Association Between Lumbar Spinal Stenosis and Accelerated Biological Aging Estimated by PhenoAge
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Comparison Between LSS and Control
2.3. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Demographic Data
3.3. Blood Biomarkers
3.4. Correlation Coefficient Between PhenoAgeAccel and Demographic Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LSS | Lumbar spinal stenosis |
| SMI | Skeletal mass index |
| BMI | Body mass index |
| CRP | C-reactive protein |
| MCV | Mean corpuscular volume |
| RDW-CV | Red cell distribution width–coefficient of variation |
| ALP | Alkaline phosphatase |
| PhenoAgeAccel | Phenotypic age acceleration |
| ANOVA | Analysis of variance |
References
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Jaul, E.; Barron, J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and over Population. Front. Public Health 2017, 5, 335. [Google Scholar] [CrossRef]
- Oblak, L.; van der Zaag, J.; Higgins-Chen, A.T.; Levine, M.E.; Boks, M.P. A Systematic Review of Biological, Social and Environmental Factors Associated with Epigenetic Clock Acceleration. Ageing Res. Rev. 2021, 69, 101348. [Google Scholar] [CrossRef]
- Horvath, S. DNA Methylation Age of Human Tissues and Cell Types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Bell, C.G.; Lowe, R.; Adams, P.D.; Baccarelli, A.A.; Beck, S.; Bell, J.T.; Christensen, B.C.; Gladyshev, V.N.; Heijmans, B.T.; Horvath, S.; et al. DNA Methylation Aging Clocks: Challenges and Recommendations. Genome Biol. 2019, 20, 249. [Google Scholar] [CrossRef]
- Fransquet, P.D.; Wrigglesworth, J.; Woods, R.L.; Ernst, M.E.; Ryan, J. The Epigenetic Clock as a Predictor of Disease and Mortality Risk: A Systematic Review and Meta-Analysis. Clin. Epigenetics 2019, 11, 62. [Google Scholar] [CrossRef]
- Horvath, S.; Raj, K. DNA Methylation-Based Biomarkers and the Epigenetic Clock Theory of Ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An Epigenetic Biomarker of Aging for Lifespan and Healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef]
- Dugue, P.A.; Bassett, J.K.; Wong, E.M.; Joo, J.E.; Li, S.; Yu, C.; Schmidt, D.F.; Makalic, E.; Doo, N.W.; Buchanan, D.D.; et al. Biological Aging Measures Based on Blood DNA Methylation and Risk of Cancer: A Prospective Study. JNCI Cancer Spect. 2021, 5, pkaa109. [Google Scholar] [CrossRef]
- Dugue, P.A.; Bodelon, C.; Chung, F.F.; Brewer, H.R.; Ambatipudi, S.; Sampson, J.N.; Cuenin, C.; Chajes, V.; Romieu, I.; Fiorito, G.; et al. Methylation-Based Markers of Aging and Lifestyle-Related Factors and Risk of Breast Cancer: A Pooled Analysis of Four Prospective Studies. Breast Cancer Res. 2022, 24, 59. [Google Scholar] [CrossRef]
- Pan, L.; Liu, Y.; Huang, C.; Huang, Y.; Lin, R.; Wei, K.; Yao, Y.; Qin, G.; Yu, Y. Association of Accelerated Phenotypic Aging, Genetic Risk, and Lifestyle with Progression of Type 2 Diabetes: A Prospective Study Using Multi-State Model. BMC Med. 2025, 23, 62. [Google Scholar] [CrossRef]
- Xie, Q.; Qu, H.; Xie, S.; Li, W.; Ouyang, R.; Zhang, C.; Du, M. Associations of Biological Aging with the Morbidity and All-Cause Mortality of Patients with Lung Cancer. Sci. Rep. 2025, 15, 17880. [Google Scholar] [CrossRef] [PubMed]
- Kwan, K.J.S.; Xie, S.S.; Li, H.L.; Lin, X.G.; Lu, Y.J.; Chen, B.; Ge, K.X.; Tang, S.Y.; Zhang, H.; Jiang, S.; et al. Evaluating the Potential of Phenotypic Age to Enhance Cardiovascular Risk Prediction over Chronological Age in the Uk Biobank. Sci. Rep. 2025, 15, 27858. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Geng, T.; Jiang, M.; Huang, N.; Zheng, Y.; Belsky, D.W.; Huang, T. Accelerated Biological Aging and Risk of Depression and Anxiety: Evidence from 424,299 Uk Biobank Participants. Nat. Commun. 2023, 14, 2277. [Google Scholar] [CrossRef]
- Yagi, M.; Mizukoshi, R.; Maruiwa, R.; Isogai, N.; Funao, H.; Fujita, R. Accelerated Biological Aging in Patients with Degenerative Spine Diseases: The Impact of Modifiable Lifestyle Factors on Phenotypic Age. Spine J. 2025, 25, 2369–2381. [Google Scholar] [CrossRef]
- Hijikata, Y.; Kamitani, T.; Otani, K.; Konno, S.; Fukuhara, S.; Yamamoto, Y. Association of Lumbar Spinal Stenosis with Severe Disability and Mortality among Community-Dwelling Older Adults: The Locomotive Syndrome and Health Outcomes in the Aizu Cohort Study. Spine 2021, 46, E784–E790. [Google Scholar] [CrossRef]
- Hwang, R.W.; Briggs, C.M.; Greenwald, S.D.; Manberg, P.J.; Chamoun, N.G.; Tromanhauser, S.G. Surgical Treatment of Single-Level Lumbar Stenosis Is Associated with Lower 2-Year Mortality and Total Cost Compared with Nonsurgical Treatment: A Risk-Adjusted, Paired Analysis. J. Bone Jt. Surg. Am. 2023, 105, 214–222. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.J.; Ko, B.G.; Chung, J.W.; Kim, S.H.; Park, S.H.; Lee, M.H.; Yeom, J.S. The Prevalence and Impact of Sarcopenia on Degenerative Lumbar Spinal Stenosis. Bone Jt. J. 2016, 98-B, 1093–1098. [Google Scholar] [CrossRef]
- Eguchi, Y.; Suzuki, M.; Yamanaka, H.; Tamai, H.; Kobayashi, T.; Orita, S.; Yamauchi, K.; Suzuki, M.; Inage, K.; Fujimoto, K.; et al. Associations between Sarcopenia and Degenerative Lumbar Scoliosis in Older Women. Scoliosis Spinal Disord. 2017, 12, 9. [Google Scholar] [CrossRef]
- Knutsson, B.; Sanden, B.; Sjoden, G.; Jarvholm, B.; Michaelsson, K. Body Mass Index and Risk for Clinical Lumbar Spinal Stenosis: A Cohort Study. Spine 2015, 40, 1451–1456. [Google Scholar] [CrossRef]
- Hirano, K.; Imagama, S.; Hasegawa, Y.; Muramoto, A.; Ishiguro, N. Impact of Spinal Imbalance and Bmi on Lumbar Spinal Canal Stenosis Determined by a Diagnostic Support Tool: Cohort Study in Community-Living People. Arch. Orthop. Trauma Surg. 2013, 133, 1477–1482. [Google Scholar] [CrossRef]
- Wandell, P.E.; Carlsson, A.C.; Theobald, H. The Association between Bmi Value and Long-Term Mortality. Int. J. Obes. 2009, 33, 577–582. [Google Scholar] [CrossRef]
- Ho, K.M. Associations between Body Mass Index, Biological Age and Frailty in the Critically Ill. Obes. Res. Clin. Pract. 2024, 18, 189–194. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, M.; Zhang, W.; Liu, X.; Pu, J.; Wei, T.; Zhu, Z.; Tang, Z.; Wei, N.; Liu, B. Association between Life’s Essential 8 and Biological Ageing among Us Adults. J. Transl. Med. 2023, 21, 622. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Zamboni, People Writing Group for the European Working Group on Sarcopenia in Older, and Ewgsop the Extended Group for. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Leigh, L.; Byles, J.E.; Jagger, C. Bmi and Healthy Life Expectancy in Old and Very Old Women. Br. J. Nutr. 2016, 116, 692–699. [Google Scholar] [CrossRef]
- Inkster, A.M.; Duarte-Guterman, P.; Albert, A.Y.; Barha, C.K.; Galea, L.A.M.; Robinson, W.P.; Alzheimer’s Disease Neuroimaging Initiative. Are Sex Differences in Cognitive Impairment Reflected in Epigenetic Age Acceleration Metrics? Neurobiol. Aging 2022, 109, 192–194. [Google Scholar] [CrossRef]
- Johnston, C.D.; Pang, A.P.S.; Siegler, E.L.; Thomas, C.; Burchett, C.O.; Crowley, M.; O’Brien, R.; Ndhlovu, L.C.; Glesby, M.J.; Corley, M.J. Sex Differences in Epigenetic Ageing for Older People Living with Hiv. eBioMedicine 2025, 113, 105588. [Google Scholar] [CrossRef]
- Hagg, S.; Jylhava, J. Sex Differences in Biological Aging with a Focus on Human Studies. eLife 2021, 10, e63425. [Google Scholar] [CrossRef]
- Sugimori, K.; Kawaguchi, Y.; Morita, M.; Kitajima, I.; Kimura, T. High-Sensitivity Analysis of Serum C-Reactive Protein in Young Patients with Lumbar Disc Herniation. J. Bone Jt. Surg. Br. 2003, 85, 1151–1154. [Google Scholar] [CrossRef]
- Sturmer, T.; Raum, E.; Buchner, M.; Gebhardt, K.; Schiltenwolf, M.; Richter, W.; Brenner, H. Pain and High Sensitivity C Reactive Protein in Patients with Chronic Low Back Pain and Acute Sciatic Pain. Ann. Rheum. Dis. 2005, 64, 921–925. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, S.H. Investigation of High-Sensitivity C-Reactive Protein and Erythrocyte Sedimentation Rate in Low Back Pain Patients. Korean J. Pain 2010, 23, 147–150. [Google Scholar] [CrossRef]
| LSS Group (n = 208) | Control Group (n = 196) | p Value | |
|---|---|---|---|
| Gender (Male/Female) | 105/103 | 103/93 | 0.691 |
| Age (y/o) | 70.2 ± 9.3 | 70.5 ± 8.6 | 0.835 |
| PhenoAge (y/o) | 65.0 ± 10.7 | 62.0 ± 9.2 | 0.003 |
| PhenoAgeAccel (y/o) | −5.7 ± 6.5 | −8.5 ± 3.7 | <0.001 |
| Height (m) | 1.60 ± 0.10 | 1.60 ± 0.09 | 0.890 |
| Weight (kg) | 64.5 ± 13.6 | 63.4 ± 12.2 | 0.395 |
| BMI (kg/m2) | 25.1 ± 4.0 | 24.6 ± 3.5 | 0.239 |
| LSS Group | Young Males (n = 52) | Old Males (n = 53) | Young Females (n = 38) | Old Females (n = 65) | p Value |
|---|---|---|---|---|---|
| Age (y/o) | 62.2 ± 5.6 | 77.0 ± 4.6 | 61.2 ± 6.9 | 77.1 ± 4.5 | <0.001 |
| PhenoAge (y/o) | 59.9 ± 8.4 * | 72.6 ± 6.4 * | 52.4 ± 8.3 | 70.2 ± 7.3 * | <0.001 |
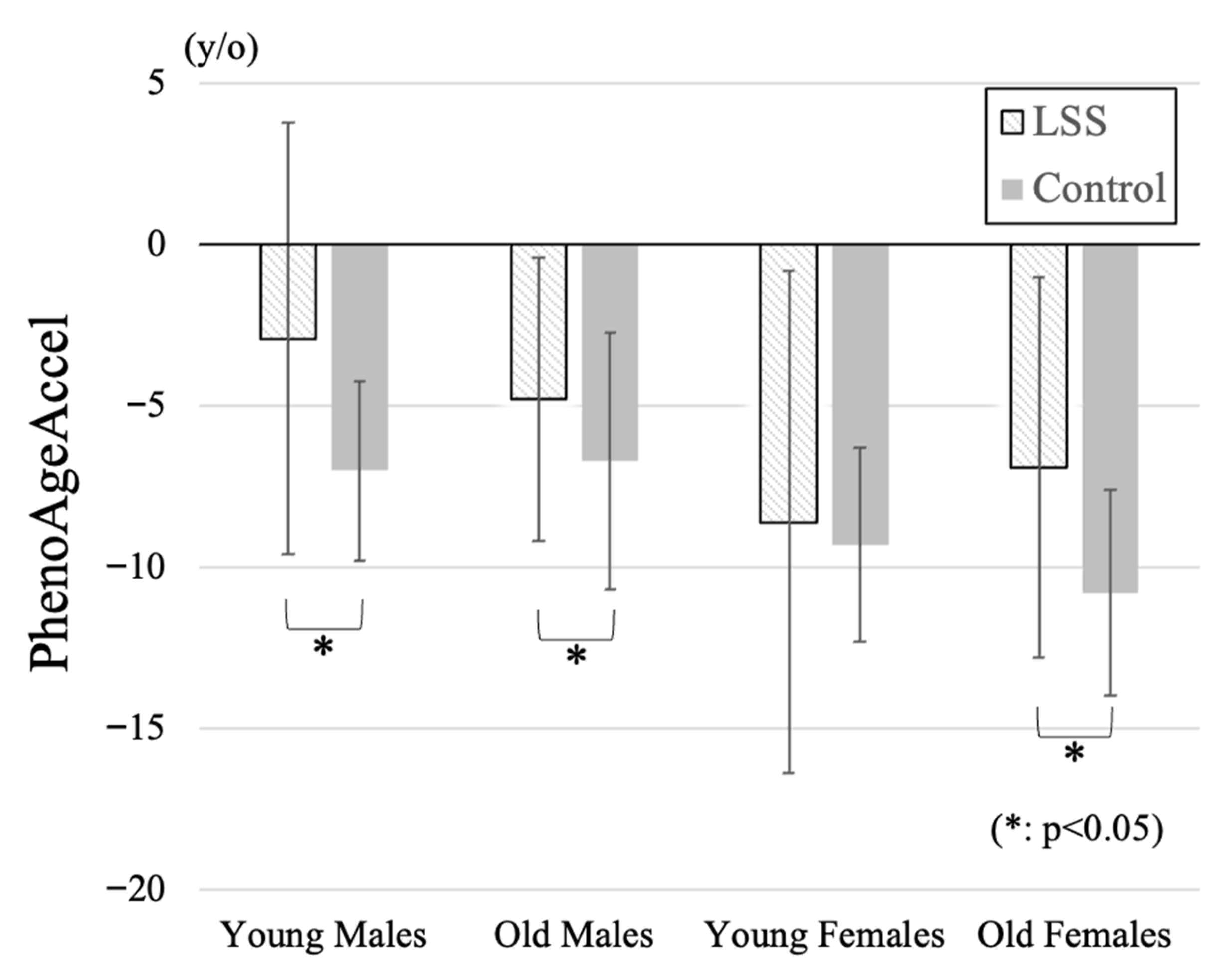
| PhenoAgeAccel (y/o) | −2.9 ± 6.7 * | −4.8 ± 4.4 * | −8.6 ± 7.8 | −6.9 ± 5.9 * | <0.001 |
| Height (m) | 1.70 ± 0.06 | 1.64 ± 0.07 | 1.57 ± 0.05 | 1.51 ± 0.05 | <0.001 |
| Weight (kg) | 74.6 ± 12.4 | 68.0 ± 9.6 | 61.9 ± 14.4 | 55.2 ± 9.8 | <0.001 |
| BMI (kg/m2) | 25.9 ± 4.0 | 25.0 ± 2.7 | 25.2 ± 5.3 | 24.3 ± 3.9 | 0.198 |
| SMI (kg/m2) | 8.1 ± 1.4 | 7.4 ± 1.0 | 6.4 ± 0.8 | 6.0 ± 0.8 | <0.001 |
| Control Group | Young Males (n = 51) | Old Males (n = 52) | Young Females (n = 36) | Old Females (n = 57) | p Value |
| Age (y/o) | 62.9 ± 5.6 | 77.1 ± 4.7 | 62.2 ± 6.3 | 76.6 ± 3.6 | <0.001 |
| PhenoAge (y/o) | 55.9 ± 6.8 | 70.3 ± 6.3 | 52.9 ± 6.7 | 65.7 ± 4.7 | <0.001 |
| PhenoAgeAccel (y/o) | −7.0 ± 2.8 | −6.7 ± 4.0 | −9.3 ± 3.0 | −10.8 ± 3.2 | <0.001 |
| Height (m) | 1.68 ± 0.06 | 1.66 ± 0.05 | 1.57 ± 0.06 | 1.50 ± 0.04 | <0.001 |
| Weight (kg) | 72.4 ± 10.9 | 68.4 ± 9.0 | 59.9 ± 10.7 | 53.1 ± 7.5 | <0.001 |
| BMI (kg/m2) | 25.6 ± 3.6 | 24.9 ± 2.6 | 24.5 ± 4.3 | 23.5 ± 3.2 | 0.016 |
| LSS Group (n = 208) | Control Group (n = 196) | p Value | |
|---|---|---|---|
| Albumin (g/dL) | 4.3 ± 0.3 | 4.4 ± 0.3 | 0.008 |
| Creatinine (mg/dL) | 0.80 ± 0.23 | 0.83 ± 0.20 | 0.315 |
| Glucose (mg/dL) | 117 ± 30 | 112 ± 21 | 0.036 |
| CRP (mg/dL) | 0.37 ± 1.33 | 0.16 ± 0.17 | 0.031 |
| Lymp (%) | 29.7 ± 8.9 | 31.5 ± 8.1 | 0.034 |
| MCV (fl) | 91.9 ± 8.0 | 92.7 ± 4.9 | 0.205 |
| RDW-CV (%) | 13.0 ± 1.1 | 12.5 ± 0.1 | <0.001 |
| ALP (U/L) | 74.2 ± 22.5 | 68.0 ± 17.7 | 0.002 |
| WBC (10 × 3/μL) | 6.20 ± 1.69 | 5.39 ± 1.36 | <0.001 |
| LSS Group | Young Males (n = 52) | Old Males (n = 53) | Young Females (n = 38) | Old Females (n = 65) | p Value |
|---|---|---|---|---|---|
| Albumin (g/dL) | 4.3 ± 0.3 * | 4.3 ± 0.3 | 4.5 ± 0.3 * | 4.2 ± 0.4 * | <0.001 |
| Creatinine (mg/dL) | 0.91 ± 0.23 | 0.91 ± 0.22 | 0.64 ± 0.11 | 0.72 ± 0.21 | <0.001 |
| Glucose (mg/dL) | 122 ± 42 | 118 ± 25 | 108 ± 26 | 118 ± 24 * | 0.164 |
| CRP (mg/dL) | 0.26 ± 0.56 | 0.44 ± 1.81 | 0.20 ± 0.44 | 0.49 ± 1.64 | 0.645 |
| Lymp (%) | 29.5 ± 7.9 * | 29.5 ± 8.5 | 29.3 ± 9.7 * | 30.3 ± 9.6 | 0.942 |
| MCV (fl) | 90.8 ± 8.8 | 94.2 ± 4.2 | 89.2 ± 13.5 | 92.5 ± 3.5 | 0.015 |
| RDW-CV (%) | 13.0 ± 1.1 * | 13.0 ± 0.9 * | 12.8 ± 1.7 * | 13.0 ± 0.8 * | 0.697 |
| ALP (U/L) | 71.5 ± 19.2 | 74.2 ± 25.4 | 74.1 ± 19.1 | 76.4 ± 24.3 * | 0.716 |
| WBC (10 × 3/μL) | 6.32 ± 1.61 * | 6.59 ± 1.67 * | 5.66 ± 1.17 | 6.10 ± 1.95 * | 0.063 |
| Control Group | Young Males (n = 51) | Old Males (n = 52) | Young Females (n = 36) | Old Females (n = 57) | p Value |
| Albumin (g/dL) | 4.5 ± 0.3 | 4.4 ± 0.3 | 4.4 ± 0.2 | 4.4 ± 0.3 | 0.003 |
| Creatinine (mg/dL) | 0.92 ± 0.15 | 0.97 ± 0.18 | 0.69 ± 0.14 | 0.70 ± 0.13 | <0.001 |
| Glucose (mg/dL) | 112 ± 20 | 121 ± 26 | 106 ± 11 | 107 ± 17 | 0.001 |
| CRP (mg/dL) | 0.13 ± 0.09 | 0.17 ± 0.14 | 0.20 ± 0.23 | 0.16 ± 0.19 | 0.219 |
| Lymp (%) | 33.1 ± 7.9 | 29.4 ± 8.2 | 33.6 ± 6.7 | 30.7 ± 8.6 | 0.036 |
| MCV (fl) | 92.3 ± 4.1 | 93.8 ± 3.8 | 90.7 ± 4.8 | 93.5 ± 6.0 | 0.012 |
| RDW-CV (%) | 12.5 ± 0.1 | 12.5 ± 0.1 | 12.5 ± 0.1 | 12.5 ± 0.1 | 0.997 |
| ALP (U/L) | 65.7 ± 15.3 | 68.7 ± 21.2 | 74.2 ± 17.2 | 65.5 ± 15.9 | 0.093 |
| WBC (10 × 3/μL) | 5.50 ± 1.38 | 5.56 ± 1.50 | 5.29 ± 1.37 | 5.20 ± 1.18 | 0.481 |
| LSS Group | Young Males (n = 52) | Old Males (n = 53) | Young Females (n = 38) | Old Females (n = 65) | p Value |
|---|---|---|---|---|---|
| Age | 0.10 | −0.02 * | −0.38 * | 0.03 | 0.260 |
| PhenoAge | 0.73 | 0.68 | 0.63 | 0.78 | 0.614 |
| Height | 0.09 | −0.12 | −0.06 | 0.15 | 0.508 |
| Weight | 0.04 | −0.11 | −0.07 | −0.20 | 0.424 |
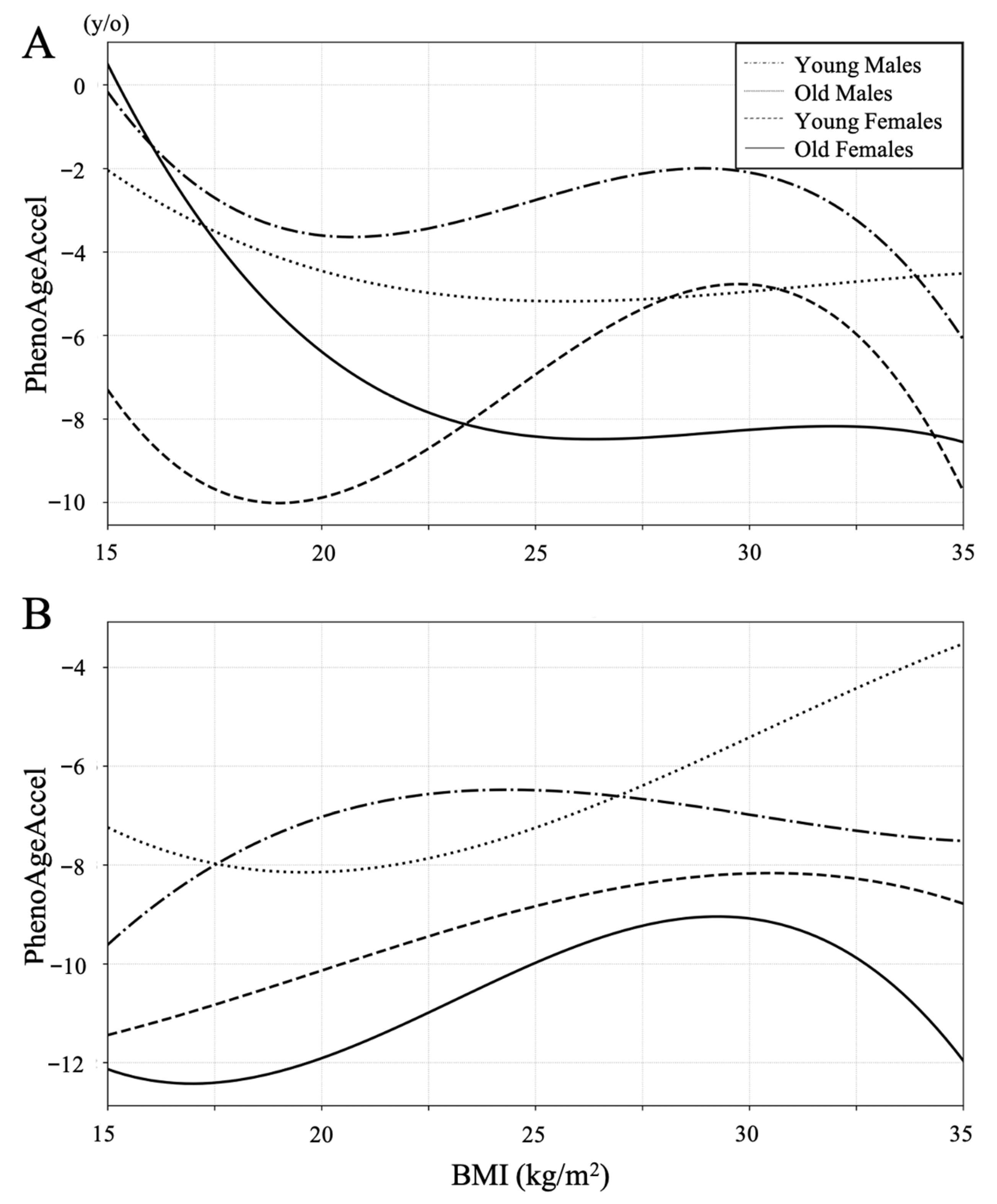
| BMI | −0.003 | −0.06 | −0.09 | −0.29 | 0.189 |
| SMI | 0.27 * | 0.06 | 0.09 | −0.25 * | 0.051 |
| Control Group | Young Males (n = 51) | Old Males (n = 52) | Young Females (n = 36) | Old Females (n = 57) | p Value |
| Age | 0.22 | 0.03 | −0.09 | −0.07 | 0.544 |
| PhenoAge | 0.60 | 0.67 | 0.36 | 0.62 | 0.006 |
| Height | −0.02 | 0.04 | −0.05 | −0.12 | 0.833 |
| Weight | −0.07 | 0.26 | 0.27 | 0.24 | 0.429 |
| BMI | 0.08 | 0.30 | 0.29 | 0.31 | 0.264 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isogai, N.; Funao, H.; Mizukoshi, R.; Ito, K.; Ebata, S.; Yagi, M. Association Between Lumbar Spinal Stenosis and Accelerated Biological Aging Estimated by PhenoAge. J. Clin. Med. 2025, 14, 7852. https://doi.org/10.3390/jcm14217852
Isogai N, Funao H, Mizukoshi R, Ito K, Ebata S, Yagi M. Association Between Lumbar Spinal Stenosis and Accelerated Biological Aging Estimated by PhenoAge. Journal of Clinical Medicine. 2025; 14(21):7852. https://doi.org/10.3390/jcm14217852
Chicago/Turabian StyleIsogai, Norihiro, Haruki Funao, Ryo Mizukoshi, Keirato Ito, Shigeto Ebata, and Mitsuru Yagi. 2025. "Association Between Lumbar Spinal Stenosis and Accelerated Biological Aging Estimated by PhenoAge" Journal of Clinical Medicine 14, no. 21: 7852. https://doi.org/10.3390/jcm14217852
APA StyleIsogai, N., Funao, H., Mizukoshi, R., Ito, K., Ebata, S., & Yagi, M. (2025). Association Between Lumbar Spinal Stenosis and Accelerated Biological Aging Estimated by PhenoAge. Journal of Clinical Medicine, 14(21), 7852. https://doi.org/10.3390/jcm14217852






