Outcomes from Referral to Transplant for Patients with MASLD: A California Liver Network Study
Abstract
1. Introduction
2. Materials and Methods
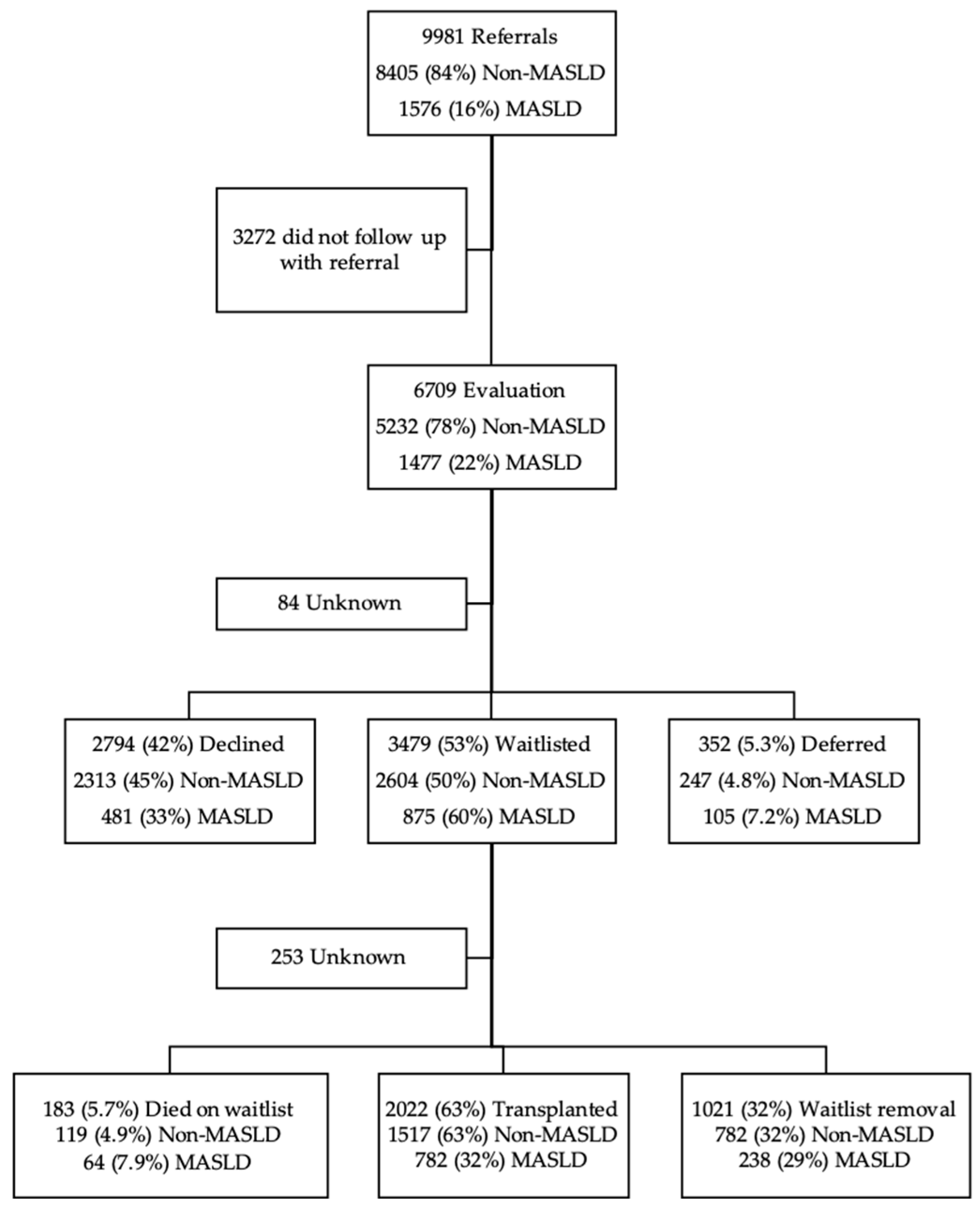
3. Results
3.1. Demographics
| Characteristic | Overall N = 6709 1 | Non-MASLD N = 5232 1 | MASLD N = 1477 1 | p-Value 2 |
|---|---|---|---|---|
| Age at Referral | 59 (50, 65) | 57 (48, 64) | 63 (56, 67) | <0.001 |
| Sex | <0.001 | |||
| female | 2693 (40.1%) | 1892 (36.2%) | 801 (54.2%) | |
| male | 4012 (59.8%) | 3336 (63.7%) | 676 (45.8%) | |
| Unknown | 4 | 4 | 0 | |
| Ethnicity | <0.001 | |||
| Hispanic/Latino | 2840 (43.8%) | 2065 (40.9%) | 775 (54.0%) | |
| Not Hispanic/Latino | 3642 (56.1%) | 2983 (59.0%) | 659 (45.9%) | |
| Unknown | 227 | 184 | 43 | |
| Race | <0.001 | |||
| African American | 234 (3.7%) | 219 (4.4%) | 15 (1.1%) | |
| American Indian/Alaskan Native | 65 (1.0%) | 48 (1.0%) | 17 (1.2%) | |
| Asian American/Pacific Islander | 692 (10.9%) | 589 (11.9%) | 103 (7.3%) | |
| White | 3706 (58.5%) | 2847 (57.7%) | 859 (61.3%) | |
| Other/Multiracial | 1637 (25.8%) | 1229 (24.9%) | 408 (29.1%) | |
| Unknown | 375 | 300 | 75 | |
| Body Mass Index (BMI) | 28 (24, 32) | 27 (23, 31) | 31 (27, 36) | <0.001 |
| Unknown | 51 | 41 | 10 | |
| BMI Class | <0.001 | |||
| Underweight | 156 (2.3%) | 136 (2.6%) | 20 (1.4%) | |
| Normal | 1999 (30.0%) | 1801 (34.6%) | 198 (13.4%) | |
| Overweight | 2143 (32.2%) | 1736 (33.4%) | 407 (27.7%) | |
| Obese | 1972 (29.6%) | 1300 (25.0%) | 672 (45.8%) | |
| Morbidly Obese | 388 (5.8%) | 218 (4.2%) | 170 (11.6%) | |
| Unknown | 51 | 41 | 10 | |
| Decompensated Cirrhosis | 4620 (68.8%) | 3465 (66.2%) | 1155 (78.2%) | <0.001 |
| Hepatocellular Carcinoma | 1530 (22.8%) | 1217 (23.2%) | 313 (21.2%) | 0.094 |
| Comorbidities | ||||
| Diabetes Mellitus | 1748 (26.1%) | 993 (19.0%) | 755 (51.2%) | <0.001 |
| Hypertension | 2013 (30.0%) | 1368 (26.1%) | 645 (43.7%) | <0.001 |
| Hyperlipidemia | 848 (12.6%) | 506 (9.7%) | 342 (23.1%) | <0.001 |
| Coronary Artery Disease | 312 (4.7%) | 213 (4.1%) | 99 (6.7%) | <0.001 |
3.2. Predictors and Interaction Analyses of Waitlisting Among MASLD and Non-MASLD Candidates
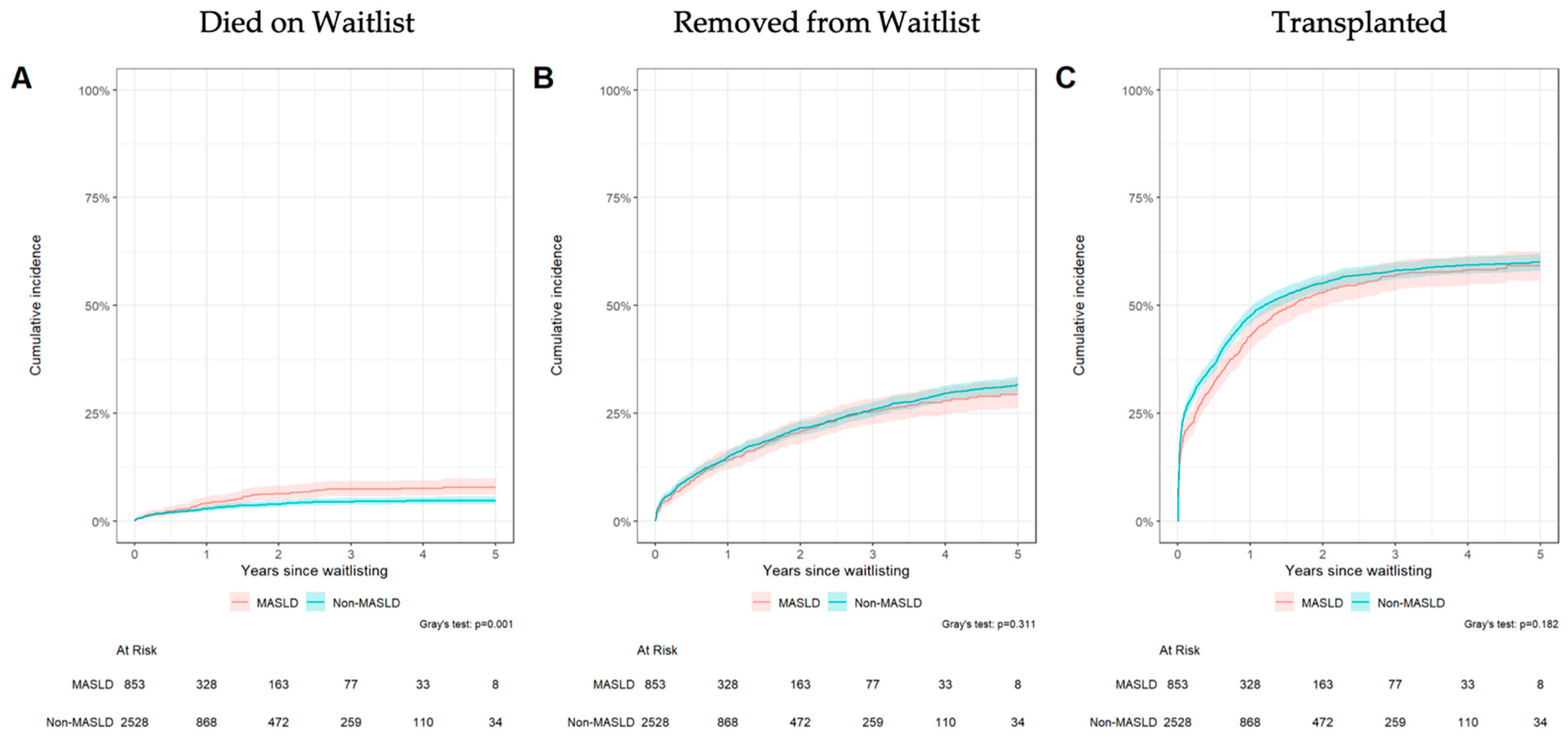
3.3. Waitlist Outcomes
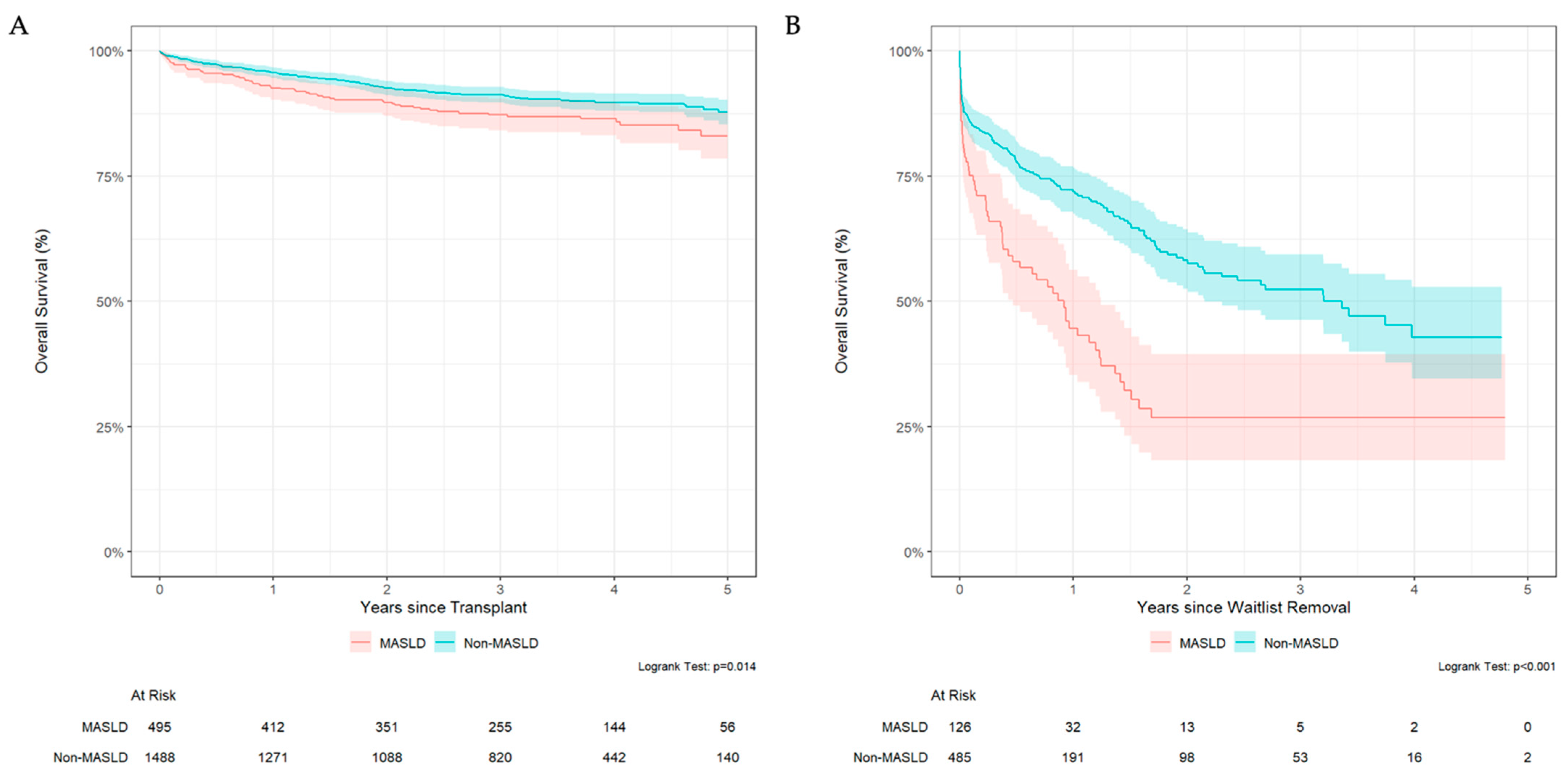
3.4. MASLD Patients Have Lower Survival Rate After Waitlist Removal and Transplantation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| LT | Liver Transplantation |
| MELD | Model for End-Stage Liver Disease |
| BMI | Body Mass Index |
| CLN | California Liver Network |
| GLP-1 | Glucagon-Like Peptide 1 |
References
- Le, P.; Tatar, M.; Dasarathy, S.; Alkhouri, N.; Herman, W.H.; Taksler, G.B.; Deshpande, A.; Ye, W.; Adekunle, O.A.; McCullough, A.; et al. Estimated Burden of Metabolic Dysfunction-Associated Steatotic Liver Disease in US Adults, 2020 to 2050. JAMA Netw. Open 2025, 8, e2454707. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Al Shabeeb, R.; Eberly, K.E.; Shah, D.; Nguyen, V.; Ong, J.; Henry, L.; Alqahtani, S.A. The changing epidemiology of adult liver transplantation in the United States in 2013–2022: The dominance of metabolic dysfunction-associated steatotic liver disease and alcohol-associated liver disease. Hepatol. Commun. 2024, 8, e0352. [Google Scholar] [CrossRef]
- Kwong, A.J.; Schnellinger, E.; Foutz, J.; Cafarella, M.; Nagai, S.; Biggins, S.W.; Pomposelli, J.; Trotter, J. Excess waitlist mortality among candidates for multivisceral liver-intestine transplant in acuity circle allocation. Am. J. Transplant. 2024, 24, 1080–1086. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Ong, J.; Trimble, G.; AlQahtani, S.; Younossi, I.; Ahmed, A.; Racila, A.; Henry, L. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin. Gastroenterol. Hepatol. 2021, 19, 580–589.e5. [Google Scholar] [CrossRef]
- Parikh, N.D.; Marrero, W.J.; Wang, J.; Steuer, J.; Tapper, E.B.; Konerman, M.; Singal, A.G.; Hutton, D.W.; Byon, E.; Lavieri, M.S. Projected Increase in Obesity and Non-Alcoholic-Steatohepatitis–Related Liver Transplantation Waitlist Additions in the United States. Hepatology 2019, 70, 487–495. [Google Scholar] [CrossRef]
- Steggerda, J.A.; Mahendraraj, K.; Todo, T.; Noureddin, M. Clinical considerations in the management of non-alcoholic steatohepatitis cirrhosis pre- And post-transplant: A multi-system challenge. World J. Gastroenterol. 2020, 26, 4018–4035. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Altshuler, P.J.; Dang, H.; Frank, A.M.; Shah, A.P.; Glorioso, J.; Zhan, T.; Rios Diaz, A.; Shaheen, O.; Ramirez, C.B.; Maley, W.R.; et al. Evaluating Outcomes Related to Donor and Recipient Metabolic Environment: Macrosteatotic Allografts and Nonalcoholic Steatohepatitis. Liver Transplant. 2022, 28, 623–635. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Marchesini, G.; Pinto-Cortez, H.; Petta, S. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: Implications for Liver Transplantation. Transplantation 2019, 103, 22–27. [Google Scholar] [CrossRef]
- van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; John Wiley & Sons: Hoboken, NJ, USA, 1987. [Google Scholar] [CrossRef]
- Gray, R.J. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann. Statist. 1988, 16, 1141–1154. [Google Scholar] [CrossRef]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests ad diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Li, L.; Yang, Y.X.; Cao, Y.; Yu, X.; Samuel, R.; Ali, B.; Desiderio, R.; Cholankeril, G.; et al. GLP-1 Receptor Agonists and Risk for Cirrhosis and Related Complications in Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease. JAMA Intern. Med. 2024, 184, 1314–1323. [Google Scholar] [CrossRef]
- Wester, A.; Shang, Y.; Grip, E.T.; Matthews, A.A.; Hagström, H. Glucagon-like peptide-1 receptor agonists and risk of major adverse liver outcomes in patients with chronic liver disease and type 2 diabetes. Gut 2024, 73, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.H.; Wong, G.; Garner, E.; Izzy, M.; Srivastava, G. Utility of glucagon-like peptide 1 receptor agonists as anti-obesity medications in liver transplant recipients. Liver Transpl. 2024, 30, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Carias, S.; Castellanos, A.L.; Vilchez, V.; Nair, R.; Dela Cruz, A.C.; Watkins, J.; Barrett, T.; Trushar, P.; Esser, K.; Gedaly, R. Nonalcoholic steatohepatitis is strongly associated with sarcopenic obesity in patients with cirrhosis undergoing liver transplant evaluation. J. Gastroenterol. Hepatol. 2016, 31, 628–633. [Google Scholar] [CrossRef]
- Satapathy, S.K.; Jiang, Y.; Agbim, U.; Wu, C.; Bernstein, D.E.; Teperman, L.W.; Kedia, S.K.; Aithal, G.P.; Bhamidimarri, K.R.; Duseja, A.; et al. Posttransplant Outcome of Lean Compared With Obese Nonalcoholic Steatohepatitis in the United States: The Obesity Paradox. Liver Transplant. 2020, 26, 68–79. [Google Scholar] [CrossRef]
- Segev, D.L.; Thompson, R.E.; Locke, J.E.; Simpkins, C.E.; Thuluvath, P.J.; Montgomery, R.A.; Maley, W.R. Prolonged waiting times for liver transplantation in obese patients. Ann. Surg. 2008, 248, 863–870. [Google Scholar] [CrossRef]
- Larson, E.L.; Ellias, S.D.; Blezek, D.J.; Klug, J.; Hartman, R.P.; Ziller, N.F.; Bamlet, H.; Mao, S.A.; Perry, D.K.; Nimma, I.R.; et al. Simultaneous liver transplant and sleeve gastrectomy provides durable weight loss, improves metabolic syndrome and reduces allograft steatosis. J. Hepatol. 2025, 83, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.G.; Bitetto, D.; Fornasiere, E.; Fumolo, E.; Ferrarese, A.; Toniutto, P. Efficacy and Safety of GLP-1 Receptor Agonists and SGLT-2 Inhibitors in the Treatment of Diabetes Mellitus and Obesity in Liver Transplant Recipients: A Systematic Review. J. Clin. Med. 2025, 14, 4619. [Google Scholar] [CrossRef]
- Karnam, R.S.; Chen, S.; Xu, W.; Chen, C.; Elangainesan, P.; Ghanekar, A.; McGilvray, I.; Reichman, T.; Sayed, B.; Selzner, M.; et al. Sex Disparity in Liver Transplant and Access to Living Donation. JAMA Surg. 2021, 156, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Croome, K.P.; Lee, D.D.; Burns, J.M.; Keaveny, A.P.; Taner, C.B. Intraregional model for end-stage liver disease score variation in liver transplantation: Disparity in our own backyard. Liver Transplant. 2018, 24, 488–496. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | OR | 95% CI | p-Value |
|---|---|---|---|
| Age | 0.99 | 0.99, 1.00 | <0.001 |
| Sex | |||
| female | — | — | |
| male | 1.02 | 0.92, 1.13 | 0.8 |
| BMI | |||
| Normal | — | — | |
| Underweight | 0.88 | 0.63, 1.23 | 0.4 |
| Overweight | 1.03 | 0.91, 1.17 | 0.7 |
| Obese | 1.08 | 0.95, 1.24 | 0.2 |
| Morbidly Obese | 0.65 | 0.52, 0.82 | <0.001 |
| MELD at evaluation | 1.00 | 1.00, 1.01 | 0.14 |
| Comorbidities Amount | |||
| 0 | — | — | |
| 1 | 1.15 | 1.01, 1.30 | 0.029 |
| 2 | 1.05 | 0.90, 1.23 | 0.6 |
| 3 | 0.94 | 0.75, 1.18 | 0.6 |
| 4 | 0.86 | 0.50, 1.46 | 0.6 |
| Liver Disease Etiology | |||
| Non-MASLD | — | — | |
| MASLD | 1.52 | 1.33, 1.74 | <0.001 |
| Estimate | p-Value | Estimate | p-Value | Estimate | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| BMI | 0.096 | Site | 0.014 | Sex | 0.571 | |||
| Normal | 1.08 (1.00, 1.16) | 0.044 | 0 | 1.01 (0.95, 1.07) | 0.834 | Non-MASLD | ||
| Underweight | 1.20 (0.97, 1.50) | 0.098 | 1 | 1.18 (1.11, 1.25) | <0.001 | Male (vs. Female) | 1.01 (0.98, 1.04) | 0.573 |
| Overweight | 1.13 (1.07, 1.19) | <0.001 | 2 | 1.12 (1.06, 1.18) | <0.001 | MASLD | ||
| Obese | 1.12 (1.07, 1.17) | <0.001 | 3 | 1.05 (0.95, 1.15) | 0.317 | Male (vs. Female) | 0.99 (0.94, 1.04) | 0.694 |
| Morbidly Obese | 0.98 (0.89, 1.08) | 0.693 | 4 | 1.08 (0.99, 1.17) | 0.091 | |||
| 5 | 1.15 (1.05, 1.26) | 0.002 | ||||||
| Died on Waitlist | Waitlist Removal | Transplanted | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age | 1.02 | 1.00, 1.03 | 0.039 | 1.00 | 0.99, 1.00 | 0.7 | 1.00 | 1.00, 1.01 | 0.12 |
| Sex | |||||||||
| female | — | — | — | — | — | — | |||
| male | 0.72 | 0.53, 0.98 | 0.036 | 0.95 | 0.84, 1.09 | 0.5 | 1.19 | 1.06, 1.32 | 0.002 |
| Site | |||||||||
| 0 | — | — | — | — | — | — | |||
| 1 | 1.62 | 1.09, 2.41 | 0.017 | 1.27 | 1.07, 1.52 | 0.007 | 0.67 | 0.57, 0.78 | <0.001 |
| 2 | 0.79 | 0.50, 1.25 | 0.3 | 1.00 | 0.83, 1.21 | >0.9 | 1.09 | 0.94, 1.26 | 0.3 |
| 3 | 0.80 | 0.45, 1.44 | 0.5 | 1.00 | 0.79, 1.28 | >0.9 | 1.08 | 0.91, 1.30 | 0.4 |
| 4 | 0.25 | 0.11, 0.59 | 0.002 | 0.94 | 0.74, 1.20 | 0.6 | 1.30 | 1.08, 1.56 | 0.005 |
| 5 | 0.45 | 0.23, 0.88 | 0.020 | 1.08 | 0.86, 1.37 | 0.5 | 1.04 | 0.87, 1.24 | 0.7 |
| BMI | |||||||||
| Normal | — | — | — | — | — | — | |||
| Underweight | 1.03 | 0.31, 3.40 | >0.9 | 1.10 | 0.72, 1.68 | 0.7 | 1.00 | 0.68, 1.46 | >0.9 |
| Overweight | 1.49 | 0.99, 2.26 | 0.058 | 0.93 | 0.79, 1.09 | 0.4 | 0.93 | 0.82, 1.05 | 0.3 |
| Obese | 1.52 | 1.00, 2.30 | 0.049 | 1.01 | 0.86, 1.19 | >0.9 | 0.86 | 0.75, 0.99 | 0.030 |
| Morbidly Obese | 1.58 | 0.79, 3.14 | 0.2 | 0.86 | 0.62, 1.19 | 0.4 | 1.08 | 0.86, 1.37 | 0.5 |
| MELD at Listing | 0.99 | 0.98, 1.01 | 0.4 | 0.97 | 0.97, 0.98 | <0.001 | 1.06 | 1.05, 1.07 | <0.001 |
| Comorbidities Amount | |||||||||
| 0 | — | — | — | — | — | — | |||
| 1 | 0.91 | 0.61, 1.34 | 0.6 | 0.95 | 0.81, 1.10 | 0.5 | 1.09 | 0.97, 1.23 | 0.2 |
| 2 | 1.54 | 1.04, 2.28 | 0.031 | 0.99 | 0.82, 1.20 | >0.9 | 0.94 | 0.81, 1.10 | 0.4 |
| 3 | 1.19 | 0.67, 2.13 | 0.6 | 0.92 | 0.69, 1.21 | 0.5 | 1.00 | 0.81, 1.22 | >0.9 |
| 4 | 0.74 | 0.11, 5.17 | 0.8 | 0.38 | 0.14, 1.02 | 0.055 | 1.37 | 0.95, 1.97 | 0.10 |
| Liver Disease Etiology | |||||||||
| Non-MASLD | — | — | — | — | — | — | |||
| MASLD | 1.20 | 0.87, 1.67 | 0.3 | 0.91 | 0.78, 1.07 | 0.3 | 1.00 | 0.89, 1.14 | >0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, T.Y.; Steggerda, J.A.; Trivedi, H.; Luu, M.; Vipani, A.; Adjei, M.A.; Singh, J.; Zhou, K.; Kwong, A.; Tincopa, M.; et al. Outcomes from Referral to Transplant for Patients with MASLD: A California Liver Network Study. J. Clin. Med. 2025, 14, 7841. https://doi.org/10.3390/jcm14217841
Lim TY, Steggerda JA, Trivedi H, Luu M, Vipani A, Adjei MA, Singh J, Zhou K, Kwong A, Tincopa M, et al. Outcomes from Referral to Transplant for Patients with MASLD: A California Liver Network Study. Journal of Clinical Medicine. 2025; 14(21):7841. https://doi.org/10.3390/jcm14217841
Chicago/Turabian StyleLim, Tiffany Y., Justin A. Steggerda, Hirsh Trivedi, Michael Luu, Aarshi Vipani, Michie A. Adjei, Jasleen Singh, Kali Zhou, Allison Kwong, Monica Tincopa, and et al. 2025. "Outcomes from Referral to Transplant for Patients with MASLD: A California Liver Network Study" Journal of Clinical Medicine 14, no. 21: 7841. https://doi.org/10.3390/jcm14217841
APA StyleLim, T. Y., Steggerda, J. A., Trivedi, H., Luu, M., Vipani, A., Adjei, M. A., Singh, J., Zhou, K., Kwong, A., Tincopa, M., Vodkin, I., Ajmera, V., Mehta, N., Freise, C. E., Yilma, M., Hirose, R., Kuo, A., & Wisel, S. A. (2025). Outcomes from Referral to Transplant for Patients with MASLD: A California Liver Network Study. Journal of Clinical Medicine, 14(21), 7841. https://doi.org/10.3390/jcm14217841







