versus First-Line (Chemo-) Immunotherapy. J. Thorac. Oncol. 2025, 20, 1328–1335. [Google Scholar] [CrossRef]
- Goethe, E.A.; Srinivasan, S.; Kumar, S.; Prabhu, S.S.; Gubbiotti, M.A.; Ferguson, S.D. High-Grade Astrocytoma with Piloid Features: A Single-Institution Case Series and Literature Review. Acta Neuropathol. Commun. 2025, 13, 82. [Google Scholar] [CrossRef]
- Kim, K.B.; Kefford, R.; Pavlick, A.C.; Infante, J.R.; Ribas, A.; Sosman, J.A.; Fecher, L.A.; Millward, M.; McArthur, G.A.; Hwu, P.; et al. Phase II Study of the MEK1/MEK2 Inhibitor Trametinib in Patients with Metastatic BRAF-Mutant Cutaneous Melanoma Previously Treated with or without a BRAF Inhibitor. J. Clin. Oncol. 2013, 31, 482–489. [Google Scholar] [CrossRef]
- Livingstone, E.; Zimmer, L.; Vaubel, J.; Schadendorf, D. BRAF, MEK and KIT Inhibitors for Melanoma: Adverse Events and Their Management. Chin. Clin. Oncol. 2014, 3, 29. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Kurzrock, R.; Valero, V.; Gonzalez, R.; Heist, R.S.; Tan, A.R.; Means-Powell, J.; Werner, T.L.; Becerra, C.; Wang, C.; et al. Phase I Dose-Escalation Trial of the Oral AKT Inhibitor Uprosertib in Combination with the Oral MEK1/MEK2 Inhibitor Trametinib in Patients with Solid Tumors. Cancer Chemother. Pharmacol. 2020, 85, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.M.; Simeone, E.; Festino, L.; Vanella, V.; Strudel, M.; Ascierto, P.A. MEK Inhibitors in the Treatment of Metastatic Melanoma and Solid Tumors. Am. J. Clin. Dermatol. 2017, 18, 745–754. [Google Scholar] [CrossRef]
- Wilmott, J.S.; Long, G.V.; Howle, J.R.; Haydu, L.E.; Sharma, R.; Thompson, J.F.; Kefford, R.F.; Hersey, P.; Scolyer, R.A. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin. Cancer Res. 2012, 18, 1386–1394. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Hamid, O.; Gonzalez, R.; Infante, J.R.; Patel, S.P.; Kudchadkar, R.R.; Sznol, M.; Kluger, H.; Maio, M.; Mitchell, T.C.; et al. Atezolizumab plus cobimetinib and vemurafenib in BRAF-mutated melanoma patients. Nat. Med. 2019, 25, 929–935. [Google Scholar] [CrossRef]
- Planchard, D.; Besse, B.; Groen, H.J.M.; Souquet, P.J.; Quoix, E.; Baik, C.S.; Barlesi, F.; Kim, T.M.; Mazieres, J.; Novello, S.; et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small cell lung cancer: An open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1307–1316. [Google Scholar] [CrossRef]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, B.C.; Schellens, J.H.M.; Soria, J.C.; Gainor, J.F.; Vansteenkiste, J.F.; Wolf, J.; Seto, T.; et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600E-mutant anaplastic thyroid cancer. J. Clin. Oncol. 2018, 36, 7–13. [Google Scholar] [CrossRef]
- Ebert, P.J.R.; Cheung, J.; Yang, Y.; McNamara, E.; Hong, R.; Moskalenko, M.; Gould, S.E.; Maecker, H.; Irving, B.A.; Kim, J.M.; et al. MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity 2016, 44, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Boni, A.; Cogdill, A.P.; Dang, P.; Udayakumar, D.; Njauw, C.N.; Sloss, C.M.; Ferrone, C.R.; Flaherty, K.T.; Lawrence, D.P.; Fisher, D.E.; et al. Selective BRAF(V600E) inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010, 70, 5213–5219. [Google Scholar] [CrossRef]
- Sumimoto, H.; Imabayashi, F.; Iwata, T.; Kawakami, Y. The BRAF–MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J. Exp. Med. 2006, 203, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Chang, J.E. Targeted Therapy for Cancers: From Ongoing Clinical Trials to FDA-Approved Drugs. Int. J. Mol. Sci. 2023, 24, 13618. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, R.B.; Atreya, C.E.; Falchook, G.S.; Kwak, E.L.; Ryan, D.P.; Bendell, J.C.; Hamid, O.; Messersmith, W.A.; Daud, A.; Kurzrock, R.; et al. Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer. J. Clin. Oncol. 2015, 33, 4023–4031. [Google Scholar] [CrossRef]
- Roberts, S.K.; Galgadas, I.; Clarke, D.T.; Zanetti-Domingues, L.C.; Gervasio, F.L.; Martin-Fernandez, M.L. Targeting Mutant EGFR in Non-Small Cell Lung Cancer in the Context of Cell Adaptation and Resistance. Drug Discov. Today 2025, 30, 104407. [Google Scholar] [CrossRef]
- Liu, S.; Zou, Q.; Chen, J.P.; Yao, X.; Guan, P.; Liang, W.; Deng, P.; Lai, X.; Yin, J.; Chen, J.; et al. Targeting Enhancer Reprogramming to Mitigate MEK Inhibitor Resistance in Preclinical Models of Advanced Ovarian Cancer. J. Clin. Investig. 2021, 131, e145035. [Google Scholar] [CrossRef] [PubMed]
- Sigaud, R.; Stefanski, A.; Selt, F.; Kocher, D.; Usta, D.; Picard, D.; Büdenbender, I.; Remke, M.; Pfister, S.M.; Jones, D.T.W.; et al. Multi-Omics Dissection of MAPK-Driven Senescence Unveils Therapeutic Vulnerabilities in KIAA1549::BRAF-Fusion Pediatric Low-Grade Glioma Models. Signal Transduct. Target. Ther. 2025, 10, 197. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, S.; Wang, J.; Zhang, N.; Wang, J.; Jiang, M.; Meng, D.; Xie, J.; Chang, X.; Cheng, H.; et al. Case Report: Successful Use of MEK Inhibitors as an Adjuvant Approach in the Treatment of Pediatric MAP4-RAF1 Fusion-Positive Solid Tumor. NPJ Precis. Oncol. 2025, 9, 201. [Google Scholar] [CrossRef]
- Hernando-Calvo, A.; Kurzrock, R.; Gonzalez, N.S.; Magidi, S.; Bresson, C.; Wunder, F.; Pretelli, G.; Casado, A.M.; El-Deiry, W.S. Case Report WIN-MTB-2023001 WIN International Molecular Tumor Board: A 62-Year-Old Male with Metastatic Colorectal Cancer with 5 Prior Lines of Treatment. Oncotarget 2025, 16, 456–466. [Google Scholar] [CrossRef]
- Hartman, M.L.; Sztiller-Sikorska, M.; Czyz, M. Synergistic Activity of S63845 and Parthenolide to Overcome Acquired Resistance to MEK1/2 Inhibitor in Melanoma Cells: Mechanisms and Therapeutic Potential. Biomed. Pharmacother. 2025, 188, 118183. [Google Scholar] [CrossRef]
- Gu, Q.; Wang, K.; Lu, T.; Xiao, Y.; Wu, Y.; Zhou, H.; Zhou, K. Single-Cell and Spatial Transcriptome Analyses Reveal MAZ+ NPC-like Clusters as Key Role Contributing to Glioma Recurrence and Drug Resistance. J. Transl. Med. 2025, 23, 657. [Google Scholar] [CrossRef] [PubMed]
- Gilmartin, A.G.; Bleam, M.R.; Groy, A.; Moss, K.G.; Stewart, A.E.; Dicker, D.T.; Hollis, A.; McMahon, G.; LoRusso, P.M.; Owens, T.D.; et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin. Cancer Res. 2011, 17, 989–1000. [Google Scholar] [CrossRef]
- Yaeger, R.; Corcoran, R.B. Targeting alterations in the RAF–MEK pathway. Cancer Discov. 2019, 9, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Ahronian, L.G.; Sennott, E.M.; Van Allen, E.M.; Wagle, N.; Whittaker, S.; Montesion, M.; Ducar, M.; Margolis, C.A.; Greenberg, M.; Godfrey, J.T.; et al. Clinical acquired resistance to RAF inhibitor combinations in BRAF-mutant colorectal cancer through MAPK pathway alterations. Cancer Discov. 2015, 5, 358–367. [Google Scholar] [CrossRef]
- King, J.W.; Nathan, P.D. Role of the MEK Inhibitor Trametinib in the Treatment of Metastatic Melanoma. Future Oncol. 2014, 10, 1559–1570. [Google Scholar] [CrossRef]
- Barrow, C.; Browning, J.; MacGregor, D.; Davis, I.D.; Sturrock, S.; Jungbluth, A.A.; Cebon, J. Tumor antigen expression in melanoma varies according to antigen and stage. Clin. Cancer Res. 2006, 12, 764–771. [Google Scholar] [CrossRef]
- Teixidó, C.; Castillo, P.; Martinez-Vila, C.; Arance, A.; Alós, L. Molecular Markers and Targets in Melanoma. Cells 2021, 10, 2320. [Google Scholar] [CrossRef]
- Druker, B.J.; Talpaz, M.; Resta, D.J.; Peng, B.; Buchdunger, E.; Ford, J.M.; Lydon, N.B.; Kantarjian, H.; Capdeville, R.; Ohno-Jones, S.; et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 2001, 344, 1031–1037. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Corless, C.L.; Duensing, A.; McGreevey, L.; Chen, C.J.; Joseph, N.; Singer, S.; Griffith, D.J.; Haley, A.; Town, A.; et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003, 299, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Carofiglio, F.; Lopalco, A.; Lopedota, A.; Cutrignelli, A.; Nicolotti, O.; Denora, N.; Stefanachi, A.; Leonetti, F. Bcr-Abl Tyrosine Kinase Inhibitors in the Treatment of Pediatric CML. Int. J. Mol. Sci. 2020, 21, 4469. [Google Scholar] [CrossRef] [PubMed]
- Buchdunger, E.; Cioffi, C.L.; Law, N.; Stover, D.; Ohno-Jones, S.; Druker, B.J.; Lydon, N.B. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J. Pharmacol. Exp. Ther. 2000, 295, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, E.; Manley, P.W.; Breitenstein, W.; Bruggen, J.; Cowan-Jacob, S.W.; Ray, A.; Huntly, B.J.P.; Fabbro, D.; Fendrich, G.; Hall-Meyers, E.; et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell 2005, 7, 129–141. [Google Scholar] [CrossRef]
- Mittal, B.; Tulsyan, S.; Kumar, S.; Mittal, R.D.; Agarwal, G. Cytochrome P450 in Cancer Susceptibility and Treatment. Adv. Clin. Chem. 2015, 71, 77–139. [Google Scholar] [CrossRef]
- Rowley, J.D. A new consistent chromosomal abnormality in chronic myelogenous leukemia identified by quinacrine fluorescence and Giemsa staining. Nature 1973, 243, 290–293. [Google Scholar] [CrossRef]
- Nowell, P.C. The Minute Chromosome (Phl) in Chronic Granulocytic Leukemia. Blut 1962, 8, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Warfvinge, R.; Geironson Ulfsson, L.; Dhapola, P.; Safi, F.; Sommarin, M.; Soneji, S.; Hjorth-Hansen, H.; Mustjoki, S.; Richter, J.; Thakur, R.K.; et al. Single-Cell Multiomics Analysis of Chronic Myeloid Leukemia Links Cellular Heterogeneity to Therapy Response. eLife 2024, 12, RP92074. [Google Scholar] [CrossRef]
- O’Brien, S.G.; Guilhot, F.; Larson, R.A.; Gathmann, I.; Baccarani, M.; Cervantes, F.; Cornelissen, J.J.; Fischer, T.; Hochhaus, A.; Hughes, T.; et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl. J. Med. 2003, 348, 994–1004. [Google Scholar] [CrossRef]
- Jackson, S.E.; Chester, J.D. Personalised cancer medicine. Int. J. Cancer 2015, 137, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Giles, F.; Bhalla, K.; O’Brien, S.; Cortes, J.; Rios, M.B.; Shan, J.; Thomas, D.; Wierda, W.; Faderl, S.; et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive acute lymphoblastic leukemia. N. Engl. J. Med. 2006, 354, 2542–2551. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Blanke, C.D.; Van den Abbeele, A.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef]
- Verweij, J.; Casali, P.G.; Zalcberg, J.; Le Cesne, A.; Reichardt, P.; Blay, J.Y.; Issels, R.; van Oosterom, A.T.; Hogendoorn, P.C.; Dimitrijevic, S.; et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: Randomised trial. Lancet 2004, 364, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Bello, D.M.; Dematteo, R.P.; Ariyan, C.E. The GIST of targeted therapy for malignant melanoma. Ann. Surg. Oncol. 2014, 21, 2059–2067. [Google Scholar] [CrossRef]
- Ben Ami, E.; Demetri, G.D. A Safety Evaluation of Imatinib Mesylate in the Treatment of Gastrointestinal Stromal Tumor. Expert Opin. Drug Saf. 2016, 15, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Sosman, J.A. Update on the targeted therapy of melanoma. Curr. Treat. Options Oncol. 2013, 14, 280–292. [Google Scholar] [CrossRef]
- Bellouard, M.; Donadieu, J.; Thiebot, P.; Giroux Leprieur, E.; Saiag, P.; Etting, I.; Dugues, P.; Abe, E.; Alvarez, J.C.; Larabi, I.A. Validation of liquid chromatography coupled with tandem mass spectrometry for the determination of 12 tyrosine kinase inhibitors (TKIs) and their application to therapeutic drug monitoring in adult and pediatric populations. Pharmaceutics 2023, 16, 5. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Z.; Li, D.; Zhang, B.; Zong, X.; Liang, X.; Li, Z. The Molecular Mechanisms of Imatinib Treatment on Acute Lung Injury in Septic Mice through Proteomic Technology. J. Immunol. Res. 2025, 2025, 4526375. [Google Scholar] [CrossRef]
- Li, Q.; Liu, T.; Lv, K.; Liao, F.; Wang, J.; Tu, Y.; Chen, Q. Malaria: Past, Present, and Future. Signal Transduct. Target. Ther. 2025, 10, 188. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Cost in the United States of FDA-approved small molecule protein kinase inhibitors used in the treatment of neoplastic and non-neoplastic diseases. Pharmacol. Res. 2024, 199, 107036. [Google Scholar] [CrossRef]
- Sposito, M.; Belluomini, L.; Pontolillo, L.; Tregnago, D.; Trestini, I.; Insolda, J.; Avancini, A.; Milella, M.; Bria, E.; Carbognin, L.; et al. Adjuvant targeted therapy in solid cancers: Pioneers and new glories. J. Pers. Med. 2023, 13, 1427. [Google Scholar] [CrossRef] [PubMed]
- Bouraqqadi, O.; Elloudi, S.; Hammas, N.; Boubekri, N.; Mernissi, F. Rapidly Evolving Cervical Dermatofibrosarcoma Protuberans with Deep Muscle Infiltration: A Rare and Aggressive Presentation. Cureus 2025, 17, e84596. [Google Scholar] [CrossRef]
- Meisenheimer, J.; Usovich, M.; Rypka, K.J.; Liu, L.; Warshaw, E.; Gravely, A.; Lynch, J.; Westanmo, A.D.; Goldfarb, N. Variations in Anatomic Locations of Squamous Cell Carcinoma Stratified by Histologic Risk: A Single-Center Retrospective Study. Int. J. Dermatol. 2025. [Google Scholar] [CrossRef]
- Curtin, J.A.; Busam, K.; Pinkel, D.; Bastian, B.C. Somatic activation of KIT in distinct subtypes of melanoma. J. Clin. Oncol. 2006, 24, 4340–4346. [Google Scholar] [CrossRef] [PubMed]
- Delyon, J.; Lebbe, C.; Dumaz, N. Targeted therapies in melanoma beyond BRAF: Targeting NRAS-mutated and KIT-mutated melanoma. Curr. Opin. Oncol. 2020, 32, 79–84. [Google Scholar] [CrossRef]
- Berger, M.; Richtig, G.; Kashofer, K.; Aigelsreiter, A.; Richtig, E. The window of opportunities for targeted therapy in BRAFwt/NRASwt/KITwt melanoma: Biology and clinical implications of fusion proteins and other mutations. G. Ital. Dermatol. Venereol. 2018, 153, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.R.; Mehnert, J.M. Mucosal Melanoma: Epidemiology, Biology and Treatment. Cancer Treat. Res. 2016, 167, 295–320. [Google Scholar] [CrossRef]
- Wei, X.; Mao, L.; Chi, Z.; Sheng, X.; Cui, C.; Kong, Y.; Dai, J.; Wang, X.; Li, S.; Tang, B.; et al. Efficacy evaluation of imatinib for the treatment of melanoma: Evidence from a retrospective study. Oncol. Res. 2019, 27, 495–501. [Google Scholar] [CrossRef]
- Spagnolo, F.; Caltabiano, G.; Queirolo, P. Uveal melanoma. Cancer Treat. Rev. 2012, 38, 549–553. [Google Scholar] [CrossRef]
- Dummer, R.; Rozati, S.; Eggmann, N.; Rinderknecht, J.; Goldinger, S.M. From chemotherapy to targeted treatment. Ann. Oncol. 2012, 23 (Suppl. 10), x101–x103. [Google Scholar] [CrossRef]
- Johnson, D.B.; Pollack, M.H.; Sosman, J.A. Emerging targeted therapies for melanoma. Expert Opin. Emerg. Drugs 2016, 21, 195–207. [Google Scholar] [CrossRef]
- Stoff, R.; Markovic, S.N.; McWilliams, R.R.; Kottschade, L.A.; Montane, H.N.; Dimou, A.; Dudek, A.Z.; Tan, W.; Dronca, R.S.; Seetharam, M.; et al. Real-world evidence on efficacy and toxicity of targeted therapy in older melanoma patients treated in a tertiary-hospital setting. Melanoma Res. 2024, 34, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Gorre, M.E.; Mohammed, M.; Ellwood, K.; Hsu, N.; Paquette, R.; Rao, P.N.; Sawyers, C.L. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001, 293, 876–880. [Google Scholar] [CrossRef]
- Shah, N.P.; Nicoll, J.M.; Nagar, B.; Gorre, M.E.; Paquette, R.; Kuriyan, J.; Sawyers, C.L. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell 2002, 2, 117–125. [Google Scholar] [CrossRef]
- Tsubaki, M.; Obana, T.; Matsuo, T.; Komori, R.; Takeda, T.; Koumoto, Y.; Usami, H.; Nagai, N.; Nishida, S. Overexpression of miR-29a and miR-29b Is Involved in Imatinib Resistance via Abrogated NF1 Expression and Increased ERK1/2 Activation in Chronic Myeloid Leukemia Cells. Med. Oncol. 2025, 42, 268. [Google Scholar] [CrossRef] [PubMed]
- Ghadyani Nejhad, L.; Sohani, M.; Ghandforoush, N.A.; Nikbakht, M.; Mohammadi, S.; Vaezi, M.; Rostami, S.; Chahardouli, B. Non-Coding RNAs: Emerging Contributors to Chemoresistance in Chronic Myeloid Leukemia. Leuk. Res. Rep. 2025, 23, 100513. [Google Scholar] [CrossRef]
- Kantarjian, H.; Sawyers, C.L.; Hochhaus, A.; Guilhot, F.; Schiffer, C.A.; Gambacorti-Passerini, C.; Niederwieser, D.; Rousselot, P.; Ottmann, O.G.; Pasquini, R.; et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N. Engl. J. Med. 2002, 346, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Kim, D.W.; Pinilla-Ibarz, J.; le Coutre, P.; Paquette, R.; Chuah, C.; Nicolini, F.E.; Apperley, J.F.; Khoury, H.J.; Talpaz, M.; et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: Final results of the phase 2 PACE trial. Blood 2018, 132, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Abdihamid, O.; Tan, F.; Zhou, H.; Liu, H.; Li, Z.; Xiao, S.; Li, B. KIT Mutations and Expression: Current Knowledge and New Insights for Overcoming IM Resistance in GIST. Cell Commun. Signal. 2024, 22, 153. [Google Scholar] [CrossRef]
- Liu, X.; Yu, J.; Li, Y.; Shi, H.; Jiao, X.; Liu, X.; Guo, D.; Li, Z.; Tian, Y.; Dai, F.; et al. Deciphering the Tumor Immune Microenvironment of Imatinib-Resistance in Advanced Gastrointestinal Stromal Tumors at Single-Cell Resolution. Cell Death Dis. 2024, 15, 190. [Google Scholar] [CrossRef]
- Gambacorti-Passerini, C.; Antolini, L.; Mahon, F.X.; Guilhot, J.; Deininger, M.; Niederwieser, D.; Soverini, S.; Hochhaus, A.; Hughes, T.P.; Clark, R.E.; et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J. Natl. Cancer Inst. 2011, 103, 553–561. [Google Scholar] [CrossRef]
- Kantarjian, H.; Shah, N.; Hochhaus, A.; Cortes, J.; Shah, N.P.; Tanaka, C.; Giles, F.; Boque, C.; O’Brien, S.; Jorgensen, J.; et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-Year follow-up from a randomized phase 3 trial (DASISION). Blood 2012, 119, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Steegmann, J.L.; Baccarani, M.; Breccia, M.; Casado, L.F.; Garcia-Gutierrez, V.; Gratwohl, A.; Huguet, F.; le Coutre, P.; Mahon, F.X.; Nicolini, F.E.; et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukemia. Leukemia 2016, 30, 1648–1671. [Google Scholar] [CrossRef] [PubMed]
- Quintás-Cardama, A.; Cortes, J. Chronic myeloid leukemia: Diagnosis and treatment. Mayo Clin. Proc. 2006, 81, 973–988. [Google Scholar] [CrossRef]
- Hughes, T.P.; Saglio, G.; Kantarjian, H.M.; Guilhot, F.; Niederwieser, D.; Rosti, G.; Nakaseko, C.; De Souza, C.A.; Kalaycio, M.E.; Meier, S.; et al. Early Molecular Response Predicts Outcomes in Patients with Chronic Myeloid Leukemia in Chronic Phase Treated with Frontline Nilotinib or Imatinib. Blood 2014, 123, 1353–1360. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Shoushtari, A.N.; Keohan, M.L.; Dickson, M.A.; Gounder, M.M.; Chi, P.; Loo, J.K.; Gaffney, L.; Schneider, L.; Patel, Z.; et al. Combined KIT and CTLA-4 Blockade in Patients with Refractory GIST and Other Advanced Sarcomas: A Phase Ib Study of Dasatinib plus Ipilimumab. Clin. Cancer Res. 2017, 23, 2972–2980. [Google Scholar] [CrossRef]
- Soria, J.C.; Mauguen, A.; Reck, M.; Hofman, P.; Osório, A.; Lena, H.; Jackman, D.M.; Sequist, L.V.; Mahé, M.; Riely, G.J.; et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Yao, Z.; Lu, L.; Xu, Q.; Hua, S.; Wang, H.; Jiang, H. Integrated analysis reveals COL4A3 as a novel diagnostic and therapeutic target in UV-related skin cutaneous melanoma. Clin. Cosmet. Investig. Dermatol. 2024, 17, 1429–1446. [Google Scholar] [CrossRef]
- Pham, T.M.; Ahmed, M.; Lai, T.H.; Bahar, M.E.; Hwang, J.S.; Maulidi, R.F.; Ngo, Q.N.; Kim, D.R. Regulation of cell cycle progression through RB phosphorylation by nilotinib and AT-9283 in human melanoma A375P cells. Int. J. Mol. Sci. 2024, 25, 2956. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ge, P.; Liu, S.; Yang, D.; Zhang, J.; Wang, X.; Liang, W. Efficacy and Safety of Bevacizumab in Patients with Malignant Melanoma: A Systematic Review and PRISMA-Compliant Meta-Analysis of Randomized Controlled Trials and Non-Comparative Clinical Studies. Front. Pharmacol. 2023, 14, 1163805. [Google Scholar] [CrossRef]
- Peters, J.; Bollaert, E.; Cloos, A.S.; Claus, M.; Essaghir, A.; Lenglez, S.; Saussoy, P.; Dachy, G.; Autin, P.; Demoulin, J.B.; et al. MiR-92a-1-5p Contributes to Cellular Proliferation and Survival in Chronic Myeloid Leukemia and Its Inhibition Enhances Imatinib Efficacy. Noncoding RNA Res. 2025, 14, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Takahashi, T.; Kurokawa, Y.; Nishida, T.; Hirota, S.; Serada, S.; Fujimoto, M.; Naka, T.; Teranishi, R.; Saito, T.; et al. Targeted Therapy for Drug-Tolerant Persister Cells after Imatinib Treatment for Gastrointestinal Stromal Tumours. Br. J. Cancer 2021, 125, 1511–1522. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Hughes, T.P.; Larson, R.A.; Kim, D.W.; Issaragrisil, S.; le Coutre, P.; Etienne, G.; Boquimpani, C.; Pasquini, R.; Clark, R.E.; et al. Long-Term Outcomes with Frontline Nilotinib versus Imatinib in Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase: ENESTnd 10-Year Analysis. Leukemia 2021, 35, 440–453. [Google Scholar] [CrossRef]
- Liu, W.Z.; Du, Y.Q.; Shen, Q.; Tao, K.X.; Zhang, P. Ripretinib for the Treatment of Advanced, Imatinib-Resistant Gastrointestinal Stromal Tumors. J. Dig. Dis. 2024, 25, 559–563. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Q.; Lin, X.; Shu, P.; Gao, X.; Shen, K. Imatinib Induces Ferroptosis in Gastrointestinal Stromal Tumors by Promoting STUB1-Mediated GPX4 Ubiquitination. Cell Death Dis. 2023, 14, 839. [Google Scholar] [CrossRef]
- Szucs, Z.; Thway, K.; Fisher, C.; Bulusu, R.; Constantinidou, A.; Benson, C.; van der Graaf, W.T.; Jones, R.L. Promising Novel Therapeutic Approaches in the Management of Gastrointestinal Stromal Tumors. Future Oncol. 2017, 13, 185–194. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, T.; Eide, C.A.; Deininger, M.W.N. BCR-ABL kinase domain mutations, drug resistance, and the road to a cure for chronic myeloid leukemia. Blood 2007, 110, 2242–2249. [Google Scholar] [CrossRef]
- Sawyers, C.L. Targeted cancer therapy. Nature 2004, 432, 294–297. [Google Scholar] [CrossRef]
- Druker, B.J. Translation of the Philadelphia chromosome into therapy for CML. Blood 2008, 112, 4808–4817. [Google Scholar] [CrossRef]
- Cohen, P.; Cross, D.; Jänne, P.A. Kinase drug discovery 20 years after imatinib: Progress and future directions. Nat. Rev. Drug Discov. 2021, 20, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Kim, K.H.; Lee, S.-C. Oral chemotherapeutic agents in current use. J. Korean Med. Assoc. 2011, 54, 1191–1198. [Google Scholar] [CrossRef]
- Amouei, A.; Daeian, N.; Khezrnia, S.S.; Mansouri, A.; Hadjibabaie, M. Imatinib efficacy, safety and resistance in Iranian patients with chronic myeloid leukemia: A review of literature. Int. J. Hematol. Oncol. Stem Cell Res. 2021, 15, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Yan, W.; Wang, C.; Liu, W.; Lin, X.; Zou, Z.; Sun, W.; Chen, Y. BRAF Inhibitor Resistance in Melanoma: Mechanisms and Alternative Therapeutic Strategies. Curr. Treat. Options Oncol. 2022, 23, 1503–1521. [Google Scholar] [CrossRef]
- Luebker, S.A.; Koepsell, S.A. Diverse Mechanisms of BRAF Inhibitor Resistance in Melanoma Identified in Clinical and Preclinical Studies. Front. Oncol. 2019, 9, 268. [Google Scholar] [CrossRef]
- Paton, E.L.; Turner, J.A.; Schlaepfer, I.R. Overcoming Resistance to Therapies Targeting the MAPK Pathway in BRAF-Mutated Tumours. J. Oncol. 2020, 2020, 1079827. [Google Scholar] [CrossRef]
- Lelliott, E.J.; McArthur, G.A.; Oliaro, J.; Sheppard, K.E. Immunomodulatory Effects of BRAF, MEK, and CDK4/6 Inhibitors: Implications for Combining Targeted Therapy and Immune Checkpoint Blockade for the Treatment of Melanoma. Front. Immunol. 2021, 12, 661737. [Google Scholar] [CrossRef]
- Idris, O.A.; Shebrain, A.; Jawad, A.; Pacione, S.C.; Haj, D.; Bzizi, H.; Ahmedfiqi, Y.O.; Saadaie Jahromi, B.; Deleon, N.; Zhang, T.; et al. Breaking Barriers: Pembrolizumab’s Role in Overcoming Targeted Therapy Resistance in BRAF-Mutant Melanoma. Clin. Surg. Oncol. 2025, 4, 100095. [Google Scholar] [CrossRef]
- Buchanan, T.; Amouzegar, A.; Luke, J.J. Next-Generation Immunotherapy Approaches in Melanoma. Curr. Oncol. Rep. 2021, 23, 116. [Google Scholar] [CrossRef]
- Zoroddu, S.; Bagella, L. Next-Generation mRNA Vaccines in Melanoma: Advances in Delivery and Combination Strategies. Cells 2025, 14, 1476. [Google Scholar] [CrossRef]
- Slusher, N.; Jones, N.; Nonaka, T. Liquid Biopsy for Diagnostic and Prognostic Evaluation of Melanoma. Front. Cell Dev. Biol. 2024, 12, 1420360. [Google Scholar] [CrossRef] [PubMed]
- Connor, C.; Carr, Q.L.; Sweazy, A.; McMasters, K.; Hao, H. Clinical Approaches for the Management of Skin Cancer: A Review of Current Progress in Diagnosis, Treatment, and Prognosis for Patients with Melanoma. Cancers 2025, 17, 707. [Google Scholar] [CrossRef]
- Song, Y.; Bi, Z.; Liu, Y.; Qin, F.; Wei, Y.; Wei, X. Targeting RAS-RAF-MEK-ERK Signaling Pathway in Human Cancer: Current Status in Clinical Trials. Genes Dis. 2022, 10, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Boutros, A.; Croce, E.; Ferrari, M.; Gili, R.; Massaro, G.; Marconcini, R.; Arecco, L.; Tanda, E.T.; Spagnolo, F. The Treatment of Advanced Melanoma: Current Approaches and New Challenges. Crit. Rev. Oncol. Hematol. 2024, 196, 104276. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Huang, W.-K.; Yeh, K.-Y.; Chang, J.W.-C.; Lin, Y.-C.; Chou, W.-C. Integrating Comprehensive Genomic Profiling in the Management of Oncology Patients: Applications and Challenges in Taiwan. Biomed. J. 2025, 48, 100851. [Google Scholar] [CrossRef]
- Nero, C.; Duranti, S.; Giacomini, F.; Minucci, A.; Giacò, L.; Piermattei, A.; Genuardi, M.; Pasciuto, T.; Urbani, A.; Daniele, G.; et al. Integrating a Comprehensive Cancer Genome Profiling into Clinical Practice: A Blueprint in an Italian Referral Center. J. Pers. Med. 2022, 12, 1746. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.T.; Pan, I.W.; Teich, N. Global Challenges in Access to and Implementation of Precision Oncology: The Health Care Manager and Health Economist Perspective. ASCO Educ. Book 2022, 42, 429–437. [Google Scholar] [CrossRef]

| Class/ Agent | Key Targets | Pivotal Trials | ORR (%) | Median PFS (mo) | Median OS (mo) | Common AEs | Distinct Features/ Resistance Notes |
|---|---|---|---|---|---|---|---|
| Vemurafenib (Zelboraf) | BRAF V600E/K | BRIM-3 | 48 | 6.9 | 13.6 | Photosensitivity, arthralgia | First-in-class; higher cutaneous toxicity; paradoxical MAPK activation |
| Dabrafenib (Tafinlar) | BRAF V600E/K | BREAK-3 | 51 | 9.3 | 18.2 | Pyrexia, fatigue | Lower rash/photosensitivity; improved tolerability |
| Encorafenib (Braftovi) | BRAF V600E/K | COLUMBUS | 60 | 14.9 | 33.6 | Arthralgia, fatigue | Longest half-life; least paradoxical activation |
| Trametinib (Mekinist) | MEK1/2 | METRIC | 22 | 4.8 | 15.6 | Rash, diarrhea | Alone or combined with dabrafenib |
| Cobimetinib (Cotellic) | MEK1 | coBRIM | — (combo) | 12.3 | 22.3 | Diarrhea, photosensitivity | Used with vemurafenib |
| Binimetinib (Mektovi) | MEK1/2 | COLUMBUS | — (combo) | 14.9 | 33.6 | Fatigue, nausea | Used with encorafenib |
| Imatinib (Gleevec) | KIT (L576P, K642E) | Phase II | 16–29 | 3–7 | 12–18 | Edema, nausea | Benefit limited to activating mutations; not amplifications |
| Nilotinib (Tasigna) | KIT (L576P, K642E) | Phase II | 20–26 | 3–6 | — | Fatigue, cytopenia | May retain efficacy post-imatinib; off-label use |
| Treatment Type | Drug/ Class | Molecular Target/ Mechanism | Indications | Biomarkers | Combination Strategies | Common Resistance Mechanisms | Key Side Effects (Including Severe/Fatal) |
|---|---|---|---|---|---|---|---|
| Targeted Therapy | Vemurafenib, Dabrafenib | BRAF V600E/K mutation, inhibits MAPK pathway at BRAF | BRAF V600+ advanced or metastatic melanoma | BRAF V600 mutation (PCR/NGS) | With MEK inhibitors (e.g., Trametinib, Cobimetinib) | BRAF amplification, NRAS mutation, MAPK reactivation | Rash, arthralgia, QT prolongation (potentially fatal arrhythmia), photosensitivity, cutaneous squamous cell carcinoma (severe) |
| Trametinib, Cobimetinib | MEK1/2 inhibition, downstream of BRAF | BRAF V600+ (always combined with BRAF inhibitors) | BRAF mutation | With BRAF inhibitors to avoid resistance | MEK/ERK mutations, RTK upregulation | Diarrhea, fatigue, cardiac toxicity (heart failure, rare fatal), retinopathy, interstitial lung disease (rare severe) | |
| KIT inhibitors (Imatinib, Nilotinib) | KIT mutations or amplifications (rare subtype of melanoma) | Acral, mucosal, or chronically sun-damaged melanoma | KIT exon 11/13 mutation | Rarely used; sometimes with immunotherapy or chemotherapy | Secondary KIT mutations | Edema, cytopenias, nausea, hepatotoxicity, cardiac toxicity (rare fatal) | |
| Immunotherapy | Anti–PD-1 (Nivolumab, Pembrolizumab) | Blocks PD-1 receptor, restores T-cell activity | Metastatic or unresectable melanoma; adjuvant setting | PD-L1 expression (optional), TMB, IFN-γ signature | With CTLA-4 inhibitors, targeted therapy, or chemo | Loss of MHC expression, JAK/STAT mutations, T cell exhaustion | Immune-related AEs: colitis, hepatitis, pneumonitis (can be severe/fatal) |
| Anti–CTLA-4 (Ipilimumab) | Blocks CTLA-4, enhances T-cell priming and activation | Metastatic or refractory melanoma | None specific | With PD-1 inhibitors (dual checkpoint blockade) | Severe immune evasion, Treg expansion | Colitis, dermatitis, hypophysitis, enteritis (severe/fatal in rare cases) | |
| Oncolytic Therapy | Talimogene laherparepvec (T-VEC) | Modified HSV-1 virus that replicates in tumors and expresses GM-CSF | Unresectable stage IIIB–IV melanoma (locoregional) | HSV-seronegative status (relative) | With PD-1 inhibitors (under investigation) | Immunosuppressive tumor microenvironment | Flu-like symptoms, injection site pain (generally mild; rare systemic viral spread possible) |
| Chemotherapy | Dacarbazine, Temozolomide | Alkylating agents causing DNA damage | Previously standard in advanced melanoma | None required | Rarely used; now replaced by immunotherapy/targeted agents | High toxicity, low response rates | Myelosuppression, nausea, fatigue (potentially severe infections) |
| Radiotherapy | Stereotactic, whole-brain, adjuvant RT | DNA damage, p53-dependent apoptosis | Brain metastases, palliation | None required | With checkpoint inhibitors (for synergy) | Radioresistance (via DNA repair enzymes) | Cognitive impairment, fatigue, dermatitis (rare severe necrosis) |
| Surgery | Wide local excision, lymph node dissection | Curative in early-stage melanoma | Stage I–II and some stage III | Tumor thickness, ulceration, SLNB results | Adjuvant immunotherapy or radiotherapy in high-risk cases | N/A | Wound complications, lymphedema, infection |
| Adjuvant/Neoadjuvant | Immunotherapy or targeted therapy | Same as above—used to reduce recurrence risk or downstage tumors | Stage III–IV resectable or high-risk patients | BRAF status, LDH, PD-L1 (optional) | Nivolumab, Dabrafenib/Trametinib as standard options | Similar to primary therapies | Dependent on regimen used (includes severe immune or cardiac events for targeted/immunotherapy) |
| RAF Isoform/Mutation | Cancer Type(s) | Clinical Relevance |
|---|---|---|
| B-RAF V600E | Melanoma, Colorectal, Thyroid (Papillary), Ovarian, NSCLC | Highly oncogenic; Targetable with B-RAF and MEK inhibitors; In colorectal cancer, associated with poor prognosis and resistance to B-RAF inhibitor monotherapy |
| B-RAF Non-V600 Mutations (general) | Melanoma | Targetable with B-RAF and MEK inhibitors |
| B-RAF F595L | Melanoma, Colorectal, Thyroid (Papillary), NSCLC | Weak/intermediate kinase activity; sometimes respond better to MEK inhibitors rather than B-RAF inhibitors |
| B-RAF L597Q | Melanoma, Colorectal, Thyroid (Papillary), NSCLC | Weak/intermediate kinase activity; sometimes respond better to MEK inhibitors rather than B-RAF inhibitors |
| B-RAF G469A | Melanoma, Colorectal, Thyroid (Papillary), NSCLC | Weak/intermediate kinase activity; sometimes respond better to MEK inhibitors rather than B-RAF inhibitors |
| B-RAF V600K | Hairy Cell Leukemia | Highly sensitive to B-RAF inhibition |
| C-RAF F133L | Melanoma, Colorectal | May respond to B-RAF inhibitors |
| C-RAF S257L | Melanoma, Colorectal | May respond to B-RAF inhibitors |
| A-RAF Rare Mutations | Colorectal, Ovarian, Lung, Pancreatic, Gliomas | Limited therapeutic targeting due to complexity of RAF dimerization; limited treatment options |
| Feature | Vemurafenib | Trametinib | Imatinib |
|---|---|---|---|
| Drug Class | BRAF inhibitor (ATP-competitive) | MEK1/2 inhibitor (non-ATP competitive) | Tyrosine kinase inhibitor (TKI) |
| Primary Molecular Target(s) | Mutant BRAF V600E | MEK1 and MEK2 (MAP2K1/2) downstream of BRAF | BCR-ABL (CML, Ph+ ALL) KIT (GIST) PDGFRα/β (DFSP, HES) |
| Primary Signaling Pathway | MAPK/ERK pathway (RAS–RAF–MEK–ERK) | MAPK/ERK pathway (inhibits downstream of RAF, at MEK level) | BCR-ABL STAT/JAK KIT/PDGFR PI3K/AKT/mTOR MAPK/ERK |
| Upstream Activators | RAS– Receptor tyrosine kinases (RTKs) | BRAF (WT or mutant) RAS mutations RTKs | BCR-ABL fusion gene SCF for KIT PDGF for PDGFR |
| Direct Action | Inhibits aberrant BRAF V600E kinase activity to block downstream MEK/ERK signaling | Inhibits MEK1/2 phosphorylation to prevent ERK activation | Inhibits phosphorylation of BCR-ABL, KIT, and PDGFR, blocking their downstream signal transduction |
| Downstream Effects | ↓ MEK1/2 and ERK1/2 phosphorylation ↓ cyclin D1 ↓ proliferation ↑ apoptosis | ↓ ERK phosphorylation ↓ proliferation ↑ apoptosis | ↓ PI3K/AKT, MAPK, and JAK/STAT signaling ↓ proliferation ↑ apoptosis |
| Cellular Consequences | G1-phase arrest Decreased cell survival and angiogenesis | G1-phase arrest Reduced tumor growth | Suppression of malignant hematopoietic cell growth Long-term disease control in CML and GIST |
| Resistance Mechanisms | BRAF amplification NRAS mutation MAPK pathway reactivation PI3K activation | MEK amplification ERK mutations Bypass via PI3K/AKT, RTKs | BCR-ABL mutations (e.g., T315I) Overexpression KIT exon 17 mutations Activation of bypass kinases |
| Combination Strategies | With MEK inhibitors (e.g., trametinib) With PI3K/mTOR inhibitors Autophagy inhibitors (HCQ) | With BRAF inhibitors With CDK4/6 inhibitors Immunotherapy | 2nd/3rd gen TKIs (dasatinib, ponatinib) Allosteric BCR-ABL inhibitors (asciminib) Epigenetic agents |
| Clinical Biomarkers | BRAF V600E mutation | BRAF V600E/K, NRAS (trial/experimental) | BCR-ABL, KIT, PDGFRA mutations (PCR, FISH, NGS) |
| Median OS (months) | 13.6 (monotherapy); up to 25 (with MEK inhibitor) | ~11 (monotherapy); up to 25 (combo) | ~12 |
| Median PFS (months) | 5.3 (monotherapy); ~12 (combo) | 4–5 (mono); 11–12 (combo) | 3.5–4 |
| ORR (%) | 50–60 (mono); ~70 (combo) | 20–25 (mono); 60–70 (combo) | 30–50 |
| Adverse Effect | Vemurafenib | Trametinib | Imatinib |
|---|---|---|---|
| Arthralgia | Very common (>30%)—Mild–Moderate | – | – |
| Rash | Very common (>30%)—Mild–Moderate | Very common (>30%)—Mild–Moderate | Common (10–30%)—Mild–Moderate |
| Photosensitivity | Very common (>30%)—Mild–Moderate | – | – |
| Fatigue | Common (10–30%)—Mild–Moderate | Very common (>30%)—Mild–Moderate | Common (10–30%)—Mild–Moderate |
| Diarrhea | – | Very common (>30%)—Mild–Moderate | Common (10–30%)—Mild–Moderate |
| Nausea | Common (10–30%)—Mild | Common (10–30%)—Mild | Very common (>30%)—Mild |
| Vomiting | – | – | Common (10–30%)—Mild–Moderate |
| Pruritus | Common (10–30%)—Mild–Moderate | – | – |
| Alopecia | Common (10–30%)—Mild | – | – |
| Edema/Periorbital edema | – | Common (10–30%)—Mild–Moderate | Very common (>30%)—Mild–Moderate |
| Hypertension | – | Common (10–30%)—Moderate–Severe | – |
| Cutaneous squamous cell carcinoma/Keratoacanthoma | Common (10–30%)—Severe | – | – |
| Cardiotoxicity (heart failure, QT prolongation) | Uncommon (1–10%)—Severe/Potentially fatal | Uncommon (1–10%)—Severe | Rare (<1%)—Severe/Potentially fatal |
| QT prolongation | Uncommon (1–10%)—Severe/Potentially fatal | – | Rare (<1%)—Severe/Potentially fatal |
| Hepatotoxicity | Uncommon (1–10%)—Severe | – | Uncommon (1–10%)—Severe/Potentially fatal |
| Cardiomyopathy/decreased ejection fraction | – | Uncommon (1–10%)—Severe | Rare (<1%)—Severe/Potentially fatal |
| Retinopathy/Retinal vein occlusion | – | Uncommon (1–10%)—Severe/Vision-threatening | – |
| Interstitial lung disease/Pneumonitis | – | Rare (<1%)—Severe/Potentially fatal | – |
| Myelosuppression (neutropenia, thrombocytopenia) | – | – | Common (10–30%)—Severe |
| Muscle cramps | – | – | Common (10–30%)—Mild–Moderate |
| Mechanism of Action | Treatment Approach |
|---|---|
| Identifies predictive markers (PD-L1, TMB, IFN-γ, LAG-3, TILs, HLA types, gut microbiota) to personalize therapy and improve treatment outcomes. | Biomarker Discovery and Validation |
| Employs genetically engineered T cells (e.g., TYP1-targeted) to recognize and kill melanoma cells; currently in preclinical and early clinical stages. | CAR-T Cell Therapy Targeting Melanoma Antigens |
| Enhances antitumor responses by integrating ICIs with radiotherapy, oncolytic viruses, vaccines, or TILs to overcome immune resistance mechanisms. | Combination Immunotherapy Approaches |
| Androgen receptor inhibition may boost NK cell activity and improve ICI effectiveness; currently under preclinical evaluation. | Natural Killer (NK) Cell-Based Immunotherapy |
| Suppresses melanoma cell growth by inhibiting mutations in key driver genes (BRAF, KIT, NRAS, TERT, CCND1); TERT and CCND1 are common in acral melanoma. | Targeted Inhibition of Oncogenic Signaling |
| Utilizes expanded TILs (e.g., lifileucel) to directly attack tumor cells; effective after ICI and BRAF/MEK inhibitor failure, though limited by cost and toxicity. | Tumor-Infiltrating Lymphocyte (TIL) Therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawczak, P.; Bączek, T. Pathway-Specific Therapeutic Modulation of Melanoma: Small-Molecule Inhibition of BRAF–MEK and KIT Signaling in Contemporary Precision Oncology with a Special Focus on Vemurafenib, Trametinib, and Imatinib. J. Clin. Med. 2025, 14, 7906. https://doi.org/10.3390/jcm14227906
Kawczak P, Bączek T. Pathway-Specific Therapeutic Modulation of Melanoma: Small-Molecule Inhibition of BRAF–MEK and KIT Signaling in Contemporary Precision Oncology with a Special Focus on Vemurafenib, Trametinib, and Imatinib. Journal of Clinical Medicine. 2025; 14(22):7906. https://doi.org/10.3390/jcm14227906
Chicago/Turabian StyleKawczak, Piotr, and Tomasz Bączek. 2025. "Pathway-Specific Therapeutic Modulation of Melanoma: Small-Molecule Inhibition of BRAF–MEK and KIT Signaling in Contemporary Precision Oncology with a Special Focus on Vemurafenib, Trametinib, and Imatinib" Journal of Clinical Medicine 14, no. 22: 7906. https://doi.org/10.3390/jcm14227906
APA StyleKawczak, P., & Bączek, T. (2025). Pathway-Specific Therapeutic Modulation of Melanoma: Small-Molecule Inhibition of BRAF–MEK and KIT Signaling in Contemporary Precision Oncology with a Special Focus on Vemurafenib, Trametinib, and Imatinib. Journal of Clinical Medicine, 14(22), 7906. https://doi.org/10.3390/jcm14227906






