The Impact of Multidisciplinary Preoperative Optimization Program on Postoperative Outcomes Among Surgical Oncology Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
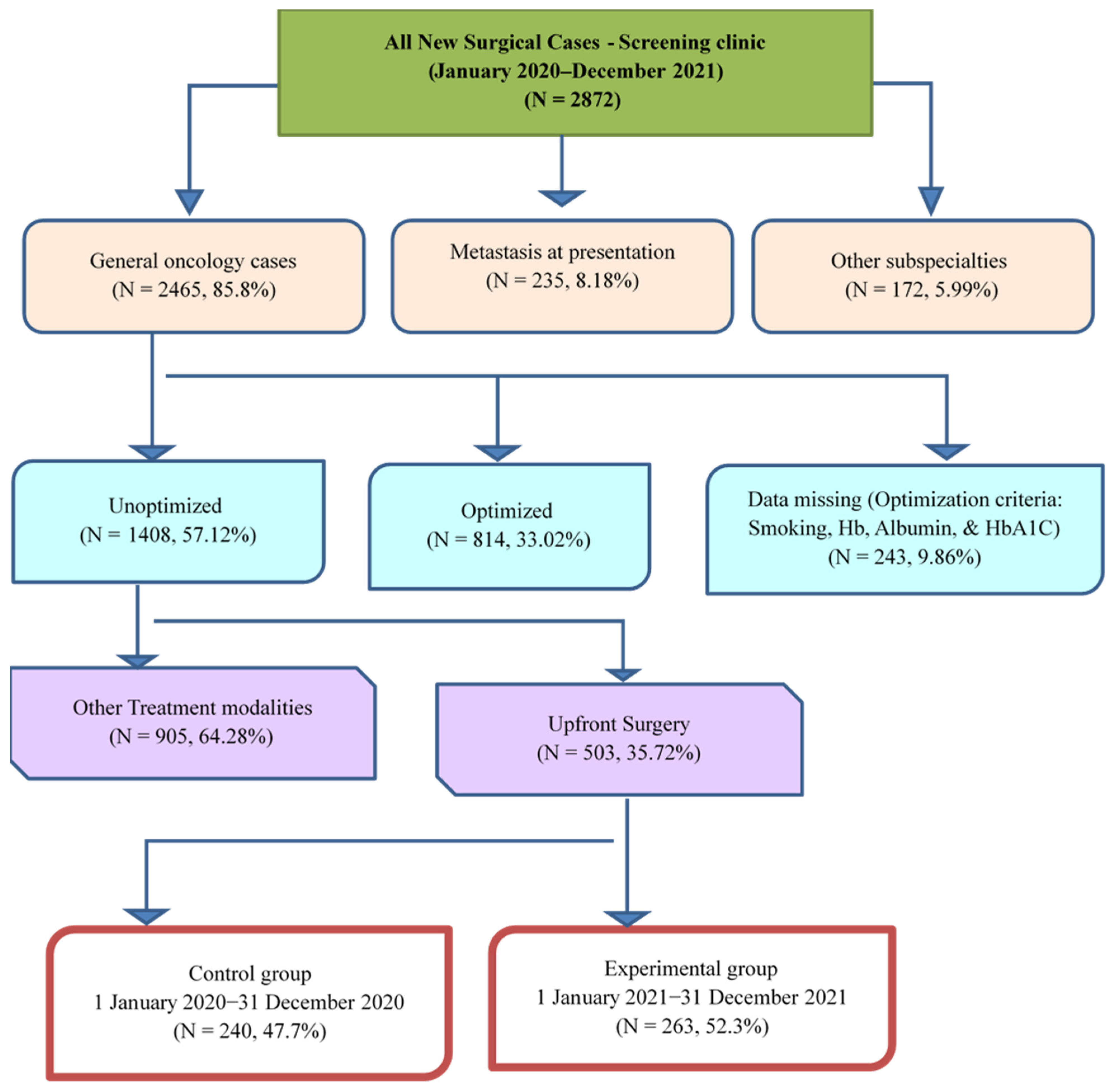
2.2. Setting and Population
2.3. Preoperative Optimization Program
- Preoperative anemia—defined as hemoglobin < 13 mg/dL for men and <12 mg/dL for non-pregnant women. While the ideal goal was to achieve Hb ≥ 13 mg/dL in men or ≥12 mg/dL in women, full correction was not always feasible prior to surgery. Patients received appropriate interventions (e.g., iron supplementation, erythropoietin) to optimize Hb levels as much as possible before surgery, and their optimization status was documented accordingly.
- Malnutrition—assessed using serum albumin (<3.5 mg/dL). Nutritional support (dietitian counseling, supplements) was provided to improve albumin levels before surgery. Full normalization to ≥3.5 mg/dL was the target, but when time constraints did not allow complete correction, patients were still considered partially optimized if interventions had been initiated and nutritional status improved as much as possible.
- Smoking cessation—current smokers were referred to cessation counseling and support programs. Patients were considered optimized if they successfully abstained from smoking for at least two weeks prior to surgery.
- Uncontrolled diabetes—defined as HbA1C > 7.5%. Medication adjustment or endocrinology consultation before surgery were considered optimized.
2.4. Data Collection and Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Program Adherence
3.3. Preoperative Parameter Changes
3.4. Perioperative and Postoperative Outcomes
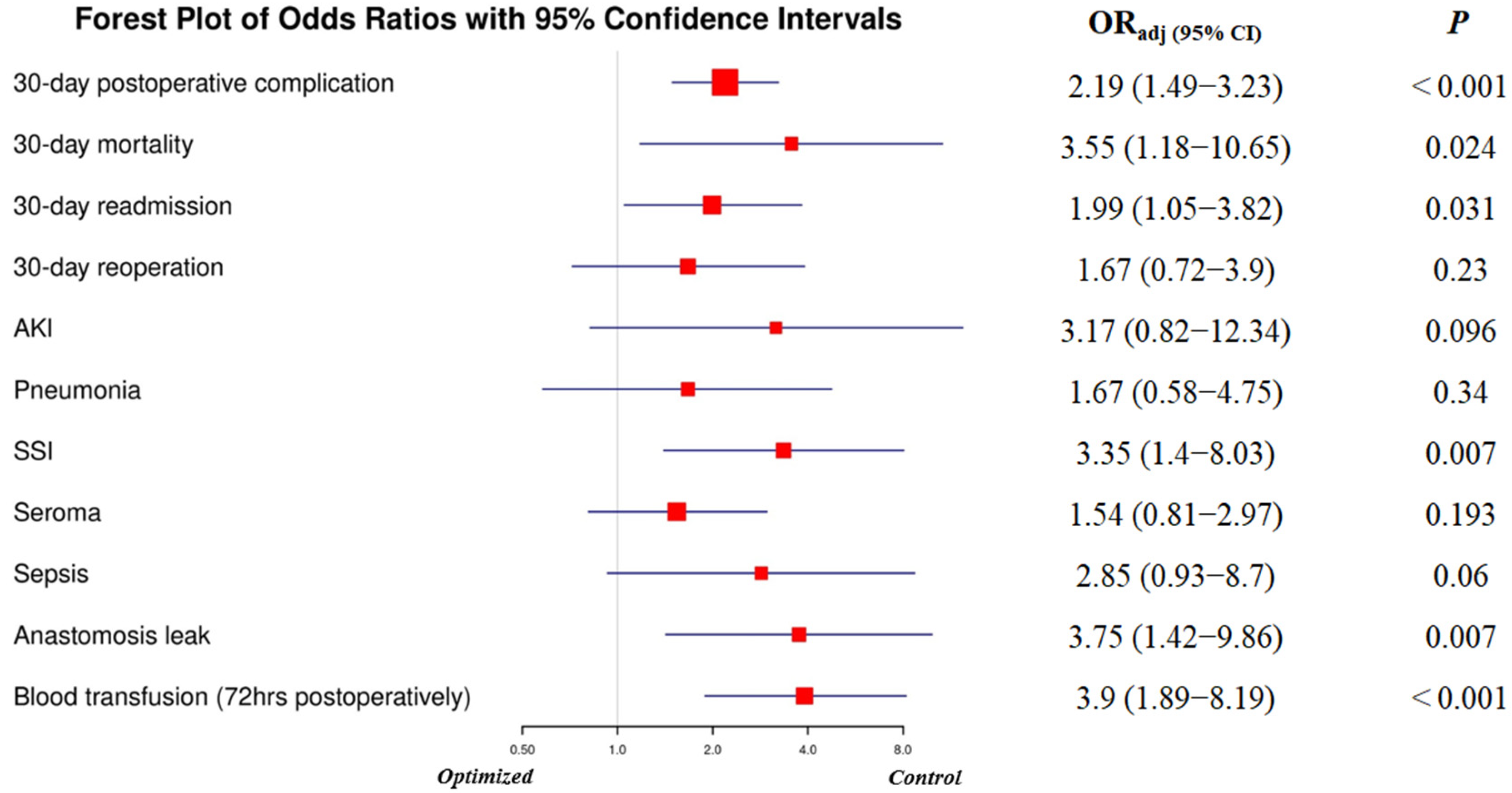
3.5. Adjusted Odds Ratios for Postoperative Complications (Control vs. Optimized Group)
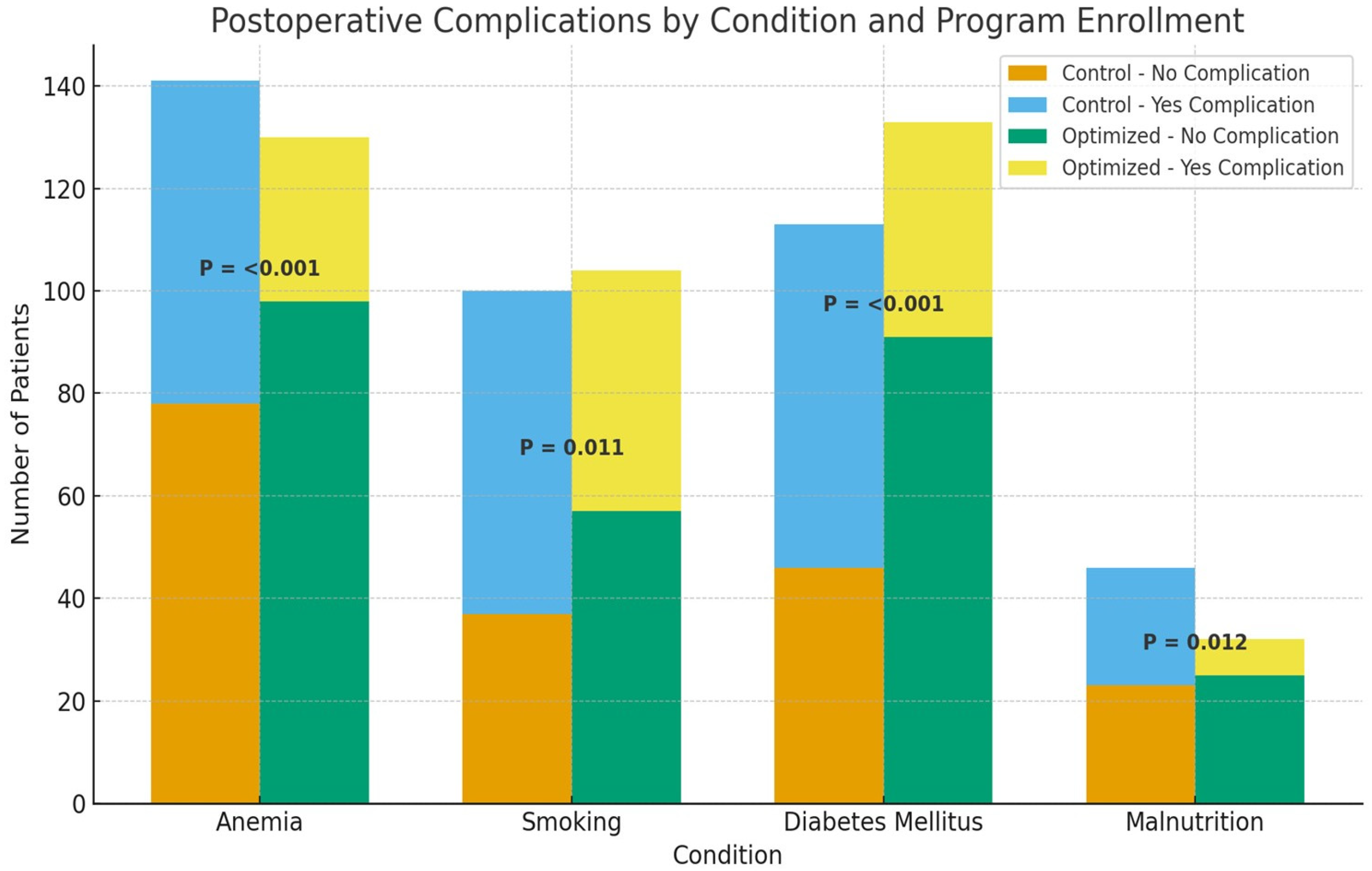
3.6. Subgroup Analysis of Postoperative Complications by Modifiable Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | American College of Surgeon |
| AKI | Acute Kidney Injury |
| AL | Anastomosis leak |
| ASA | American Society of Anesthesiologists Score |
| AUC | Area Under Curve |
| CD | Clavien–Dindo Classification |
| CDC | Centers For Disease Prevention and Control |
| CRC | Colorectal Cancer |
| DM | Diabetes Mellitus |
| EMR | Electronic Medical Record |
| UGI | Upper Gastrointestinal |
| HB | Hemoglobin |
| HBA1C | Hba1c |
| NSQIP | National Surgical Quality Improvement Program |
| OP | Optimization program |
| OR | Odd Ratio |
| ORadj | Adjusted Odd Ration |
| POC | Postoperative Complications |
| QI | Quality Improvement |
| ROC | Receiver Operating Characteristic Curve |
| ROR | Return To Operation Room |
| RPP | Retrospective Pretest-Posttest |
| S4S | Strong for Surgery |
| SSI | Surgical Site Infection |
| WHO | World Health Organization |
Appendix A

References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 1 May 2025).
- Ministry of Health Non-Communicable Diseases Directorate Jordan Cancer Registry. Cancer Incidence in Jordan; Ministry of Health: Amman, Jordan, 2019.
- Abbas, Z.; Rehman, S. An Overview of Cancer Treatment Modalities. In Neoplasm; Shahzad, H.N., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Leeds, I.L.; Canner, J.K.; Gani, F.; Meyers, P.M.; Haut, E.R.; Efron, J.E.; Johnston, F.M. Increased Healthcare Utilization for Medical Comorbidities Prior to Surgery Improves Postoperative Outcomes. Ann. Surg. 2020, 271, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Martos-Benítez, F.D.; Gutiérrez-Noyola, A.; Echevarría-Víctores, A. Postoperative Complications and Clinical Outcomes among Patients Undergoing Thoracic and Gastrointestinal Cancer Surgery: A Prospective Cohort Study. Rev. Bras. Ter. Intensiv. 2016, 28, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Dencker, E.E.; Bonde, A.; Troelsen, A.; Varadarajan, K.M.; Sillesen, M. Postoperative Complications: An Observational Study of Trends in the United States from 2012 to 2018. BMC Surg. 2021, 21, 393. [Google Scholar] [CrossRef]
- Pak, H.; Maghsoudi, L.H.; Soltanian, A.; Gholami, F. Surgical Complications in Colorectal Cancer Patients. Ann. Med. Surg. 2020, 55, 13–18. [Google Scholar] [CrossRef]
- Woodfield, J.C.; Jamil, W.; Sagar, P.M. Incidence and Significance of Postoperative Complications Occurring between Discharge and 30 Days: A Prospective Cohort Study. J. Surg. Res. 2016, 206, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Aronson, S.; Murray, S.; Martin, G.; Blitz, J.; Crittenden, T.; Lipkin, M.E.; Mantyh, C.R.; Lagoo-Deenadayalan, S.A.; Flanagan, E.M.; Attarian, D.E.; et al. Roadmap for Transforming Preoperative Assessment to Preoperative Optimization. Anesth. Analg. 2020, 130, 811–819. [Google Scholar] [CrossRef]
- Varghese, T.K.; Chishimba, S.; Ma, M.; Ko, C.Y.; Flum, D.R. The ACS Strong for Surgery Program: Changing Clinician and System Behavior to Optimize Health Before Surgery; ACS Bulletin: Chicago, IL, USA, 2019; pp. 1–21. Available online: https://www.facs.org/for-medical-professionals/news-publications/news-and-articles/bulletin/2019/10/the-acs-strong-for-surgery-program-changing-clinician-and-system-behavior-to-optimize-health-before-surgery/ (accessed on 13 July 2024).
- Christie, C.A.; Fleischer, D.N. Insight Into Evaluation Practice: A Content Analysis of Designs and Methods Used in Evaluation Studies Published in North American Evaluation-Focused Journals. Am. J. Eval. 2010, 31, 326–346. [Google Scholar] [CrossRef]
- Young, J. Retrospective Pre/Posttest Design and Response-Shift Bias in an Urban After-School Program for Teens: A Mixed Methods Study. Ph.D. Thesis, Loyola University Chicago, Chicago, IL, USA, 2016. Available online: https://ecommons.luc.edu/luc_diss/2156 (accessed on 13 July 2024).
- Robson, M.; Alexopoulou, P. Pre-Optimisation of the Cancer Patient. Dig. Med. Res. 2020, 3, 29. [Google Scholar] [CrossRef]
- Snowden, C.P.; Anderson, H. Preoperative Optimization: Rationale and Process: Is It Economic Sense? Curr. Opin. Anaesthesiol. 2012, 25, 210–216. [Google Scholar] [CrossRef]
- Vine, M.; Joseph, K.; Gibson, D.; Lim, B.; Chua, M.; Siu, A.H.Y.; Dooreemeah, D.; Lee, A.; Cuomo, R.; Seth, I. Innovative Approaches to Preoperative Care Including Feasibility, Efficacy, and Ethical Implications: A Narrative Review. AME Surg. J. 2024, 4, 1. [Google Scholar] [CrossRef]
- Stokes, S.M.; Wakeam, E.; Antonoff, M.B.; Backhus, L.M.; Meguid, R.A.; Odell, D.; Varghese, T.K. Optimizing Health before Elective Thoracic Surgery: Systematic Review of Modifiable Risk Factors and Opportunities for Health Services Research. J. Thorac. Dis. 2019, 11, S537–S554. [Google Scholar] [CrossRef]
- MacMahon, A.; Rao, S.S.; Chaudhry, Y.P.; Hasan, S.A.; Epstein, J.A.; Hegde, V.; Valaik, D.J.; Oni, J.K.; Sterling, R.S.; Khanuja, H.S. Preoperative Patient Optimization in Total Joint Arthroplasty—The Paradigm Shift from Preoperative Clearance: A Narrative Review. HSS J. 2022, 18, 418–427. [Google Scholar] [CrossRef]
- Musallam, K.M.; Tamim, H.M.; Richards, T.; Spahn, D.R.; Rosendaal, F.R.; Habbal, A.; Khreiss, M.; Dahdaleh, F.S.; Khavandi, K.; Sfeir, P.M.; et al. Preoperative Anaemia and Postoperative Outcomes in Non-Cardiac Surgery: A Retrospective Cohort Study. Lancet 2011, 378, 1396–1407. [Google Scholar] [CrossRef]
- Yan, T.; Lei, S.; Zhou, B.; Huang, Y.; Li, X.; Zhang, J.; Huang, Q.; Zhang, L. Association between Preoperative Anemia and Postoperative Short-Term Outcomes in Patients Undergoing Colorectal Cancer Surgery—A Propensity Score Matched Retrospective Cohort Study. BMC Anesthesiol. 2023, 23, 307. [Google Scholar] [CrossRef]
- Srivastava, V.; Basu, S.; Shukla, V.K. Seroma Formation after Breast Cancer Surgery: What We Have Learned in the Last Two Decades. J. Breast Cancer 2012, 15, 373–380. [Google Scholar] [CrossRef]
- Berriós-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef]
- Goltsman, D.; Munabi, N.C.O.; Ascherman, J.A. The Association between Smoking and Plastic Surgery Outcomes in 40,465 Patients: An Analysis of the American College of Surgeons National Surgical Quality Improvement Program Data Sets. Plast. Reconstr. Surg. 2017, 139, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Gräsbeck, H.L.; Reito, A.R.P.; Ekroos, H.J.; Aakko, J.A.; Hölsä, O.; Vasankari, T.M. Smoking Is a Predictor of Complications in All Types of Surgery: A Machine Learning-Based Big Data Study. BJS Open 2023, 7, zrad016. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.B.; Martin, D.P.; Thompson, R.; Schroeder, D.R.; Hanson, A.C.; Warner, D.O. Association between Smoking Status, Preoperative Exhaled Carbon Monoxide Levels, and Postoperative Surgical Site Infection in Patients Undergoing Elective Surgery. JAMA Surg. 2017, 152, 476–483. [Google Scholar] [CrossRef]
- Sandy-Hodgetts, K. Surgical Wound Complications: A 21st Century Problem? J. Wound Care 2019, 28, 645. [Google Scholar] [CrossRef]
- Sørensen, L.T. The Clinical Impact of Smoking and Smoking Cessation: A Systematic Rivew and Meta-Analysis. Arch. Surg. 2012, 147, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, R.; Katada, J. Effects of Active Smoking on Postoperative Outcomes in Hospitalised Patients Undergoing Elective Surgery: A Retrospective Analysis of an Administrative Claims Database in Japan. BMJ Open 2019, 9, e029913. [Google Scholar] [CrossRef]
- Badiani, S.; Diab, J.; Woodford, E.; Natarajan, P.; Berney, C.R. Impact of Preoperative Smoking on Patients Undergoing Right Hemicolectomies for Colon Cancer. Langenbeck’s Arch. Surg. 2022, 407, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Fan Chiang, Y.H.; Lee, Y.W.; Lam, F.; Liao, C.C.; Chang, C.C.; Lin, C.S. Smoking Increases the Risk of Postoperative Wound Complications: A Propensity Score-Matched Cohort Study. Int. Wound J. 2023, 20, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, K.; Li, X.; Jin, X.; An, P.; Fang, Y.; Mu, Y. Postoperative Adverse Events in Patients with Diabetes Undergoing Orthopedic and General Surgery. Medicine 2019, 98, e15089. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.L.; Ke, Y.; Ong, Y.J.; Li, H.; Wong, T.H.; Abdullah, H.R. The Impact of Preoperative Glycated Hemoglobin (HbA1c) on Postoperative Complications after Elective Major Abdominal Surgery: A Meta-Analysis. Korean J. Anesthesiol. 2022, 75, 47–60. [Google Scholar] [CrossRef]
- Riad, A.; Knight, S.R.; Ghosh, D.; Kingsley, P.A.; Lapitan, M.C.; Parreno-Sacdalan, M.D.; Sundar, S.; Qureshi, A.U.; Valparaiso, A.P.; Pius, R.; et al. Impact of Malnutrition on Early Outcomes after Cancer Surgery: An International, Multicentre, Prospective Cohort Study. Lancet Glob. Health 2023, 11, e341–e349. [Google Scholar] [CrossRef]
| Variable | Level | Optimized Group 263 (52.3%) | Control Group 240 (47.7%) | p Value | ||
|---|---|---|---|---|---|---|
| Age (years) | Mean (±Std.) | 54.18 (±14.64) | 54.34 (±14.40) | 0.905 | ||
| Median (Min–Max) | 55 (22–87) | 55 (19–85) | ||||
| Gender | Female | 167 | 49.40% | 173 | 50.60% | 0.06 |
| Male | 94 | 58.40% | 67 | 41.60% | ||
| Diagnosis Groups | Breast cancer | 84 | 41.40% | 119 | 58.60% | <0.001 |
| CRC | 91 | 59.50% | 62 | 40.50% | ||
| GI | 58 | 69% | 26 | 31% | ||
| Thyroid cancer | 30 | 47.60% | 33 | 52.40% | ||
| Disease stage | I | 65 | 43.90% | 83 | 56.10% | 0.041 |
| II | 101 | 57.70% | 74 | 42.30% | ||
| III | 97 | 53.90% | 83 | 46.10% | ||
| Comorbidity | No | 82 | 54.30% | 69 | 45.70% | 0.553 |
| Yes | 181 | 51.40% | 171 | 48.60% | ||
| Smoking status | No | 159 | 53.20% | 140 | 46.80% | 0.628 |
| Yes | 104 | 51% | 100 | 49% | ||
| Uncontrolled DM (HbA1c > 7.5) | No | 151 | 52.40% | 137 | 47.60% | 0.94 |
| Yes | 112 | 52.10% | 103 | 47.90% | ||
| HbA1c | Mean (±Std.) | 7.26 (±2.33) | 7.36 (±2.53) | 0.627 | ||
| Hb level | Mean (±Std.) | 12.24 (±2.3) | 11.9 (±2.5) | 0.076 | ||
| Albumin | Mean (±Std.) | 4.4 (±0.95) | 4.6 (±1.3) | 0.125 | ||
| Variable | Level | Optimized Group N (%) | Control Group N (%) | p Value |
|---|---|---|---|---|
| Nutritional referrals | No | 0 (0%) | 39 (84.1%) | <0.0001 |
| Yes | 32 (100%) | 7 (15.2%) | ||
| Endocrine referrals | No | 0 (0%) | 90 (87.4%) | <0.0001 |
| Yes | 112 (100%) | 13 (12.6%) | ||
| Anemia management | No | 49 (37.3%) | 90 (63.8%) | <0.0001 |
| Yes | 81 (62.7%) | 51 (36.2%) | ||
| Types of anemia management | Blood transfusion | 9 (11.1%) | 33 (64.7%) | <0.0001 |
| IV | 41 (50.6%) | 3 (5.9%) | ||
| Oral | 31 (38.3%) | 15 (29.4%) | ||
| Smoking cessation program | No | 0 (0%) | 79 (79.0%) | <0.0001 |
| Yes | 104 (100%) | 21 (21.0%) | ||
| Quit smoking | No | 37 (35.6%) | 73 (73.0%) | <0.0001 |
| Yes | 67 (64.4%) | 27 (27.0%) | ||
| Quitting duration (weeks) | <2 weeks | 15 (83.3%) | 3 (16.7%) | 0.035 |
| 2–4 weeks | 43 (64.2%) | 24 (35.8%) | ||
| >4 weeks | 9 (100%) | 0 (0%) |
| Variable | Level | Optimized Arm | Control Arm | ||||
|---|---|---|---|---|---|---|---|
| First Encounter | Pre-Op | t-Test | First Encounter | Pre-Op | t-Test | ||
| Hb level (Anemic Group) | Mean (±SD) | 10.28 (±1.8) | 10.65 (±2.1) | 2.67 | 10.13 (±1.9) | 9.28 (±2.2) | −8.14 |
| p-value | 0.008 | <0.0001 | |||||
| Albumin level (Malnourished Group) | Mean (±SD) | 2.74 (±0.16) | 3.35 (±0.32) | 6.95 | 2.8 (±0.16) | 2.7 (±0.26) | −0.89 |
| p-value | <0.0001 | 0.38 | |||||
| Smoking status | Non-smoker | 159 (60.5%) | 226 (85.9%) | 140 (58.3%) | 167 (69.6%) | ||
| Smoker | 104 (39.5%) | 37 (14.1%) | 100 (41.7%) | 73 (30.4%) | |||
| p-value | <0.0001 | <0.0001 | |||||
| Variable | Level | Optimized Group (N = 263, 52.3%) | Control Group (N = 240, 47.7%) | p-Value |
|---|---|---|---|---|
| ASA | II | 242 (92%) | 219 (91.3%) | 0.757 |
| III | 21 (8%) | 21 (8.8%) | ||
| Severity of Surgery | Major | 139 (52.9%) | 100 (41.7%) | 0.016 |
| Medium | 98 (37.3%) | 120 (50%) | ||
| Minor | 26 (9.8%) | 20 (8.3%) | ||
| Time to surgery (days) | Mean ± SD | 44.91 ± 19.8 | 43.3 ± 16.3 | 0.326 |
| LOS (days) | Mean ± SD | 4.1 ± 2.5 | 3.9 ± 2.8 | 0.39 |
| 30-Day Postoperative Complications | No | 196 (74.5%) | 145 (60.4%) | 0.001 |
| Yes | 67 (25.5%) | 95 (39.6%) | ||
| Clavien–Dindo Grade | 0 | 191 (72.7%) | 133 (55.4%) | 0.006 |
| I | 9 (3.4%) | 10 (4.2%) | ||
| II | 30 (11.4%) | 43 (17.9%) | ||
| III | 24 (9.1%) | 38 (15.8%) | ||
| IV | 4 (1.5%) | 4 (1.6%) | ||
| V | 5 (1.9%) | 12 (5%) | ||
| 30-Day Mortality | No | 258 (98.1%) | 228 (95%) | 0.081 |
| Yes | 5 (1.9%) | 12 (5%) | ||
| Postoperative Blood Transfusion (≤72 h) | No | 249 (94.7%) | 215 (89.6%) | 0.044 |
| Yes | 14 (5.3%) | 25 (10.4%) | ||
| SSI | No | 255 (97.0%) | 219 (91.3%) | 0.006 |
| Yes | 8 (3.0%) | 21 (8.8%) | ||
| Pneumonia | No | 257 (97.7%) | 230 (95.8%) | 0.229 |
| Yes | 6 (2.3%) | 10 (4.2%) | ||
| Anastomosis Leak | No | 256 (97.3%) | 226 (94.2%) | 0.076 |
| Yes | 7 (2.7%) | 14 (5.8%) | ||
| Seroma | No | 244 (92.8%) | 203 (84.6%) | 0.004 |
| Yes | 19 (7.2%) | 37 (15.4%) | ||
| Sepsis | No | 258 (98.1%) | 229 (95.4%) | 0.1 |
| Yes | 5 (1.9%) | 11 (4.6%) | ||
| AKI | No | 260 (98.9%) | 232 (96.7%) | 0.12 |
| Yes | 3 (1.1%) | 8 (3.3%) | ||
| 30-Day Readmission | No | 246 (93.5%) | 211 (87.9%) | 0.029 |
| Yes | 17 (6.5%) | 29 (12.1%) | ||
| 30-Day Reoperation | No | 254 (96.6%) | 223 (92.9%) | 0.06 |
| Yes | 9 (3.4%) | 17 (7.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safi, Y.; Alyahya, M.S.; Al-sheyab, N.A.; Suliman, M.; Al-Masri, M. The Impact of Multidisciplinary Preoperative Optimization Program on Postoperative Outcomes Among Surgical Oncology Patients. J. Clin. Med. 2025, 14, 7820. https://doi.org/10.3390/jcm14217820
Safi Y, Alyahya MS, Al-sheyab NA, Suliman M, Al-Masri M. The Impact of Multidisciplinary Preoperative Optimization Program on Postoperative Outcomes Among Surgical Oncology Patients. Journal of Clinical Medicine. 2025; 14(21):7820. https://doi.org/10.3390/jcm14217820
Chicago/Turabian StyleSafi, Yasmin, Mohammad S. Alyahya, Nihaya A. Al-sheyab, Mohammad Suliman, and Mahmoud Al-Masri. 2025. "The Impact of Multidisciplinary Preoperative Optimization Program on Postoperative Outcomes Among Surgical Oncology Patients" Journal of Clinical Medicine 14, no. 21: 7820. https://doi.org/10.3390/jcm14217820
APA StyleSafi, Y., Alyahya, M. S., Al-sheyab, N. A., Suliman, M., & Al-Masri, M. (2025). The Impact of Multidisciplinary Preoperative Optimization Program on Postoperative Outcomes Among Surgical Oncology Patients. Journal of Clinical Medicine, 14(21), 7820. https://doi.org/10.3390/jcm14217820






