Treatment Patterns by Physiologic Age in Older Adults with Early-Stage Breast Cancer: A Single Institution Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HR | Hormone receptor |
| HER-2 | Human epidermal growth factor receptor-2 |
| SLNB | Sentinel lymph node biopsy |
| RT | Radiation therapy |
| TNBC | Triple negative breast cancer |
| COQD | Clinical Operations Quality Database |
| CDK | Cyclin dependent kinase |
| ET | Endocrine therapy |
References
- Bluethmann, S.M.; Mariotto, A.B.; Rowland, J.H. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1029–1036. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology and End Results (SEER) Stat Fact Sheets: Female Breast Cancer. Available online: http://seer.cancer.gov/statfacts/html/breast.html (accessed on 3 May 2019).
- DuMontier, C.; Loh, K.P.; Bain, P.A.; Silliman, R.A.; Hshieh, T.; Abel, G.A.; Djulbegovic, B.; Driver, J.A.; Dale, W. Defining Undertreatment and Overtreatment in Older Adults with Cancer: A Scoping Literature Review. J. Clin. Oncol. 2020, 38, 2558–2569. [Google Scholar] [CrossRef]
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Schonberg, M.A.; Boyd, C.M.; Burhenn, P.S.; Canin, B.; Cohen, H.J.; Holmes, H.M.; Hopkins, J.O.; et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J. Clin. Oncol. 2018, 36, 2326–2347. [Google Scholar] [CrossRef] [PubMed]
- Biganzoli, L.; Battisti, N.M.L.; Wildiers, H.; McCartney, A.; Colloca, G.; Kunkler, I.H.; Cardoso, M.-J.; Cheung, K.-L.; de Glas, N.A.; Trimboli, R.M.; et al. Updated recommendations regarding the management of older patients with breast cancer: A joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG). Lancet Oncol. 2021, 22, e327–e340. [Google Scholar] [CrossRef] [PubMed]
- Rudenstam, C.M.; Zahrieh, D.; Forbes, J.F.; Crivellari, D.; Holmberg, S.B.; Rey, P.; Dent, D.; Campbell, I.; Bernhard, J.; Price, K.N.; et al. Randomized trial comparing axillary clearance versus no axillary clearance in older patients with breast cancer: First results of International Breast Cancer Study Group Trial 10-93. J. Clin. Oncol. 2006, 24, 337–344. [Google Scholar] [CrossRef]
- Martelli, G.; Boracchi, P.; Ardoino, I.; Lozza, L.; Bohm, S.; Vetrella, G.; Agresti, R. Axillary dissection versus no axillary dissection in older patients with T1N0 breast cancer: 15-year results of a randomized controlled trial. Ann. Surg. 2012, 256, 920–924. [Google Scholar] [CrossRef]
- Hughes, K.S.; Schnaper, L.A.; Bellon, J.R.; Cirrincione, C.T.; Berry, D.A.; McCormick, B.; Muss, H.B.; Smith, B.L.; Hudis, C.A.; Winer, E.P.; et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: Long-term follow-up of CALGB 9343. J. Clin. Oncol. 2013, 31, 2382–2387. [Google Scholar] [CrossRef]
- Kunkler, I.H.; Williams, L.J.; Jack, W.J.L.; Cameron, D.A.; Dixon, J.M. Breast-Conserving Surgery with or without Irradiation in Early Breast Cancer. N. Engl. J. Med. 2023, 388, 585–594. [Google Scholar] [CrossRef]
- Lorentzen, E.H.; Minami, C.A. Avoiding Locoregional Overtreatment in Older Adults with Early-Stage Breast Cancer. Clin. Breast Cancer 2024, 24, 319–327. [Google Scholar] [CrossRef]
- Minami, C.A.; Jin, G.; Freedman, R.A.; Schonberg, M.A.; King, T.A.; Mittendorf, E.A. Trends in Locoregional Therapy in Older Women with Early-Stage Hormone Receptor-Positive Breast Cancer by Frailty and Life Expectancy. Ann. Surg. Oncol. 2023, 31, 920–930. [Google Scholar] [CrossRef]
- Bellera, C.A.; Rainfray, M.; Mathoulin-Pélissier, S.; Mertens, C.; Delva, F.; Fonck, M.; Soubeyran, P.L. Screening older cancer patients: First evaluation of the G-8 geriatric screening tool. Ann. Oncol. 2012, 23, 2166–2172. [Google Scholar] [CrossRef]
- Schonberg, M.A.; Davis, R.B.; McCarthy, E.P.; Marcantonio, E.R. External validation of an index to predict up to 9-year mortality of community-dwelling adults aged 65 and older. J. Am. Geriatr. Soc. 2011, 59, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, J.; Long, S.; Carter, B.; Bach, S.; McCarthy, K.; Clegg, A. The prevalence of frailty and its association with clinical outcomes in general surgery: A systematic review and meta-analysis. Age Ageing 2018, 47, 793–800. [Google Scholar] [CrossRef]
- Minami, C.A.; Cooper, Z. The Frailty Syndrome: A Critical Issue in Geriatric Oncology. Crit. Care Clin. 2021, 37, 151–174. [Google Scholar] [CrossRef]
- Handforth, C.; Clegg, A.; Young, C.; Simpkins, S.; Seymour, M.T.; Selby, P.J.; Young, J. The prevalence and outcomes of frailty in older cancer patients: A systematic review. Ann. Oncol. 2015, 26, 1091–1101. [Google Scholar] [CrossRef]
- Soto-Perez-de-Celis, E.; Li, D.; Yuan, Y.; Lau, Y.M.; Hurria, A. Functional versus chronological age: Geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018, 19, e305–e316. [Google Scholar] [CrossRef] [PubMed]
- Hurria, A.; Wong, F.L.; Villaluna, D.; Bhatia, S.; Chung, C.T.; Mortimer, J.; Hurvitz, S.; Naeim, A. Role of age and health in treatment recommendations for older adults with breast cancer: The perspective of oncologists and primary care providers. J. Clin. Oncol. 2008, 26, 5386–5392. [Google Scholar] [CrossRef]
- Minami, C.A.; Bryan, A.F.; Freedman, R.A.; Revette, A.C.; Schonberg, M.A.; King, T.A.; Mittendorf, E.A. Assessment of Oncologists’ Perspectives on Omission of Sentinel Lymph Node Biopsy in Women 70 Years and Older with Early-Stage Hormone Receptor-Positive Breast Cancer. JAMA Netw. Open 2022, 5, e2228524. [Google Scholar] [CrossRef]
- Dharmarajan, K.V.; Presley, C.J.; Wyld, L. Care Disparities Across the Health Care Continuum for Older Adults: Lessons from Multidisciplinary Perspectives. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 2021, 41, e215–e224. [Google Scholar] [CrossRef]
- McKenzie, G.A.G.; Bullock, A.F.; Greenley, S.L.; Lind, M.J.; Johnson, M.J.; Pearson, M. Implementation of geriatric assessment in oncology settings: A systematic realist review. J. Geriatr. Oncol. 2021, 12, 22–33. [Google Scholar] [CrossRef]
- Kunkler, I.H.; Williams, L.J.; Jack, W.J.; Cameron, D.A.; Dixon, J.M. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): A randomised controlled trial. Lancet Oncol. 2015, 16, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, A.; Sedrak, M.S.; Gross, C.P.; Tew, W.P.; Klepin, H.D.; Wildes, T.M.; Muss, H.B.; Dotan, E.; Freedman, R.A.; O’Connor, T.; et al. Development and Validation of a Risk Tool for Predicting Severe Toxicity in Older Adults Receiving Chemotherapy for Early-Stage Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 608–618. [Google Scholar] [CrossRef]
- Li, W.; Frydman, J.L.; Li, Y.; Liu, B. Characterizing delayed care among US older adults by self-rated health during the COVID-19 pandemic. Prev. Med. 2022, 164, 107308. [Google Scholar] [CrossRef]
- Yilmaz, S.; Koita, F.; Zittel, J.; Wells, M.; Mohile, S.; Wittink, M.; Kelly, J.M.; Sedrak, M.; DeCaporale-Ryan, L.; DiGiovanni, G.; et al. The role of self-perceived age in older adults considering adjuvant chemotherapy. J. Geriatr. Oncol. 2023, 14, 101429. [Google Scholar] [CrossRef]
- Giri, S.; Mir, N.; Al-Obaidi, M.; Clark, D.; Kenzik, K.M.; McDonald, A.; Young-Smith, C.; Paluri, R.; Nandagopal, L.; Gbolahan, O.; et al. Use of Single-Item Self-Rated Health Measure to Identify Frailty and Geriatric Assessment-Identified Impairments Among Older Adults with Cancer. Oncologist 2022, 27, e45–e52. [Google Scholar] [CrossRef]
- Puts, M.; Monette, J.; Girre, V.; Sourial, N.; Wolfson, C.; Monette, M.; Batist, G.; Bergman, H. The relationship of self-rated health with functional status, toxicity and mortality: Results of a prospective pilot study of older patients with newly-diagnosed cancer. J. Geriatr. Oncol. 2013, 4, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.Y.; Stephens, E.K.; Novotny, P.; Price, K.; Salayi, M.; Roeker, L.; Peethambaram, P.; Jatoi, A. Self-perceptions of age among 292 chemotherapy-treated cancer patients: Exploring associations with symptoms and survival. J. Geriatr. Oncol. 2013, 4, 249–254. [Google Scholar] [CrossRef]
- Damián, J.; Pastor-Barriuso, R.; Valderrama-Gama, E.; de Pedro-Cuesta, J. Discordance between physician-rated health and an objective health measure among institutionalized older people. BMC Geriatr. 2015, 15, 78. [Google Scholar] [CrossRef]
- Gramling, R.; Fiscella, K.; Xing, G.; Hoerger, M.; Duberstein, P.; Plumb, S.; Mohile, S.; Fenton, J.J.; Tancredi, D.J.; Kravitz, R.L.; et al. Determinants of Patient-Oncologist Prognostic Discordance in Advanced Cancer. JAMA Oncol. 2016, 2, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Bredbeck, B.C.; Sinco, B.; Shubeck, S.; Baskin, A.S.; Skolarus, T.; Dossett, L.A. Variations in Persistent Use of Low-Value Breast Cancer Surgery. JAMA Surg. 2021, 156, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Minami, C.A.; Jin, G.; Schonberg, M.A.; Freedman, R.A.; King, T.A.; Mittendorf, E.A. Variation in Deescalated Axillary Surgical Practices in Older Women with Early-Stage Breast Cancer. Ann. Surg. Oncol. 2022, 29, 4181–4194. [Google Scholar] [CrossRef]
- Hershman, D.L.; Kushi, L.H.; Shao, T.; Buono, D.; Kershenbaum, A.; Tsai, W.-Y.; Fehrenbacher, L.; Gomez, S.L.; Miles, S.; Neugut, A.I. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 4120–4128. [Google Scholar] [CrossRef] [PubMed]
- Meattini, I.; De Santis, M.C.; Visani, L.; Scorsetti, M.; Fozza, A.; Meduri, B.; De Rose, F.; Bonzano, E.; Prisco, A.; Masiello, V.; et al. Single-modality endocrine therapy versus radiotherapy after breast-conserving surgery in women aged 70 years and older with luminal A-like early breast cancer (EUROPA): A preplanned interim analysis of a phase 3, non-inferiority, randomised trial. Lancet Oncol. 2025, 26, 37–50. [Google Scholar] [CrossRef] [PubMed]
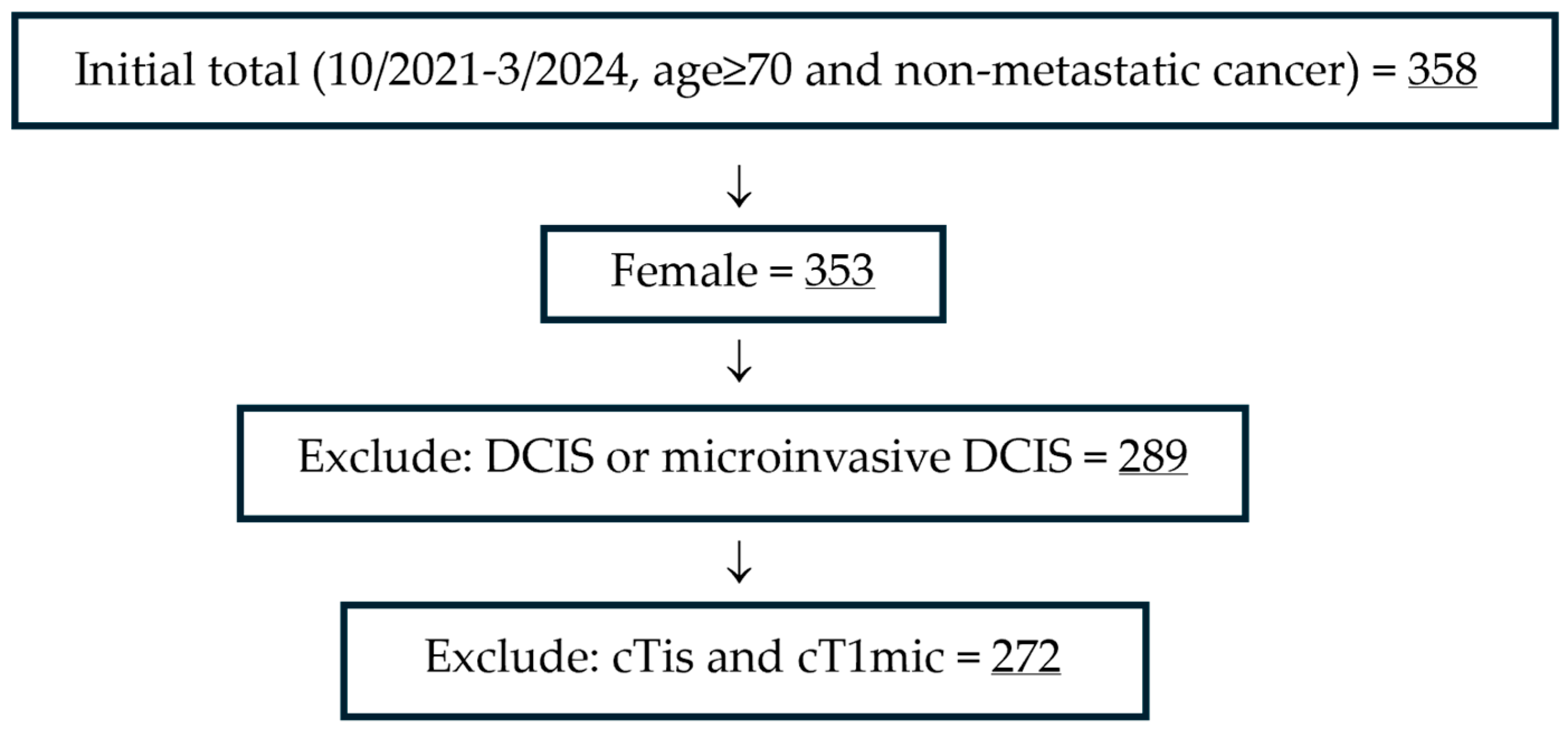
| Total N = 272 | Life Expectancy ≥ 10 Years N = 208 (76.5%) | Life Expectancy < 10 Years N = 64 (23.5%) | p-Value | Age <80 Years N = 215 (79.0%) | Age ≥80 Years N = 57 (21.0%) | p-Value | |
|---|---|---|---|---|---|---|---|
| Age | <0.0001 | ||||||
| 70–74 | 112 (41.2%) | 104 (50.0%) | 8 (12.5%) | ||||
| 75–79 | 103 (37.9%) | 75 (36.1%) | 28 (43.8%) | ||||
| 80–84 | 37 (13.6%) | 25 (12.0%) | 12 (18.8%) | ||||
| ≥85 | 20 (7.4%) | 4 (1.9%) | 16 (25.0%) | ||||
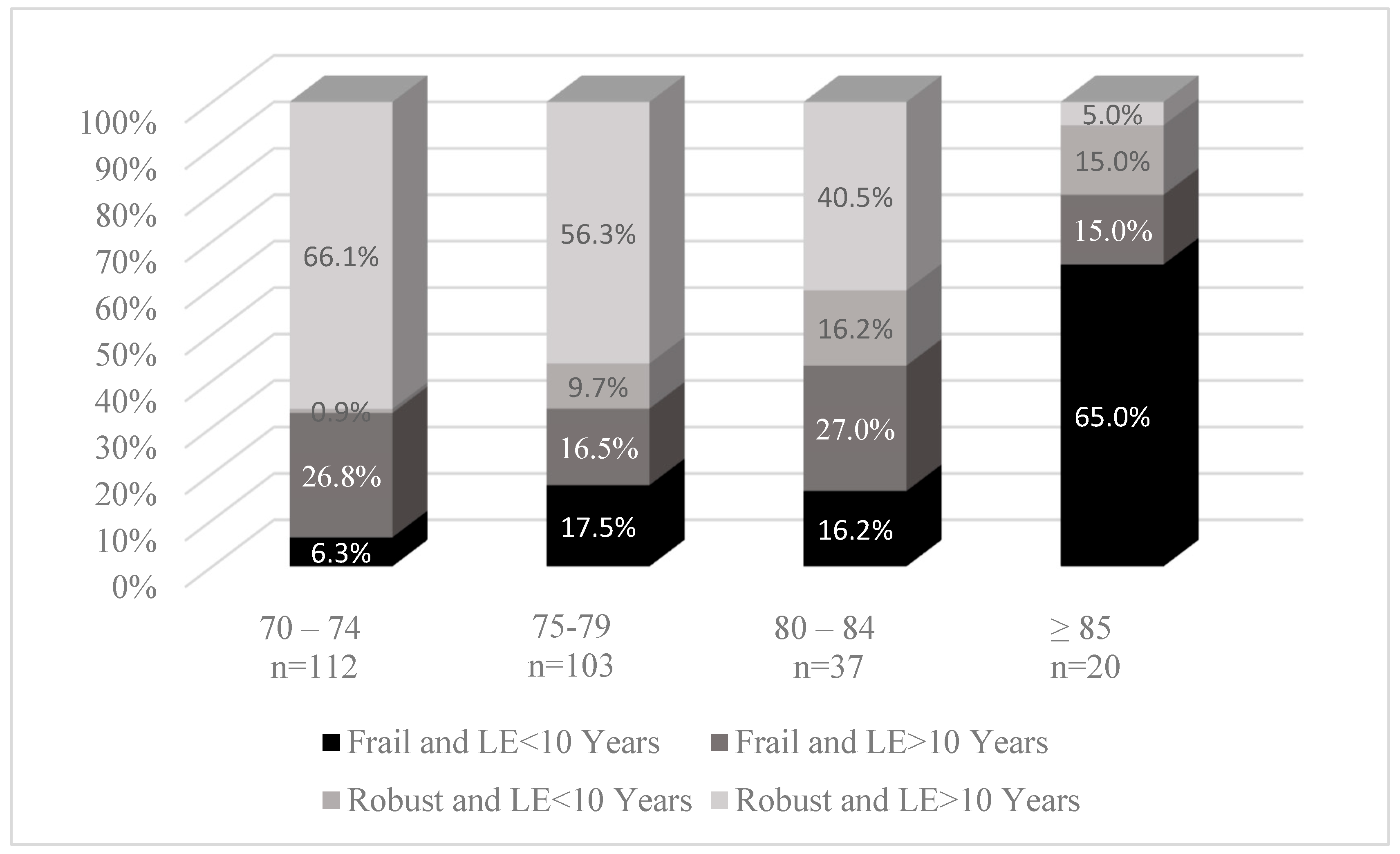
| Frailty Status | <0.0001 | 0.002 | |||||
| Robust | 168 (61.8%) | 148 (71.2%) | 20 (31.3%) | 143 (66.5%) | 25 (43.9%) | ||
| At risk of being frail | 104 (38.2%) | 60 (28.8%) | 44 (68.8%) | 72 (33.5%) | 32 (56.1%) | ||
| Self-Reported Health | <0.0001 | 0.72 | |||||
| Better than others your age | 134 (49.3%) | 116 (55.8%) | 18 (28.1%) | 103 (47.9%) | 31 (54.4%) | ||
| As good as others your age | 107 (39.3%) | 84 (40.4%) | 23 (35.9%) | 88 (40.9%) | 19 (33.3%) | ||
| Not as good as others your age | 14 (5.1%) | 1 (0.5%) | 13 (20.3%) | 11 (5.1%) | 3 (5.3%) | ||
| Does not know | 17 (6.3%) | 7 (3.4%) | 10 (15.6%) | 13 (6.0%) | 4 (7.0%) | ||
| Race/Ethnicity | 0.51 | 0.50 | |||||
| Non-Hispanic White | 255 (93.8%) | 197 (94.7%) | 58 (90.6%) | 202 (94.0%) | 53 (93.0%) | ||
| Hispanic White | 1 (0.4%) | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) | 1 (1.8%) | ||
| Black (Hispanic and Non-Hispanic) | 10 (3.7%) | 6 (2.9%) | 4 (6.3%) | 8 (3.7%) | 2 (3.5%) | ||
| Asian/Pacific Islander | 1 (0.4%) | 1 (0.5%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | ||
| Other/Unknown | 5 (1.8%) | 3 (1.4%) | 2 (3.1%) | 4 (1.9%) | 1 (1.8%) | ||
| Tumor Grade | 0.19 | 0.41 | |||||
| Grade 1 | 87 (32.0%) | 72 (34.6%) | 15 (23.4%) | 70 (32.6%) | 17 (29.8%) | ||
| Grade 2 | 125 (46.0%) | 95 (45.7%) | 30 (46.9%) | 101 (47.0%) | 24 (42.1%) | ||
| Grade 3 | 56 (20.6%) | 38 (18.3%) | 18 (28.1%) | 40 (18.6%) | 16 (28.1%) | ||
| Unknown | 4 (1.5%) | 3 (1.4%) | 1 (1.6%) | 4 (1.9%) | 0 (0.0%) | ||
| Pathologic T Stage | 0.30 | 0.09 | |||||
| T1 | 194 (71.3%) | 153 (73.6%) | 41 (64.1%) | 161 (74.9%) | 33 (57.9%) | ||
| T2 | 33 (12.1%) | 22 (10.6%) | 11 (17.2%) | 21 (9.8%) | 12 (21.1%) | ||
| T3 | 8 (2.9%) | 6 (2.9%) | 2 (3.1%) | 6 (2.8%) | 2 (3.5%) | ||
| T4 | 1 (0.4%) | 1 (0.5%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | ||
| Tx | 1 (0.4%) | 0 (0.0%) | 1 (1.6%) | 1 (0.5%) | 0 (0.0%) | ||
| Unknown | 35 (12.9%) | 26 (12.5%) | 9 (14.1%) | 25 (11.6%) | 10 (17.5%) | ||
| Pathologic N Stage | 0.71 | 0.23 | |||||
| N0 | 44 (16.2%) | 36 (17.3%) | 8 (12.5%) | 40 (18.6%) | 4 (7.0%) | ||
| N1 | 16 (5.9%) | 14 (6.7%) | 2 (3.1%) | 12 (5.6%) | 4 (7.0%) | ||
| N2 | 3 (1.1%) | 3 (1.4%) | 0 (0.0%) | 3 (1.4%) | 0 (0.0%) | ||
| N3 | 1 (0.4%) | 1 (0.5%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | ||
| Nx | 173 (63.6%) | 128 (61.5%) | 45 (70.3%) | 134 (62.3%) | 39 (68.4%) | ||
| Unknown | 35 (12.9%) | 26 (12.5%) | 9 (14.1%) | 25 (11.6%) | 10 (17.5%) | ||
| Tumor Subtype | 0.08 | 0.10 | |||||
| HR+/HER-2− | 226 (83.1%) | 179 (86.1%) | 47 (73.4%) | 180 (83.7%) | 46 (80.7%) | ||
| HER-2+ | 10 (3.7%) | 6 (2.9%) | 4 (6.3%) | 5 (2.3%) | 5 (8.8%) | ||
| TNBC | 33 (12.1%) | 21 (10.1%) | 12 (18.8%) | 28 (13.0%) | 5 (8.8%) | ||
| Other/unknown | 3 (1.1%) | 2 (1.0%) | 1 (1.6%) | 2 (0.9%) | 1 (1.8%) | ||
| OncotypeDX Score | 0.06 | 1.00 | |||||
| ≤25 | 43 (82.7%) | 40 (87.0%) | 3 (50.0%) | 38 (82.6%) | 5 (83.3%) | ||
| >25 | 9 (17.3%) | 6 (13.0%) | 3 (50.0%) | 8 (17.4%) | 1 (16.7%) | ||
| Missing (Not performed) | 220 | 162 | 58 | 169 | 51 |
| Total N = 226 | Life Expectancy ≥ 10 Years N = 179 (79.2%) | Life Expectancy < 10 Years N = 47 (20.8%) | p-Value | Age <80 Years N = 180 (79.7%) | Age ≥80 Years N = 46 (20.3%) | p-Value | |
|---|---|---|---|---|---|---|---|
| Neoadjuvant Chemotherapy | 1.00 | 1.00 | |||||
| Yes | 2 (0.9%) | 2 (1.1%) | 0 (0.0%) | 2 (1.1%) | 0 (0.0%) | ||
| No | 224 (99.1%) | 177 (98.9%) | 47 (100.0%) | 178 (98.9%) | 46 (100.0%) | ||
| Neoadjuvant Endocrine Therapy | 0.74 | 0.04 | |||||
| Yes | 14 (6.2%) | 12 (6.7%) | 2 (4.3%) | 8 (4.4%) | 6 (13.0%) | ||
| No | 212 (93.8%) | 167 (93.3%) | 45 (95.7%) | 172 (95.6%) | 40 (87.0%) | ||
| Surgery | 0.78 | 0.37 | |||||
| Lumpectomy only | 171 (75.7%) | 132 (73.7%) | 39 (83.0%) | 135 (75.0%) | 36 (78.3%) | ||
| Lumpectomy + Axillary Surgery | 19 (8.4%) | 17 (9.5%) | 2 (4.3%) | 14 (7.8%) | 5 (10.9%) | ||
| Mastectomy a | 9 (4.0%) | 8 (4.5%) | 1 (2.1%) | 6 (3.3%) | 3 (6.5%) | ||
| Mastectomy + Axillary Surgery | 22 (9.7%) | 18 (10.1%) | 4 (8.5%) | 20 (11.1%) | 2 (4.3%) | ||
| Other/unknown | 5 (2.2%) | 4 (2.2%) | 1 (2.1%) | 5 (2.8%) | 0 (0.0%) | ||
| Reconstruction b | 1.00 | 1.00 | |||||
| Yes | 4 (12.9%) | 4 (15.4%) | 0 (0.0%) | 4 (15.4%) | 0 (0.0%) | ||
| No | 27 (87.1%) | 22 (84.6%) | 5 (100.0%) | 22 (84.6%) | 5 (100.0%) | ||
| Adjuvant Chemotherapy | 1.00 | 1.00 | |||||
| Yes | 8 (3.5%) | 7 (3.9%) | 1 (2.1%) | 7 (3.9%) | 1 (2.2%) | ||
| No | 218 (96.5%) | 172 (96.1%) | 46 (97.9%) | 173 (96.1%) | 45 (97.8%) | ||
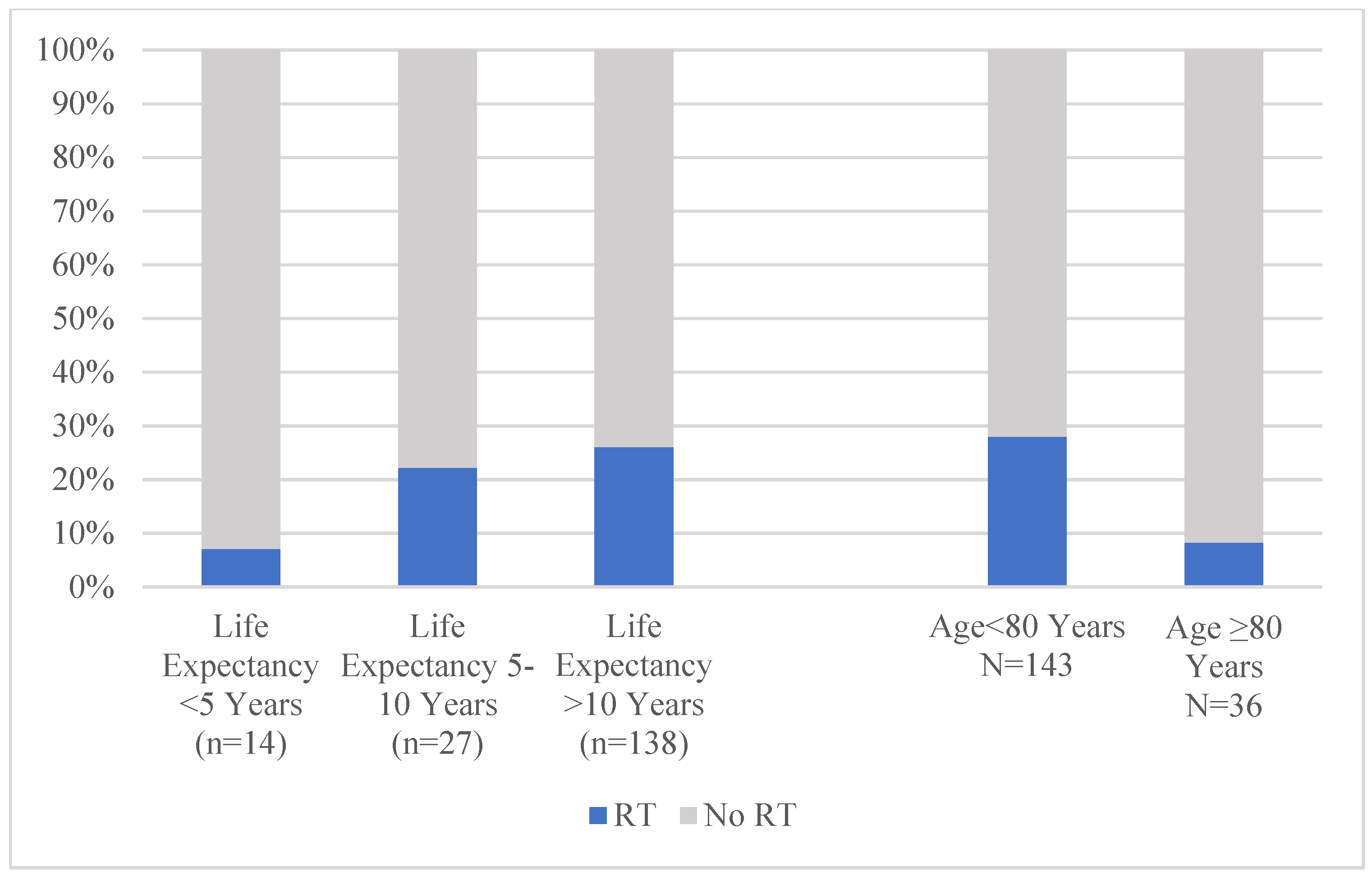
| Adjuvant Radiation Therapy | 0.24 | 0.02 | |||||
| Yes | 50 (22.1%) | 43 (24.0%) | 7 (14.9%) | 46 (25.6%) | 4 (8.7%) | ||
| No | 176 (77.9%) | 136 (76.0%) | 40 (85.1%) | 134 (74.4%) | 42 (91.3%) | ||
| Adjuvant Combination CDK 4/6 Inhibitor and Endocrine Therapy | 0.59 | 0.59 | |||||
| Yes | 5 (2.2%) | 5 (2.8%) | 0 (0.0%) | 5 (2.8%) | 0 (0.0%) | ||
| No | 221 (97.8%) | 174 (97.2%) | 47 (100.0%) | 175 (97.2%) | 46 (100.0%) | ||
| Adjuvant Endocrine Therapy | 0.84 | 0.41 | |||||
| Yes | 182 (80.5%) | 143 (79.9%) | 39 (83.0%) | 147 (81.7%) | 35 (76.1%) | ||
| No | 44 (19.5%) | 36 (20.1%) | 8 (17.0%) | 33 (18.3%) | 11 (23.9%) |
| Total N = 43 | Life Expectancy ≥10 Years N = 27 (62.8%) | Life Expectancy <10 Years N = 16 (37.2%) | p-Value | Age <80 Years N = 33 (76.7%) | Age ≥80 Years N = 10 (23.3%) | p-Value | |
|---|---|---|---|---|---|---|---|
| Neoadjuvant Chemotherapy | 1.00 | 1.00 | |||||
| Yes | 19 (44.2%) | 12 (44.4%) | 7 (43.8%) | 15 (45.5%) | 4 (40.0%) | ||
| No | 24 (55.8%) | 15 (55.6%) | 9 (56.3%) | 18 (54.5%) | 6 (60.0%) | ||
| Surgery | 0.01 | 0.02 | |||||
| Lumpectomy only | 7 (16.3%) | 2 (7.4%) | 5 (31.3%) | 4 (12.1%) | 3 (30.0%) | ||
| Lumpectomy + Axillary Surgery a | 24 (55.8%) | 19 (70.4%) | 5 (31.3%) | 22 (66.7%) | 2 (20.0%) | ||
| Mastectomy b | 5 (11.6%) | 1 (3.7%) | 4 (25.0%) | 2 (6.1%) | 3 (30.0%) | ||
| Mastectomy + Axillary Surgery | 5 (11.6%) | 3 (11.1%) | 2 (12.5%) | 3 (9.1%) | 2 (20.0%) | ||
| Other/unknown | 2 (4.7%) | 2 (7.4%) | 0 (0.0%) | 2 (6.1%) | 0 (0.0%) | ||
| Reconstruction c | 0.40 | 1.00 | |||||
| Yes | 1 (10.0%) | 1 (25.0%) | 0 (0.0%) | 1 (20.0%) | 0 (0.0%) | ||
| No | 9 (90.0%) | 3 (75.0%) | 6 (100.0%) | 4 (80.0%) | 5 (100.0%) | ||
| Adjuvant Chemotherapy | 0.09 | 0.25 | |||||
| Yes | 29 (67.4%) | 21 (77.8%) | 8 (50.0%) | 24 (72.7%) | 5 (50.0%) | ||
| No | 14 (32.6%) | 6 (22.2%) | 8 (50.0%) | 9 (27.3%) | 5 (50.0%) | ||
| Adjuvant Radiation Therapy | 0.76 | 0.03 | |||||
| Yes | 23 (53.5%) | 15 (55.6%) | 8 (50.0%) | 21 (63.6%) | 2 (20.0%) | ||
| No | 20 (46.5%) | 12 (44.4%) | 8 (50.0%) | 12 (36.4%) | 8 (80.0%) |
| N = 179 | OR (95% CI) |
|---|---|
| Life Expectancy | |
| <10 Years | Ref |
| ≥10 Years | 0.81 (0.20–3.28) |
| Self-Reported Health | |
| Better than others your age | Ref |
| As good as others your age | 0.51 (0.15–1.78) |
| Not as good as others your age | 0.44 (0.02–10.93) |
| Does not know | 0.29 (0.02–4.81) |
| Race | |
| White | Ref |
| Black | 1.36 (0.13–14.02) |
| Asian/Pacific Islander and Others | 3.19 (0.25–41.55) |
| Tumor Grade | |
| Grade 1 | Ref |
| Grade 2 | 0.92 (0.24–3.50) |
| Grade 3 | 3.92 (0.72–21.26) |
| Pathologic T Stage | |
| T1 | Ref |
| T2 | 1.79 (0.38–8.40) |
| Adjuvant Radiation Therapy | |
| Yes | Ref |
| No | 0.24 (0.08–0.76) |
| Adjuvant Endocrine Therapy | |
| Yes | Ref |
| No | 1.54 (0.40–5.94) |
| N = 179 | OR (95% CI) |
|---|---|
| Life Expectancy | |
| <10 Years | Ref |
| ≥10 Years | 1.14 (0.44–2.93) |
| Self-Reported Health | |
| Better than others your age | Ref |
| As good as others your age | 0.98 (0.47–2.06) |
| Not as good as others your age | 0.17 (0.008–3.71) |
| Does not know | 1.3 (0.29–5.79) |
| Race | |
| White | Ref |
| Black | 2.21 (0.44–11.25) |
| Asian/Pacific Islander and Others | 2.50 (0.26–23.74) |
| Tumor Grade | |
| Grade 1 | Ref |
| Grade 2 | 1.33 (0.61–2.87) |
| Grade 3 | 1.00 (0.25–3.91) |
| Pathologic T Stage | |
| T1 | Ref |
| T2 | 0.73 (0.23–2.37) |
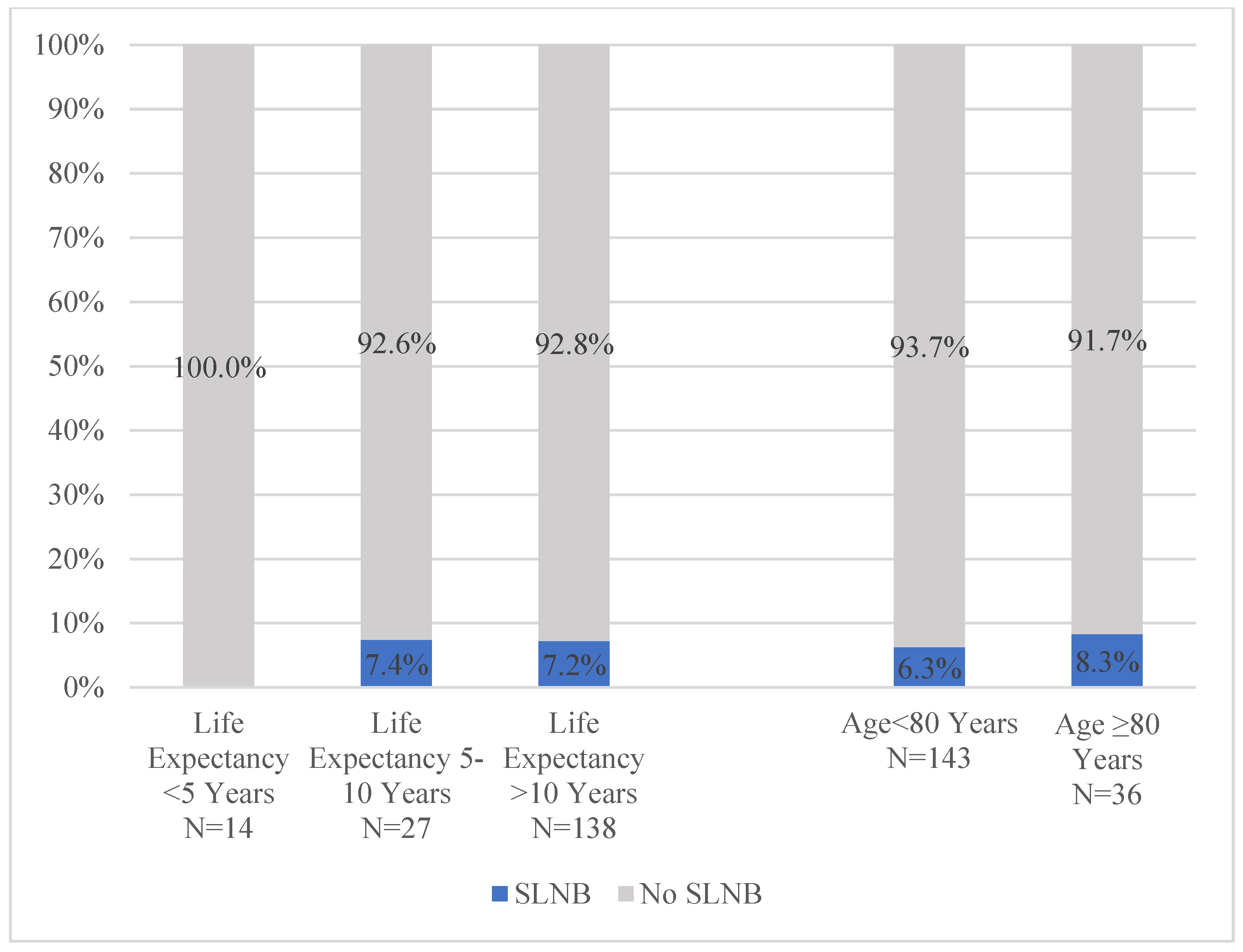
| SLNB Receipt | |
| Yes | Ref |
| No | 0.23 (0.07–0.83) |
| Adjuvant Endocrine Therapy | |
| Yes | Ref |
| No | 0.91 (0.36–2.30) |
| N = 43 | OR (95% CI) |
|---|---|
| Life Expectancy | |
| <10 Years | Ref |
| ≥10 Years | 0.99 (0.21–4.61) |
| Pathologic T Stage | |
| T1 | Ref |
| T2 | 0.25 (0.02–2.76) |
| Unknown | 3.76 (0.74–18.98) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorentzen, E.H.; Chen, Y.-J.; Harvey, M.; Minami, C.A. Treatment Patterns by Physiologic Age in Older Adults with Early-Stage Breast Cancer: A Single Institution Retrospective Study. J. Clin. Med. 2025, 14, 7853. https://doi.org/10.3390/jcm14217853
Lorentzen EH, Chen Y-J, Harvey M, Minami CA. Treatment Patterns by Physiologic Age in Older Adults with Early-Stage Breast Cancer: A Single Institution Retrospective Study. Journal of Clinical Medicine. 2025; 14(21):7853. https://doi.org/10.3390/jcm14217853
Chicago/Turabian StyleLorentzen, Eliza H., Yu-Jen Chen, Maria Harvey, and Christina A. Minami. 2025. "Treatment Patterns by Physiologic Age in Older Adults with Early-Stage Breast Cancer: A Single Institution Retrospective Study" Journal of Clinical Medicine 14, no. 21: 7853. https://doi.org/10.3390/jcm14217853
APA StyleLorentzen, E. H., Chen, Y.-J., Harvey, M., & Minami, C. A. (2025). Treatment Patterns by Physiologic Age in Older Adults with Early-Stage Breast Cancer: A Single Institution Retrospective Study. Journal of Clinical Medicine, 14(21), 7853. https://doi.org/10.3390/jcm14217853






