Clinical, Radiological, and Endoscopic Features of Pancreatic Pseudocyst and Walled-Off Necrosis: How to Diagnose and How to Drain Them
Abstract
1. Introduction
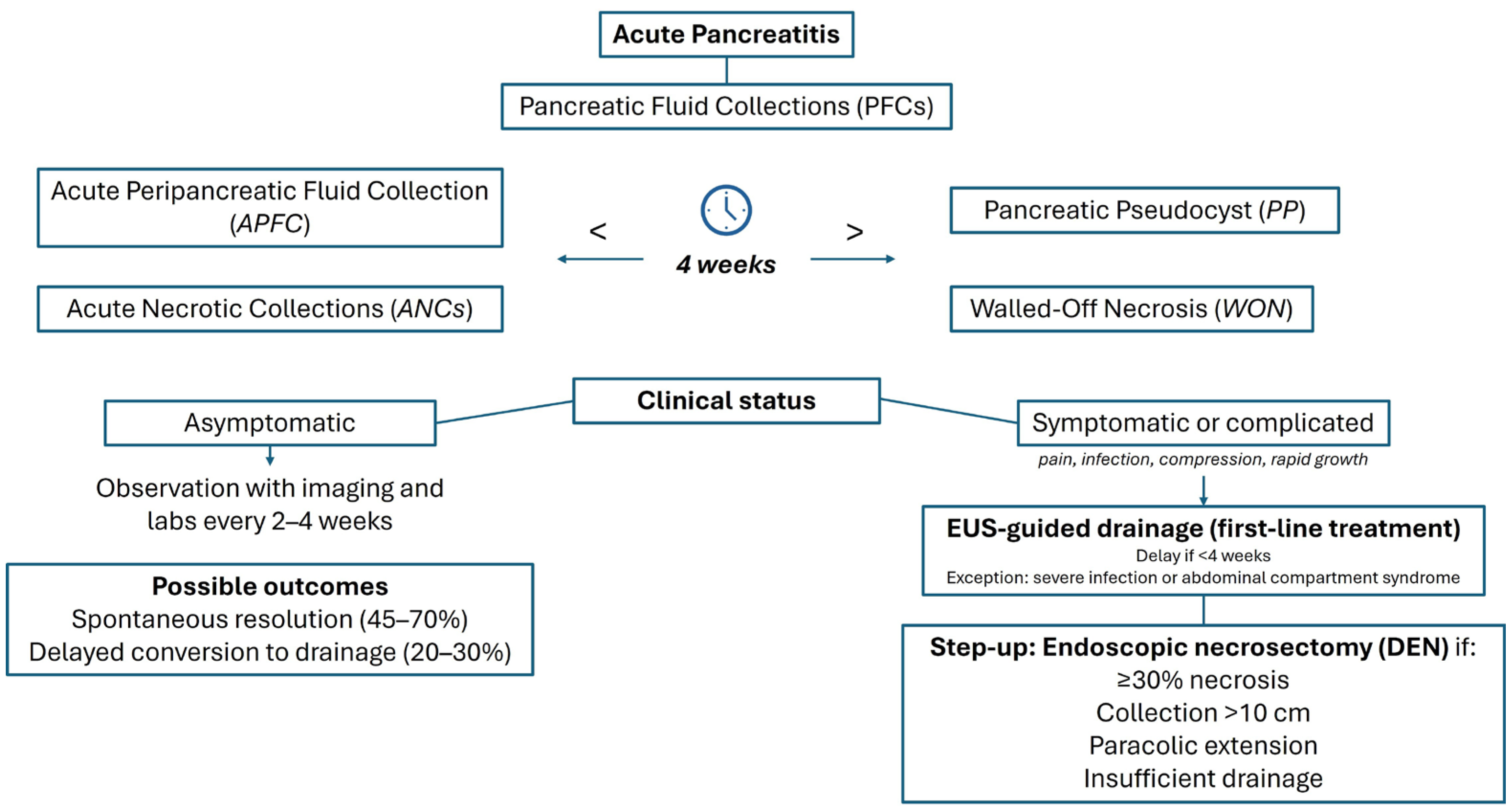
1.1. Classification and Definitions of Pancreatic Fluid Collections
1.2. Pathophysiology of Pancreatic Pseudocyst (PP) and Walled-Off Necrosis (WON)
2. Materials and Methods
3. Clinical Features and Natural History
3.1. Symptoms and Presentation
3.2. Natural History of PP and WON
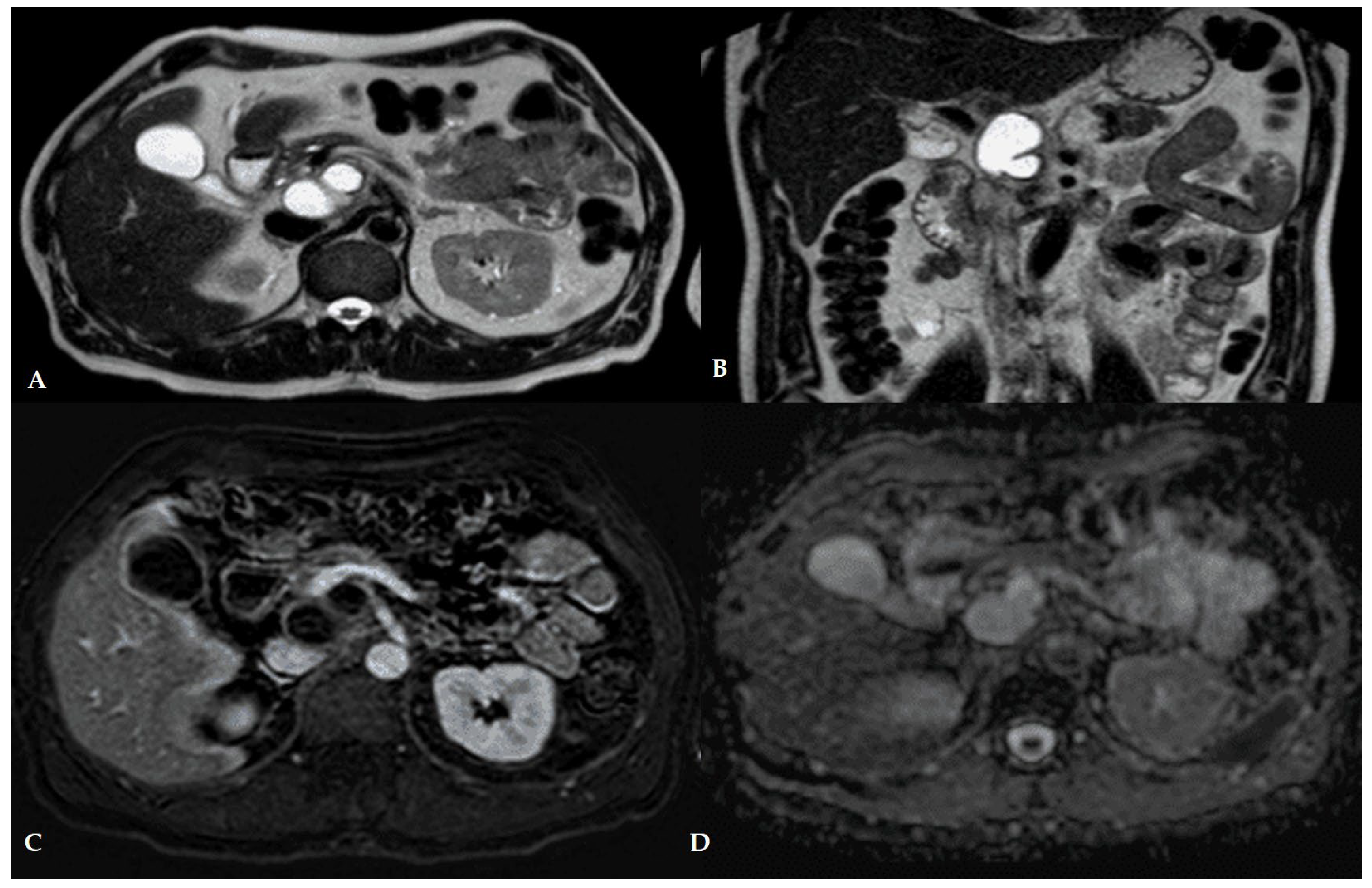
4. Imaging and Diagnostic Approach
4.1. Role of Computed Tomography (CT)
4.2. Role of Magnetic Resonance Imaging
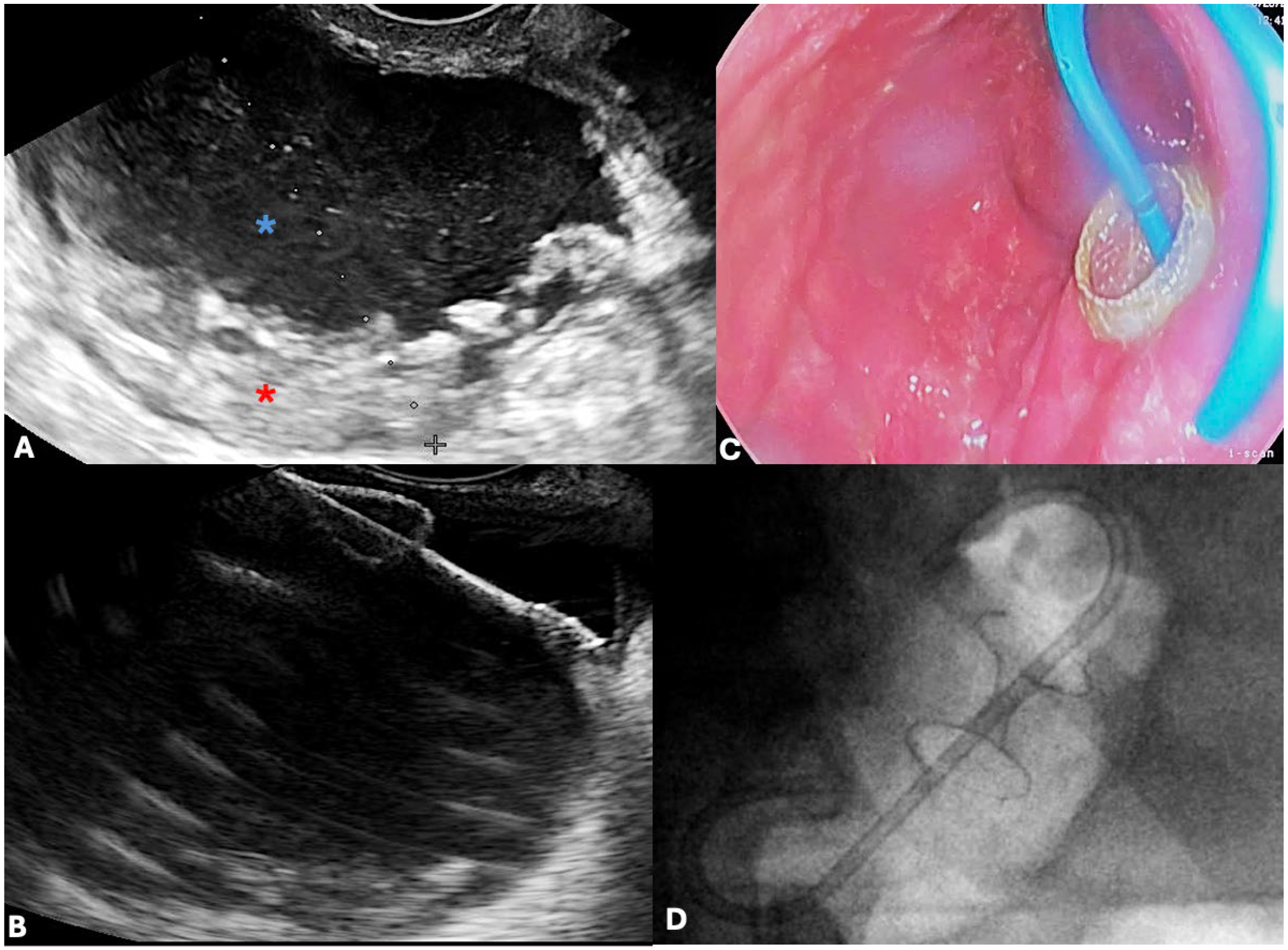
4.3. Role of Endoscopic Ultrasound (EUS)
5. Management Strategies
5.1. Conservative Management
5.2. Endoscopic Drainage
5.2.1. EUS-Guided Transmural Drainage
5.2.2. Double-Pigtail Plastic Stents (DPPSs) vs. Lumen-Apposing Metal Stents (LAMS)
Efficacy Outcomes Across Studies
Safety Outcomes and Adverse Events
Indications and Technical Considerations
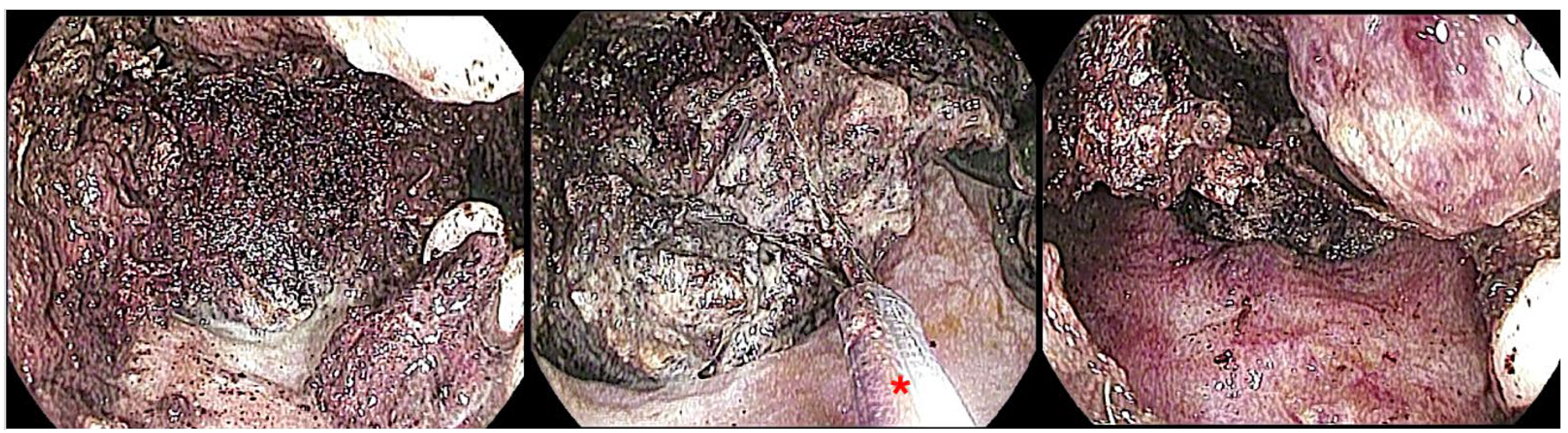
5.2.3. Endoscopic Necrosectomy for WON
Direct Endoscopic Necrosectomy (DEN) Techniques
- The EndoRotor® Powered Endoscopic Debridement System, capable of simultaneously cutting and aspirating necrotic debris, avoiding repeated scope passages [123];
- The OTSG Xcavator™, a large grasper mounted at the endoscope tip, can remove up to 1 cm3 of necrosis per pass while preserving the working channel for irrigation [124];
- The Necrolit® system, combining a stiff monofilament snare with a Dormia basket in a single catheter, is designed to reduce procedure time.
Step-Up Approach vs. Primary Necrosectomy
Safety Outcomes and Complications
5.3. Percutaneous Drainage
5.3.1. Indications and Technical Approach
5.3.2. Limitations and Role in Step-Up Strategies
6. Comparison of Different Treatment Approaches
6.1. Endoscopic Drainage vs. Percutaneous Drainage
6.1.1. Efficacy Outcome
6.1.2. Safety and Adverse Events
6.2. Endoscopic Drainage vs. Surgical Approaches
6.2.1. Efficacy Outcome
6.2.2. Safety and Adverse Events
6.2.3. Impact on Patient Morbidity and Length of Hospital Stay
7. Future Perspectives and Conclusions
8. Future Directions
9. Summary of Key Findings
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bradley, E.L. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch. Surg. 1993, 128, 586–590. [Google Scholar] [CrossRef]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Cui, M.L.; Kim, K.H.; Kim, H.G.; Han, J.; Kim, H.; Cho, K.B.; Jung, M.K.; Cho, C.M.; Kim, T.N. Incidence, risk factors and clinical course of pancreatic fluid collections in acute pancreatitis. Dig. Dis. Sci. 2014, 59, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Manrai, M.; Kochhar, R.; Gupta, V.; Yadav, T.D.; Dhaka, N.; Kalra, N.; Sinha, S.K.; Khandelwal, N. Outcome of Acute Pancreatic and Peripancreatic Collections Occurring in Patients with Acute Pancreatitis. Ann. Surg. 2018, 267, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Umapathy, C.; Gajendran, M.; Mann, R.; Boregowda, U.; Theethira, T.; Elhanafi, S.; Perisetti, A.; Goyal, H.; Saligram, S. Pancreatic fluid collections: Clinical manifestations, diagnostic evaluation and management. Dis. Mon. 2020, 66, 100986. [Google Scholar] [CrossRef] [PubMed]
- Ranson, J.H.; Balthazar, E.; Caccavale, R.; Cooper, M. Computed tomography and the prediction of pancreatic abscess in acute pancreatitis. Ann. Surg. 1985, 201, 656–665. [Google Scholar] [CrossRef]
- Baron, T.H.; DiMaio, C.J.; Wang, A.Y.; Morgan, K.A. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology 2020, 158, 67–75.e1. [Google Scholar] [CrossRef]
- ASGE Standards of Practice Committee; Muthusamy, V.R.; Chandrasekhara, V.; Acosta, R.D.; Bruining, D.H.; Chathadi, K.V.; Eloubeidi, M.A.; Faulx, A.L.; Fonkalsrud, L.; Gurudu, S.R.; et al. The role of endoscopy in the diagnosis and treatment of inflammatory pancreatic fluid collections. Gastrointest. Endosc. 2016, 83, 481–488. [Google Scholar]
- Leppäniemi, A.; Tolonen, M.; Tarasconi, A.; Segovia-Lohse, H.; Gamberini, E.; Kirkpatrick, A.W.; Ball, C.G.; Parry, N.; Sartelli, M.; Wolbrink, D.; et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J. Emerg. Surg. 2019, 14, 27. [Google Scholar] [CrossRef]
- Umapathy, C.; Raina, A.; Saligram, S.; Tang, G.; Papachristou, G.I.; Rabinovitz, M.; Chennat, J.; Zeh, H.; Zureikat, A.H.; Hogg, M.E.; et al. Natural History After Acute Necrotizing Pancreatitis: A Large US Tertiary Care Experience. J. Gastrointest. Surg. 2016, 20, 1844–1853. [Google Scholar] [CrossRef]
- Ikarashi, S.; Kawai, H.; Hayashi, K.; Kohisa, J.; Sato, T.; Nozawa, Y.; Morita, S.; Oka, H.; Sato, M.; Aruga, Y.; et al. Risk factors for walled-off necrosis associated with severe acute pancreatitis: A multicenter retrospective observational study. J. Hepatobiliary Pancreat. Sci. 2020, 27, 887–895. [Google Scholar] [CrossRef]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Capurso, G.; Tacelli, M.; Vanella, G.; Pisani, R.P.d.L.; Dell’aNna, G.; Abati, M.; Mele, R.; Lauri, G.; Panaitescu, A.; Nunziata, R.; et al. Managing complications of chronic pancreatitis: A guide for the gastroenterologist. Expert. Rev. Gastroenterol. Hepatol. 2023, 17, 1267–1283. [Google Scholar] [CrossRef] [PubMed]
- Szakó, L.; Gede, N.; Váradi, A.; Tinusz, B.; Vörhendi, N.; Mosztbacher, D.; Vincze, Á.; Takács, T.; Czakó, L.; Izbéki, F.; et al. Early occurrence of pseudocysts in acute pancreatitis—A multicenter international cohort analysis of 2275 cases. Pancreatology 2021, 21, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.O.; Kim, T.N. Acute pancreatic pseudocyst: Incidence, risk factors, and clinical outcomes. Pancreas 2012, 41, 577–581. [Google Scholar] [CrossRef]
- Lankisch, P.G.; Weber-Dany, B.; Maisonneuve, P.; Lowenfels, A.B. Pancreatic pseudocysts: Prognostic factors for their development and their spontaneous resolution in the setting of acute pancreatitis. Pancreatology 2012, 12, 85–90. [Google Scholar] [CrossRef]
- Ji, F.; Tang, W.; Yan, W.; Huang, J.; Liu, Y.; Zhou, J.; Qin, S.; Dai, S.; Ji, Y.; Yin, G. A nomogram to predict the occurrence of pseudocyst in patients with acute pancreatitis. Pancreatology 2024, 24, 863–869. [Google Scholar] [CrossRef]
- Hou, S.B.; Wang, S.; You, Y.B.; Yang, L.B.; Dou, M.M.; Zhang, Y. New model for predicting the development of pancreatic pseudocyst secondary to acute pancreatitis. Medicine 2023, 102, e36102. [Google Scholar] [CrossRef]
- Koo, J.G.; Liau, M.Y.Q.; A Kryvoruchko, I.; Habeeb, T.A.; Chia, C.; Shelat, V.G. Pancreatic pseudocyst: The past, the present, and the future. World J. Gastrointest. Surg. 2024, 16, 1986–2002. [Google Scholar] [CrossRef]
- Wade, J.W. Twenty-five year experience with pancreatic pseudocysts. Are we making progress? Am. J. Surg. 1985, 149, 705–708. [Google Scholar] [CrossRef]
- Sankaran, S.; Walt, A.J. The natural and unnatural history of pancreatic pseudocysts. Br. J. Surg. 1975, 62, 37–44. [Google Scholar] [CrossRef]
- Habashi, S.; Draganov, P.V. Pancreatic pseudocyst. World J. Gastroenterol. 2009, 15, 38–47. [Google Scholar] [CrossRef]
- Bradley, E.L.; Clements, J.L.; Gonzalez, A.C. The natural history of pancreatic pseudocysts: A unified concept of management. Am. J. Surg. 1979, 137, 135–141. [Google Scholar] [CrossRef]
- D’Egidio, A.; Schein, M. Pancreatic pseudocysts: A proposed classification and its management implications. Br. J. Surg. 1991, 78, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Nealon, W.H.; Walser, E. Main pancreatic ductal anatomy can direct choice of modality for treating pancreatic pseudocysts (surgery versus percutaneous drainage). Ann. Surg. 2002, 235, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Warshaw, A.L.; Rattner, D.W. Timing of surgical drainage for pancreatic pseudocyst. Clinical and chemical criteria. Ann. Surg. 1985, 202, 720–724. [Google Scholar] [CrossRef]
- Cannon, J.W.; Callery, M.P.; Vollmer, C.M. Diagnosis and management of pancreatic pseudocysts: What is the evidence? J. Am. Coll. Surg. 2009, 209, 385–393. [Google Scholar] [CrossRef]
- Stamatakos, M.; Stefanaki, C.; Kontzoglou, K.; Stergiopoulos, S.; Giannopoulos, G.; Safioleas, M. Walled-off pancreatic necrosis. World J. Gastroenterol. 2010, 16, 1707–1712. [Google Scholar] [CrossRef]
- Beger, H.G.; Rau, B.M. Severe acute pancreatitis: Clinical course and management. World J. Gastroenterol. 2007, 13, 5043–5051. [Google Scholar] [CrossRef]
- Bruni, A.; Colecchia, L.; Dell’anna, G.; Scalvini, D.; Mandarino, F.V.; Lisotti, A.; Fuccio, L.; Cecinato, P.; Marasco, G.; Donatelli, G.; et al. Nutritional Management in Chronic Pancreatitis: From Exocrine Pancreatic Insufficiency to Precision Therapy. Nutrients 2025, 17, 2720. [Google Scholar] [CrossRef]
- Papachristou, G.I.; Takahashi, N.; Chahal, P.; Sarr, M.G.; Baron, T.H. Peroral endoscopic drainage/debridement of walled-off pancreatic necrosis. Ann. Surg. 2007, 245, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Wroński, M.; Cebulski, W.; Pawłowski, W.; Krasnodębski, I.W.; Słodkowski, M. Walled-off necrosis: Safety of watchful waiting. Dig. Dis. Sci. 2015, 60, 1081–1086. [Google Scholar] [CrossRef]
- Boerma, D.; van Gulik, T.M.; Obertop, H.; Gouma, D.J. Internal drainage of infected pancreatic pseudocysts: Safe or sorry? Dig. Surg. 1999, 16, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Isenmann, R.; Schwarz, M.; Rau, B.; Trautmann, M.; Schober, W.; Beger, H.G. Characteristics of infection with Candida species in patients with necrotizing pancreatitis. World J. Surg. 2002, 26, 372–376. [Google Scholar] [CrossRef]
- He, Y.-M.; Lv, X.-S.; Ai, Z.-L.; Liu, Z.-S.; Qian, Q.; Sun, Q.; Chen, J.-W.; Lei, D.-X.; Jiang, C.-Q.; Yuan, Y.-F. Prevention and therapy of fungal infection in severe acute pancreatitis: A prospective clinical study. World J. Gastroenterol. 2003, 9, 2619–2621. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.E.L.; Lahchich, M.; Werge, M.P.; Hadi, A.; Ebrahim, M.; Schmidt, P.N.; Karstensen, J.G.; Novovic, S. Vascular Complications in Patients with Pancreatic Walled-Off Necrosis-A Retrospective, Single Cohort Study. Pancreas 2025, 54, e144–e149. [Google Scholar] [CrossRef]
- Risti, B.; Marincek, B.; Jost, R.; Decurtins, M.; Ammann, R. Hemosuccus pancreaticus as a source of obscure upper gastrointestinal bleeding: Three cases and literature review. Am. J. Gastroenterol. 1995, 90, 1878–1880. [Google Scholar]
- Serafin, M.; Kluszczyk, P.; Maślanka, S.; Kowalczyk, T.; Jabłońska, B.; Mrowiec, S. Hemorrhagic Cysts in the Pancreas: Risk Factors, Treatment, and Outcomes—Insights from a Single-Center Study. Med. Sci. Monit. 2024, 30, e941955. [Google Scholar] [CrossRef]
- Maurer, L.R.; Fagenholz, P.J. Contemporary Surgical Management of Pancreatic Necrosis. JAMA Surg. 2023, 158, 81–88. [Google Scholar] [CrossRef]
- Santos, J.C.; Feres, O.; Rocha, J.J.; Aracava, M.M. Massive lower gastrointestinal hemorrhage caused by pseudocyst of the pancreas ruptured into the colon. Report of two cases. Dis. Colon. Rectum 1992, 35, 75–77. [Google Scholar] [CrossRef]
- Johst, P.; Tsiotos, G.G.; Sarr, M.G. Pancreatic ascites: A rare complication of necrotizing pancreatitis. A case report and review of the literature. Int. J. Pancreatol. 1997, 22, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Bawany, M.Z.; Rafiq, E.; Ahmad, S.; Chaudhry, Q.; Nawras, A. Endoscopic therapy for significant gastric outlet obstruction caused by a small pancreatic pseudocyst with a unique shape and location. J. Interv. Gastroenterol. 2012, 2, 196–198. [Google Scholar] [CrossRef]
- Christensen, N.M.; Demling, R.; Mathewson, C. Unusual manifestations of pancreatic pseudocysts and their surgical management. Am. J. Surg. 1975, 130, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Rasch, S.; Nötzel, B.; Phillip, V.; Lahmer, T.; Schmid, R.M.; Algül, H. Management of pancreatic pseudocysts-A retrospective analysis. PLoS ONE 2017, 12, e0184374. [Google Scholar] [CrossRef]
- O’Malley, V.P.; Cannon, J.P.; Postier, R.G. Pancreatic pseudocysts: Cause, therapy, and results. Am. J. Surg. 1985, 150, 680–682. [Google Scholar] [CrossRef]
- McConnell, D.B.; Gregory, J.R.; Sasaki, T.M.; Vetto, R.M. Pancreatic pseudocyst. Am. J. Surg. 1982, 143, 599–601. [Google Scholar] [CrossRef]
- Vitas, G.J.; Sarr, M.G. Selected management of pancreatic pseudocysts: Operative versus expectant management. Surgery 1992, 111, 123–130. [Google Scholar]
- Maringhini, A.; Uomo, G.; Patti, R.; Rabitti, P.; Termini, A.; Cavallera, A.; Dardanoni, G.; Manes, G.; Ciambra, M.; Laccetti, M.; et al. Pseudocysts in acute nonalcoholic pancreatitis: Incidence and natural history. Dig. Dis. Sci. 1999, 44, 1669–1673. [Google Scholar] [CrossRef]
- Soliani, P.; Ziegler, S.; Franzini, C.; Dell’aBate, P.; Del Rio, P.; Di Mario, F.; Cavestro, M.; Sianesi, M. The size of pancreatic pseudocyst does not influence the outcome of invasive treatments. Dig. Liver Dis. 2004, 36, 135–140. [Google Scholar] [CrossRef]
- Nealon, W.H.; Bhutani, M.; Riall, T.S.; Raju, G.; Ozkan, O.; Neilan, R. A unifying concept: Pancreatic ductal anatomy both predicts and determines the major complications resulting from pancreatitis. J. Am. Coll. Surg. 2009, 208, 790–799, discussion 799–801. [Google Scholar] [CrossRef]
- Rana, S.S.; Sharma, R.K.; Gupta, P.; Gupta, R. Natural course of asymptomatic walled off pancreatic necrosis. Dig. Liver Dis. 2019, 51, 730–734. [Google Scholar] [CrossRef]
- Patra, P.S.; Das, K.; Bhattacharyya, A.; Ray, S.; Hembram, J.; Sanyal, S.; Dhali, G.K. Natural resolution or intervention for fluid collections in acute severe pancreatitis. Br. J. Surg. 2014, 101, 1721–1728. [Google Scholar] [CrossRef]
- Vetro, V.; Agnello, F.; Raitano, E.; Calsi, S.L.; Pinzone, S.; Galia, M. CT and MRI of pancreatic cystic lesions: Tricks of the trade. J. Med. Imaging Interv. Radiol. 2024, 11, 39. [Google Scholar] [CrossRef]
- Ortiz Morales, C.M.; Girela Baena, E.L.; Olalla Muñoz, J.R.; Parlorio de Andrés, E.; López Corbalán, J.A. Radiology of acute pancreatitis today: The Atlanta classification and the current role of imaging in its diagnosis and treatment. Radiologia 2019, 61, 453–466. [Google Scholar] [CrossRef]
- Patel, A.; Lalwani, N.; Kielar, A. Use of oral contrast in 2024: Primer for radiologists. Abdom. Radiol. 2024, 49, 2953–2959. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, N.; Samanta, J.; Kochhar, S.; Kalra, N.; Appasani, S.; Manrai, M.; Kochhar, R. Pancreatic fluid collections: What is the ideal imaging technique? World J. Gastroenterol. 2015, 21, 13403–13410. [Google Scholar] [CrossRef] [PubMed]
- Mayo-Smith, W.W.; Hara, A.K.; Mahesh, M.; Sahani, D.V.; Pavlicek, W. How I do it: Managing radiation dose in CT. Radiology 2014, 273, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.R.; Jensen, K.K.; Bakis, G.; Shaaban, A.M.; Coakley, F.V. Revised Atlanta Classification for Acute Pancreatitis: A Pictorial Essay. Radiographics 2016, 36, 675–687. [Google Scholar] [CrossRef]
- Yacoub, J.H.; Clark, J.A.; Paal, E.E.; Manning, M.A. Approach to Cystic Lesions in the Abdomen and Pelvis, with Radiologic-Pathologic Correlation. Radiographics 2021, 41, 1368–1386. [Google Scholar] [CrossRef]
- Thoeni, R.F. The revised Atlanta classification of acute pancreatitis: Its importance for the radiologist and its effect on treatment. Radiology 2012, 262, 751–764. [Google Scholar] [CrossRef]
- Pitchumoni, C.S.; Agarwal, N. Pancreatic pseudocysts. When and how should drainage be performed? Gastroenterol. Clin. North Am. 1999, 28, 615–639. [Google Scholar] [CrossRef]
- Takahashi, N.; Papachristou, G.I.; Schmit, G.D.; Chahal, P.; LeRoy, A.J.; Sarr, M.G.; Vege, S.S.; Mandrekar, J.N.; Baron, T.H. CT findings of walled-off pancreatic necrosis (WOPN): Differentiation from pseudocyst and prediction of outcome after endoscopic therapy. Eur. Radiol. 2008, 18, 2522–2529. [Google Scholar] [CrossRef]
- Sahu, S.K.; Giri, S.; Das, S.; Patro, C.D.; Praharaj, D.L.; Mallick, B.; Nath, P.; Panigrahi, S.C.; Anand, A.C. Approach to the Diagnosis and Management of Infected Pancreatic Necrosis: A Narrative Review. Cureus 2025, 17, e83020. [Google Scholar] [CrossRef] [PubMed]
- Schima, W.; Böhm, G.; Rösch, C.S.; Klaus, A.; Függer, R.; Kopf, H. Mass-forming pancreatitis versus pancreatic ductal adenocarcinoma: CT and MR imaging for differentiation. Cancer Imaging 2020, 20, 52. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, K.; Shinde, R.K.; Nagtode, T.; Jivani, A.; Goel, S.; Samuel, J. Role of Necrosectomy in Necrotizing Pancreatitis: A Narrative Review. Cureus 2024, 16, e70470. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.; Freitas Ferreira, A.C.; Raimundo, P.; Maio, R. Walled-off pancreatic necrosis: A staged multidisciplinary step-up approach. BMJ Case Rep. 2020, 13, e232952. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Zhou, M.; Luo, J.; Zhu, Z. Current Progress in the CT- and MRI-Based Detection and Evaluation of Acute Pancreatitis Complications. Med. Sci. Monit. 2025, 31, e948306. [Google Scholar] [CrossRef]
- Macari, M.; Finn, M.E.; Bennett, G.L.; Cho, K.C.; Newman, E.; Hajdu, C.H.; Babb, J.S. Differentiating pancreatic cystic neoplasms from pancreatic pseudocysts at MR imaging: Value of perceived internal debris. Radiology 2009, 251, 77–84. [Google Scholar] [CrossRef]
- Griffin, N.; Charles-Edwards, G.; Grant, L.A. Magnetic resonance cholangiopancreatography: The ABC of MRCP. Insights Imaging 2012, 3, 11–21. [Google Scholar] [CrossRef]
- Sgantzou, I.K.; A Samara, A.; Diamantis, A.; Karagiorgas, G.P.; Zacharoulis, D.; Rountas, C. Pseudoaneurysm of gastroduodenal artery and pulmonary embolism: Rare co-incidence of two complications of pancreatitis. J. Surg. Case Rep. 2020, 2020, rjz407. [Google Scholar] [CrossRef]
- Ardeshna, D.R.; Cao, T.; Rodgers, B.; Onongaya, C.; Jones, D.; Chen, W.; Koay, E.J.; Krishna, S.G. Recent advances in the diagnostic evaluation of pancreatic cystic lesions. World J. Gastroenterol. 2022, 28, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Grassedonio, E.; Toia, P.; La Grutta, L.; Palmucci, S.; Smeraldi, T.; Cutaia, G.; Albano, D.; Midiri, F.; Galia, M.; Midiri, M. Role of computed tomography and magnetic resonance imaging in local complications of acute pancreatitis. Gland. Surg. 2019, 8, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.L.; Silva, M.A. Mind the Gap-Disconnected Duct Syndrome: A Review of Current Diagnostic and Management Strategies. Pancreas 2022, 51, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Bofill, A.; Law, R.; Storm, A.C.; Vargas, E.J.; Martin, J.A.; Petersen, B.T.; Majumder, S.; Vege, S.; Abu Dayyeh, B.K.; Chandrasekhara, V. The role of MRCP for preventing pancreatic fluid collection recurrence after EUS-guided drainage of walled-off necrosis. Gastrointest. Endosc. 2025, 101, 608–616. [Google Scholar] [CrossRef]
- Vitali, F.; Zundler, S.; Jesper, D.; Strobel, D.; Wildner, D.; de Pretis, N.; Frulloni, L.; Crinó, S.F.; Neurath, M.F. Endoscopic Ultrasound in Pancreatology: Focus on Inflammatory Diseases and Interventions. Visc. Med. 2023, 39, 131–139. [Google Scholar] [CrossRef]
- Medarapalem, J.B.; Appasani, S.; Gulati, A.; Manrai, M.; Siddappa, P.K.K.; Khandelwal, N.; Sinha, S.K.; Gupta, V.; Yadav, T.D.; Kochhar, R. Mo1460 Characterization of Fluid Collections Using Quantification of Solid Debris in Acute Pancreatitis—A Comparative Study of EUS vs. CT for Prediction of Intervention. Gastrointest. Endosc. 2014, 79, AB445. [Google Scholar] [CrossRef]
- Capurso, G.; Rizzo, G.E.M.; Coluccio, C.; Crinò, S.F.; Cucchetti, A.; Facciorusso, A.; Hassan, C.; Amato, A.; Auriemma, F.; Bertani, H.; et al. The i-EUS consensus on the management of pancreatic fluid collections—Part 1. Dig. Liver Dis. 2024, 56, 1663–1674. [Google Scholar] [CrossRef]
- Capurso, G.; Coluccio, C.; Rizzo, G.E.M.; Crinò, S.F.; Cucchetti, A.; Facciorusso, A.; Hassan, C.; Amato, A.; Auriemma, F.; Bertani, H.; et al. The 1st i-EUS consensus on the management of pancreatic fluid collections—Part 2. Dig. Liver Dis. 2024, 56, 1819–1827. [Google Scholar] [CrossRef]
- Chiang, K.C.; Chen, T.H.; Hsu, J.T. Management of chronic pancreatitis complicated with a bleeding pseudoaneurysm. World J. Gastroenterol. WJG 2014, 20, 16132. [Google Scholar] [CrossRef]
- Brimhall, B.; Han, S.; Tatman, P.D.; Clark, T.J.; Wani, S.; Brauer, B.; Edmundowicz, S.; Wagh, M.S.; Attwell, A.; Hammad, H.; et al. Increased Incidence of Pseudoaneurysm Bleeding with Lumen-Apposing Metal Stents Compared to Double-Pigtail Plastic Stents in Patients with Peripancreatic Fluid Collections. Clin. Gastroenterol. Hepatol. 2018, 16, 1521–1528. [Google Scholar] [CrossRef]
- Herman, T.; Karna, R.; Vanek, P.; Azeem, N.; Trikudanathan, G. An unusual presentation of necrotizing pancreatitis. Gastrointest. Endosc. 2024, 100, 952–954. [Google Scholar] [CrossRef]
- Rana, S.S.; Kumar, A.; Lal, A.; Sharma, R.; Kang, M.; Gorsi, U.; Gupta, R. Safety and efficacy of angioembolisation followed by endoscopic ultrasound guided transmural drainage for pancreatic fluid collections associated with arterial pseudoaneurysm. Pancreatology 2017, 17, 658–662. [Google Scholar] [CrossRef]
- Goordeen, A.; Sharbatji, M.; Khalid, S.; Abbass, A.; Majeed, U. A Case of Pancreatic Pseudocyst Complicated by Pseudoaneurysm. Cureus 2018, 10, e2512. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takahashi, K.; Aruga, Y.; Yamazaki, F.; Kumaki, D.; Yamakawa, M.; Hirano, M.; Funakoshi, K.; Terai, S. A case of pancreatic pseudocysts accompanied by infection, pseudoaneurysm ruptures, and pseudocystocolonic fistulae. Clin. J. Gastroenterol. 2019, 12, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Negm, A.A.; Poos, H.; Kruck, E.; Vonberg, R.-P.; Domagk, D.; Madisch, A.; Voigtländer, T.; Manns, M.P.; Wedemeyer, J.; Lankisch, T.O. Microbiologic analysis of peri-pancreatic fluid collected during EUS in patients with pancreatitis: Impact on antibiotic therapy. Gastrointest. Endosc. 2013, 78, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Marocchi, G.; Stigliano, S.; Baldaro, F.; Di Matteo, F.M. Timing of lumen-apposing metal stents removal in pancreatic fluid collections: Could we go beyond? Pancreatology 2023, 23, e75–e76. [Google Scholar] [CrossRef]
- Ramai, D.; Enofe, I.; Deliwala, S.S.; Mozell, D.; Facciorusso, A.; Gkolfakis, P.; Mohan, B.P.; Chandan, S.; Previtera, M.; Maida, M.; et al. Early (<4 weeks) versus standard (≥4 weeks) endoscopic drainage of pancreatic walled-off fluid collections: A systematic review and meta-analysis. Gastrointest. Endosc. 2023, 97, 415–421.e5. [Google Scholar]
- Sheth, S.G.; Machicado, J.D.; Chalhoub, J.M.; Forsmark, C.; Zyromski, N.; Thosani, N.C.; Thiruvengadam, N.R.; Ruan, W.; Pawa, S.; Ngamruengphong, S.; et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in the management of chronic pancreatitis: Summary and recommendations. Gastrointest. Endosc. 2024, 100, 584–594. [Google Scholar] [CrossRef]
- Dumonceau, J.-M.; Delhaye, M.; Tringali, A.; Arvanitakis, M.; Sanchez-Yague, A.; Vaysse, T.; Aithal, G.P.; Anderloni, A.; Bruno, M.; Cantú, P.; et al. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline-Updated August 2018. Endoscopy 2019, 51, 179–193. [Google Scholar] [CrossRef]
- Zhu, H.; Du, Y.; Wang, K.; Li, Z.; Jin, Z. Consensus guidelines on the diagnosis and treatment of pancreatic pseudocyst and walled-off necrosis from a Chinese multiple disciplinary team expert panel. Endosc. Ultrasound 2024, 13, 205. [Google Scholar] [CrossRef]
- Van Santvoort, H.C.; Besselink, M.G.; Bakker, O.J.; Hofker, H.S.; Boermeester, M.A.; Dejong, C.H.; van Goor, H.; Schaapherder, A.F.; van Eijck, C.H.; Bollen, T.L.; et al. A Step-up Approach or Open Necrosectomy for Necrotizing Pancreatitis. N. Engl. J. Med. 2010, 362, 1491–1502. [Google Scholar] [CrossRef]
- Seifert, H.; Biermer, M.; Schmitt, W.; Jurgensen, C.; Will, U.; Gerlach, R.; Kreitmair, C.; Meining, A.; Wehrmann, T.; Rosch, T. Transluminal endoscopic necrosectomy after acute pancreatitis: A multicentre study with long-term follow-up (the GEPARD Study). Gut 2009, 58, 1260–1266. [Google Scholar] [CrossRef]
- Cremer, M.; Deviere, J.; Engelholm, L. Endoscopic management of cysts and pseudocysts in chronic pancreatitis: Long-term follow-up after 7 years of experience. Gastrointest. Endosc. 1989, 35, 1–9. [Google Scholar] [CrossRef]
- Binda, C.; Fabbri, S.; Perini, B.; Boschetti, M.; Coluccio, C.; Giuffrida, P.; Gibiino, G.; Petraroli, C.; Fabbri, C. Endoscopic Ultrasound-Guided Drainage of Pancreatic Fluid Collections: Not All Queries Are Already Solved. Medicina 2024, 60, 333. [Google Scholar] [CrossRef]
- Varadarajulu, S.; Christein, J.D.; Tamhane, A.; Drelichman, E.R.; Wilcox, C.M. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest. Endosc. 2008, 68, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, M.; Dumonceau, J.-M.; Albert, J.; Badaoui, A.; Bali, M.A.; Barthet, M.; Besselink, M.; Deviere, J.; Ferreira, A.O.; Gyökeres, T.; et al. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy 2018, 50, 524–546. [Google Scholar] [CrossRef] [PubMed]
- Abu Dayyeh, B.K.; Mukewar, S.; Majumder, S.; Zaghlol, R.; Valls, E.J.V.; Bazerbachi, F.; Levy, M.J.; Baron, T.H.; Gostout, C.J.; Petersen, B.T.; et al. Large-caliber metal stents versus plastic stents for the management of pancreatic walled-off necrosis. Gastrointest. Endosc. 2018, 87, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Talreja, J.P.; Shami, V.M.; Ku, J.; Morris, T.D.; Ellen, K.; Kahaleh, M. Transenteric drainage of pancreatic-fluid collections with fully covered self-expanding metallic stents (with video). Gastrointest. Endosc. 2008, 68, 1199–1203. [Google Scholar] [CrossRef]
- Dell’aNna, G.; Bruni, A.; Fanizza, J.; Biamonte, P.; Bencardino, S.; Mandarino, F.V.; Fasulo, E.; Barchi, A.; Gallo, C.; Dhar, J.; et al. Current status and further directions of endoscopic ultrasound-directed transgastric ERCP and endoscopic ultrasound-directed transenteric ERCP in the management of pancreaticobiliary diseases in surgically altered anatomy: A comprehensive review. Ther. Adv. Gastroenterol. 2025, 18, 17562848251359006. [Google Scholar] [CrossRef]
- Dell’anna, G.; Nunziata, R.; Delogu, C.; Porta, P.; Grassini, M.V.; Dhar, J.; Barà, R.; Bencardino, S.; Fanizza, J.; Mandarino, F.V.; et al. The Role of Therapeutic Endoscopic Ultrasound in Management of Malignant Double Obstruction (Biliary and Gastric Outlet): A Comprehensive Review with Clinical Scenarios. J. Clin. Med. 2024, 13, 7731. [Google Scholar] [CrossRef]
- Sharaiha, R.Z.; Tyberg, A.; Khashab, M.A.; Kumta, N.A.; Karia, K.; Nieto, J.; Siddiqui, U.D.; Waxman, I.; Joshi, V.; Benias, P.C.; et al. Endoscopic Therapy with Lumen-apposing Metal Stents Is Safe and Effective for Patients with Pancreatic Walled-off Necrosis. Clin. Gastroenterol. Hepatol. 2016, 14, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Bekkali, N.L.H.; Nayar, M.K.; Leeds, J.S.; Charnley, R.M.; Huggett, M.T.; Oppong, K.W. A comparison of outcomes between a lumen-apposing metal stent with electrocautery-enhanced delivery system and a bi-flanged metal stent for drainage of walled-off pancreatic necrosis. Endosc. Int. Open 2017, 5, E1189–E1196. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.Y.; Navaneethan, U.; Hasan, M.K.; Sutton, B.; Hawes, R.; Varadarajulu, S. Non-superiority of lumen-apposing metal stents over plastic stents for drainage of walled-off necrosis in a randomised trial. Gut 2019, 68, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Boxhoorn, L.; Verdonk, R.C.; Besselink, M.G.; Boermeester, M.; Bollen, T.L.; Bouwense, S.A.; Cappendijk, V.C.; Curvers, W.L.; Dejong, C.H.; van Dijk, S.M.; et al. Comparison of lumen-apposing metal stents versus double-pigtail plastic stents for infected necrotising pancreatitis. Gut 2023, 72, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Karstensen, J.G.; Novovic, S.; Hansen, E.F.; Jensen, A.B.; Joergensen, H.L.; Lauritsen, M.L.; Werge, M.P.; Schmidt, P.N. EUS-guided drainage of large walled-off pancreatic necroses using plastic versus lumen-apposing metal stents: A single-centre randomised controlled trial. Gut 2023, 72, 1167–1173. [Google Scholar] [CrossRef]
- Chen, Y.-I.; Yang, J.; Friedland, S.; Holmes, I.; Law, R.; Hosmer, A.; Stevens, T.; Franco, M.C.; Jang, S.; Pawa, R.; et al. Lumen apposing metal stents are superior to plastic stents in pancreatic walled-off necrosis: A large international multicenter study. Endosc. Int. Open 2019, 7, E347–E354. [Google Scholar] [CrossRef]
- Chen, Y.-I.; Barkun, A.N.; Adam, V.; Bai, G.; Singh, V.K.; Bukhari, M.; Gutierrez, O.B.; Elmunzer, B.J.; Moran, R.; Fayad, L.; et al. Cost-effectiveness analysis comparing lumen-apposing metal stents with plastic stents in the management of pancreatic walled-off necrosis. Gastrointest. Endosc. 2018, 88, 267–276.e1. [Google Scholar] [CrossRef]
- Vanella, G.; Dell’Anna, G.; Arcidiacono, P.G. Plastic Versus Metal EUS-Guided Drainage of Pancreatic Fluid Collections: Do We Really Know When to Use the Hard Way? Clin. Gastroenterol. Hepatol. 2022, 20, e1507–e1508. [Google Scholar] [CrossRef]
- Tyberg, A.; Karia, K.; Gabr, M.; Desai, A.; Doshi, R.; Gaidhane, M.; Sharaiha, R.Z.; Kahaleh, M. Management of pancreatic fluid collections: A comprehensive review of the literature. World J. Gastroenterol. 2016, 22, 2256–2270. [Google Scholar] [CrossRef]
- Trikudanathan, G.; Tawfik, P.; Amateau, S.K.; Mbbs, S.M.; Arain, M.; Attam, R.; Beilman, G.; Flanagan, S.; Freeman, M.L.; Mallery, S. Early (<4 Weeks) Versus Standard (≥4 Weeks) Endoscopically Centered Step-Up Interventions for Necrotizing Pancreatitis. Am. J. Gastroenterol. 2018, 113, 1550–1558. [Google Scholar]
- Nakai, Y.; Shiomi, H.; Hamada, T.; Ota, S.; Takenaka, M.; Iwashita, T.; Sato, T.; Saito, T.; Masuda, A.; Matsubara, S.; et al. Early versus delayed interventions for necrotizing pancreatitis: A systematic review and meta-analysis. DEN Open 2023, 3, e171. [Google Scholar] [CrossRef]
- AbiMansour, J.P.; Jaruvongvanich, V.; Velaga, S.; Law, R.J.; Storm, A.C.; Topazian, M.D.; Levy, M.J.; Alexander, R.; Vargas, E.J.; Bofill-Garcia, A.; et al. Lumen-apposing metal stents with or without coaxial plastic stent placement for the management of pancreatic fluid collections. Gastrointest. Endosc. 2024, 99, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Puga, M.; Consiglieri, C.; Busquets, J.; Pallarès, N.; Secanella, L.; Peláez, N.; Fabregat, J.; Castellote, J.; Gornals, J. Safety of lumen-apposing stent with or without coaxial plastic stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: A retrospective study. Endoscopy 2018, 50, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Vanek, P.; Falt, P.; Vitek, P.; Zoundjiekpon, V.; Horinkova, M.; Zapletalova, J.; Lovecek, M.; Urban, O. EUS-guided transluminal drainage using lumen-apposing metal stents with or without coaxial plastic stents for treatment of walled-off necrotizing pancreatitis: A prospective bicentric randomized controlled trial. Gastrointest. Endosc. 2023, 97, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Dabizzi, E.; Anderloni, A.; Cennamo, V.; Fiscaletti, M.; Fugazza, A.; Jovine, E.; Ercolani, G.; Gasbarrini, A.; Fabbri, C. Single-step endoscopic ultrasound-guided multiple gateway drainage of complex walled-off necrosis with lumen apposing metal stents. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1401–1404. [Google Scholar] [CrossRef]
- Varadarajulu, S.; Phadnis, M.A.; Christein, J.D.; Wilcox, C.M. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest. Endosc. 2011, 74, 74–80. [Google Scholar] [CrossRef]
- Bang, J.Y.; Wilcox, C.M.; Arnoletti, J.P.; Peter, S.; Christein, J.; Navaneethan, U.; Hawes, R.; Varadarajulu, S. Validation of the Orlando Protocol for endoscopic management of pancreatic fluid collections in the era of lumen-apposing metal stents. Dig. Endosc. 2022, 34, 612–621. [Google Scholar] [CrossRef]
- Parsa, N.; Nieto, J.M.; Powers, P.; Mitsuhashi, S.; Abdelqader, A.; Hadzinakos, G.; Anderloni, A.A.; Fugazza, A.; James, T.W.; Arlt, A.; et al. Endoscopic ultrasound-guided drainage of pancreatic walled-off necrosis using 20-mm versus 15-mm lumen-apposing metal stents: An international, multicenter, case-matched study. Endoscopy 2020, 52, 211–219. [Google Scholar] [CrossRef]
- Chandrasekhara, V.; Elhanafi, S.; Storm, A.C.; Takahashi, N.; Lee, N.J.; Levy, M.J.; Kaura, K.; Wang, L.; Majumder, S.; Vege, S.S.; et al. Predicting the Need for Step-Up Therapy After EUS-Guided Drainage of Pancreatic Fluid Collections with Lumen-Apposing Metal Stents. Clin. Gastroenterol. Hepatol. 2021, 19, 2192–2198. [Google Scholar] [CrossRef]
- Puli, S.R.; Graumlich, J.F.; Pamulaparthy, S.R.; Kalva, N. Endoscopic transmural necrosectomy for walled-off pancreatic necrosis: A systematic review and meta-analysis. Can. J. Gastroenterol. Hepatol. 2014, 28, 50–53. [Google Scholar] [CrossRef]
- Baroud, S.; Chandrasekhara, V.; Storm, A.C.; Law, R.J.; Vargas, E.J.; Levy, M.J.; Mahmoud, T.; Bazerbachi, F.; Bofill-Garcia, A.; Ghazi, R.; et al. Novel classification system for walled-off necrosis: A step toward standardized nomenclature and risk-stratification framework. Gastrointest. Endosc. 2023, 97, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Rizzatti, G.; Rimbas, M.; Impagnatiello, M.; Gasbarrini, A.; Costamagna, G.; Larghi, A. Endorotor-Based Endoscopic Necrosectomy as a Rescue or Primary Treatment of Complicated Walled-off Pancreatic Necrosis. A Case Series. J. Gastrointest. Liver Dis. 2020, 29, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.; Bachmann, J.; Schlag, C.; Huegle, U.; Rahman, I.; Wedi, E.; Walter, B.; Möschler, O.; Sturm, L.; Meining, A. Over-the-scope-grasper: A new tool for pancreatic necrosectomy and beyond—First multicenter experience. World J. Gastrointest. Surg. 2022, 14, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.; Yan, L.; Dargan, A.; Nieto, J.; Shariaha, R.; Binmoeller, K.; Adler, D.; DeSimone, M.; Berzin, T.; Swahney, M.; et al. Direct endoscopic necrosectomy at the time of transmural stent placement results in earlier resolution of complex walled-off pancreatic necrosis: Results from a large multicenter United States trial. Endosc. Ultrasound 2019, 8, 172–179. [Google Scholar] [CrossRef]
- Bang, J.Y.; Lakhtakia, S.; Thakkar, S.; Buxbaum, J.L.; Waxman, I.; Sutton, B.; Memon, S.F.; Singh, S.; Basha, J.; Singh, A.; et al. Upfront endoscopic necrosectomy or step-up endoscopic approach for infected necrotising pancreatitis (DESTIN): A single-blinded, multicentre, randomised trial. Lancet Gastroenterol. Hepatol. 2024, 9, 22–33. [Google Scholar] [CrossRef]
- Olsen, G.A.; Schmidt, P.N.; Hadi, A.; Prahm, A.P.; Werge, M.P.; Roug, S.; Schefte, D.F.; Lauritsen, M.L.; Hansen, E.F.; Novovic, S.; et al. Accelerated vs Step-Up Endoscopic Treatment for Pancreatic Walled-Off Necrosis: A Randomized Controlled Trial (ACCELERATE). Clin. Gastroenterol. Hepatol. 2025, in press. [Google Scholar] [CrossRef]
- van Brunschot, S.; Fockens, P.; Bakker, O.J.; Besselink, M.G.; Voermans, R.P.; Poley, J.W.; Gooszen, H.G.; Bruno, M.; van Santvoort, H.C. Endoscopic transluminal necrosectomy in necrotising pancreatitis: A systematic review. Surg. Endosc. 2014, 28, 1425–1438. [Google Scholar] [CrossRef]
- Maatman, T.K.; Heimberger, M.A.; Lewellen, K.A.; Roch, A.M.; Colgate, C.L.; House, M.G.; Nakeeb, A.; Ceppa, E.P.; Schmidt, C.M.; Zyromski, N.J. Visceral artery pseudoaneurysm in necrotizing pancreatitis: Incidence and outcomes. Can. J. Surg. 2020, 63, E272–E277. [Google Scholar] [CrossRef]
- Fugazza, A.; Colombo, M.; Gabbiadini, R.; Carrara, S.; Maselli, R.; Anderloni, A.; Repici, A. Repositioning Rather than Replacing: The Management of a Dislodged Lumen-Apposing Metal Stent in a Walled off Necrosis. Am. J. Gastroenterol. 2020, 115, 811. [Google Scholar] [CrossRef]
- Voermans, R.P.; Besselink, M.G.; Fockens, P. Endoscopic management of walled-off pancreatic necrosis. J. Hepatobiliary Pancreat. Sci. 2015, 22, 20–26. [Google Scholar] [CrossRef]
- Boxhoorn, L.; van Dijk, S.M.; van Grinsven, J.; Verdonk, R.C.; Boermeester, M.A.; Bollen, T.L.; Bouwense, S.A.; Bruno, M.J.; Cappendijk, V.C.; Dejong, C.H.; et al. Immediate versus Postponed Intervention for Infected Necrotizing Pancreatitis. N. Engl. J. Med. 2021, 385, 1372–1381. [Google Scholar] [CrossRef]
- De Filippo, M.; Puglisi, S.; D’Amuri, F.; Gentili, F.; Paladini, I.; Carrafiello, G.; Maestroni, U.; Del Rio, P.; Ziglioli, F.; Pagnini, F. CT-guided percutaneous drainage of abdominopelvic collections: A pictorial essay. Radiol. Med. 2021, 126, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wen, X.-D.; Ma, X.; Zhu, Y.-Q.; Jiang, Z.-W.; Huang, S.-Q.; Wang, T.; Liu, W.-H. Triple guidance of choledochoscopy, ultrasonography, and computed tomography facilitates percutaneous catheter drainage of infected walled-off necrosis. Insights Imaging 2021, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Ganaie, K.H.; Choh, N.A.; Parry, A.H.; Shaheen, F.A.; Robbani, I.; Gojwari, T.A.; Singh, M.; Shah, O.J. The effectiveness of image-guided percutaneous catheter drainage in the management of acute pancreatitis-associated pancreatic collections. Pol. J. Radiol. 2021, 86, e359–e365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Ding, Y.-X.; Wu, Y.-D.; Gao, C.-C.; Li, F. A meta-analysis and systematic review of percutaneous catheter drainage in treating infected pancreatitis necrosis. Medicine 2018, 97, e12999. [Google Scholar] [CrossRef]
- Onnekink, A.M.; Boxhoorn, L.; Timmerhuis, H.C.; Bac, S.T.; Besselink, M.G.; Boermeester, M.A.; Bollen, T.L.; Bosscha, K.; Bouwense, S.A.; Bruno, M.J.; et al. Endoscopic Versus Surgical Step-Up Approach for Infected Necrotizing Pancreatitis (ExTENSION): Long-term Follow-up of a Randomized Trial. Gastroenterology 2022, 163, 712–722.e14. [Google Scholar] [CrossRef]
- Bansal, A.; Gupta, P.; Singh, A.K.; Shah, J.; Samanta, J.; Mandavdhare, H.S.; Sharma, V.; Sinha, S.K.; Dutta, U.; Sandhu, M.S.; et al. Drainage of pancreatic fluid collections in acute pancreatitis: A comprehensive overview. World J. Clin. Cases 2022, 10, 6769–6783. [Google Scholar] [CrossRef]
- Luckhurst, C.M.; El Hechi, M.; Elsharkawy, A.E.; Eid, A.I.; Maurer, L.R.; Kaafarani, H.M.; Thabet, A.; Forcione, D.G.; Castillo, C.F.-D.; Lillemoe, K.D.; et al. Improved Mortality in Necrotizing Pancreatitis with a Multidisciplinary Minimally Invasive Step-Up Approach: Comparison with a Modern Open Necrosectomy Cohort. J. Am. Coll. Surg. 2020, 230, 873–883. [Google Scholar] [CrossRef]
- Maatman, T.K.; Flick, K.F.; Roch, A.M.; Zyromski, N.J. Operative pancreatic debridement: Contemporary outcomes in changing times. Pancreatology 2020, 20, 968–975. [Google Scholar] [CrossRef]
- Wan, J.; Wu, D.; He, W.; Zhu, Y.; Zhu, Y.; Zeng, H.; Liu, P.; Xia, L.; Lu, N. Comparison of percutaneous vs endoscopic drainage in the management of pancreatic fluid collections: A prospective cohort study. J. Gastroenterol. Hepatol. 2020, 35, 2170–2175. [Google Scholar] [CrossRef]
- Keane, M.G.; Sze, S.F.; Cieplik, N.; Murray, S.; Johnson, G.J.; Webster, G.J.; Thorburn, D.; Pereira, S.P. Endoscopic versus percutaneous drainage of symptomatic pancreatic fluid collections: A 14-year experience from a tertiary hepatobiliary centre. Surg. Endosc. 2016, 30, 3730–3740. [Google Scholar] [CrossRef]
- Samanta, J.; Dhar, J.; Muktesh, G.; Gupta, P.; Kumar-M, P.; Das, A.; Agarwala, R.; Bellam, B.L.; Chauhan, R.; Kumar, K.H.; et al. Endoscopic drainage versus percutaneous drainage for the management of infected walled-off necrosis: A comparative analysis. Expert. Rev. Gastroenterol. Hepatol. 2022, 16, 297–305. [Google Scholar] [CrossRef]
- Khizar, H.; Zhicheng, H.; Chenyu, L.; Yanhua, W.; Jianfeng, Y. Efficacy and safety of endoscopic drainage versus percutaneous drainage for pancreatic fluid collection; a systematic review and meta-analysis. Ann. Med. 2023, 55, 2213898. [Google Scholar] [CrossRef] [PubMed]
- Bakker, O.J.; van Santvoort, H.C.; van Brunschot, S.; Geskus, R.B.; Besselink, M.G.; Bollen, T.L.; van Eijck, C.H.; Fockens, P.; Hazebroek, E.J.; Nijmeijer, R.M.; et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: A randomized trial. JAMA 2012, 307, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.Y.; Arnoletti, J.P.; Holt, B.A.; Sutton, B.; Hasan, M.K.; Navaneethan, U.; Feranec, N.; Wilcox, C.M.; Tharian, B.; Hawes, R.H.; et al. An Endoscopic Transluminal Approach, Compared with Minimally Invasive Surgery, Reduces Complications and Costs for Patients with Necrotizing Pancreatitis. Gastroenterology 2019, 156, 1027–1040.e3. [Google Scholar] [CrossRef] [PubMed]
- Van Brunschot, S.; van Grinsven, J.; van Santvoort, H.C.; Bakker, O.J.; Besselink, M.G.; Boermeester, M.A.; Bollen, T.L.; Bosscha, K.; Bouwense, S.A.; Bruno, M.J.; et al. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: A multicentre randomised trial. Lancet 2018, 391, 51–58. [Google Scholar] [CrossRef]
- Tan, H.L.; Zhao, Y.; Chua, D.W.; Lim, S.J.M.; Wu, C.C.H.; Tan, D.M.Y.; Khor, C.J.L.; Goh, B.K.P.; Koh, Y.X. Comparison of treatment approaches for infected necrotizing pancreatitis: A systematic review and network meta-analysis. J. Gastrointest. Surg. 2025, 29, 102152. [Google Scholar] [CrossRef]
- Dell’anna, G.; Fanizza, J.; Mandarino, F.V.; Barchi, A.; Fasulo, E.; Vespa, E.; Fanti, L.; Azzolini, F.; Battaglia, S.; Puccetti, F.; et al. The Endoscopic Management of Anastomotic Strictures After Esophagogastric Surgery: A Comprehensive Review of Emerging Approaches Beyond Endoscopic Dilation. J. Pers. Med. 2025, 15, 111. [Google Scholar] [CrossRef]
- Rana, S.S.; Verma, S.; Kang, M.; Gorsi, U.; Sharma, R.; Gupta, R. Comparison of endoscopic versus percutaneous drainage of symptomatic pancreatic necrosis in the early (< 4 weeks) phase of illness. Endosc. Ultrasound 2020, 9, 402–409. [Google Scholar] [CrossRef]
- Bernardi, F.; Dell’anna, G.; Biamonte, P.; Barchi, A.; Fanti, L.; Malesci, A.; Fuccio, L.; Sinagra, E.; Calabrese, G.; Facciorusso, A.; et al. Stents and Emerging Alternatives in Upper Gastrointestinal Endoscopy: A Comprehensive Review. Diagnostics 2025, 15, 2344. [Google Scholar] [CrossRef]
- Ross, A.S.; Irani, S.; Gan, S.I.; Rocha, F.; Siegal, J.; Fotoohi, M.; Hauptmann, E.; Robinson, D.; Crane, R.; Kozarek, R.; et al. Dual-modality drainage of infected and symptomatic walled-off pancreatic necrosis: Long-term clinical outcomes. Gastrointest. Endosc. 2014, 79, 929–935. [Google Scholar] [CrossRef]
- Gralnek, I.M.; Fuccio, L.; Coluccio, C.; Jacques, J.; Hritz, I.; Boskoski, I.; Abdelrahim, M.; Bove, V.; Neves, J.A.C.; de Jonge, P.J.F.; et al. Simulators and training models for diagnostic and therapeutic gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Technical and Technology Review. Endoscopy 2025, 57, 796–813. [Google Scholar] [CrossRef]

| Sequence | Purpose/Diagnostic Value |
|---|---|
| T2-weighted (axial and coronal, ±fat-sat) | Depicts fluid as high signal intensity (bright). Detects internal septations or debris as low-signal (dark) areas within fluid. Fat-suppressed T2 helps distinguish fluid from fat; necrotic fat remains hypointense on fat-sat T2. |
| MR Cholangiopancreatography (MRCP) | Heavily T2-weighted sequences optimized to visualize pancreatic and biliary ducts. Demonstrates ductal anatomy, communication between collections and the pancreatic duct, duct leaks, disconnected duct syndrome, or obstructive calculi. |
| T1-weighted (unenhanced) | Simple fluid appears hypointense (dark). Proteinaceous or hemorrhagic fluid shows intermediate to high signal. Useful for differentiating fluid composition. |
| Dynamic contrast-enhanced T1 (3D fat-sat GRE, arterial/portal/delayed phases) | Evaluates wall enhancement, internal septa, or solid components. Pseudocysts typically show thin rim enhancement, whereas WON walls are thicker. Non-enhancing internal material confirms necrotic debris. |
| Fat-saturated T1 (post-contrast) | Improves detection of subtle wall enhancement and hemorrhage. The fibrous capsule of mature collections usually enhances on delayed phases. |
| Diffusion-Weighted Imaging (DWI) (optional) | Identifies viscous/cellular debris and may suggest infection. Restricted diffusion (high DWI signal, low ADC) can correlate with infected or proteinaceous necrosis. Still emerging but complements CT signs (e.g., gas). |
| Study | Design & Patients | Primary Endpoint | Main Results | Safety/AEs |
|---|---|---|---|---|
| Bang et al. [105] | RCT, n = 60 (LAMS = 31, DPPS = 29) | No. of procedures to achieve treatment success (clinical + CT at 6 months) | No significant difference (2 vs. 3, p = 0.192). Procedure time shorter with LAMS (15 vs. 40 min, p < 0.001). Higher costs with LAMS (USD 12,155 vs. 6609). | Stent-related AEs more frequent with LAMS (32.3% vs. 6.9%, p = 0.01): buried stents, severe bleeding, biliary strictures. |
| Boxhoorn et al. [106] | Multicenter prospective cohort vs. historical RCT cohort (n = 53 LAMS, n = 51 DPPS) | Requirement for ETN | ETN needed in 64% LAMS vs. 53% DPPS (NS). No differences in mortality, major AEs, LOS, or costs. | Bleeding: 9% LAMS vs. 22% DPPS (NS). |
| Karstensen et al. [107] | Single-center RCT, (n = 22 DPPS, n = 20 LAMS) | No. of necrosectomies to achieve treatment success (clinical + CT) | No significant differences: necrosectomies (2.2 vs. 3.2, p = 0.42), LOS (43 vs. 58 days, p = 0.71), mortality 4.8%. | AEs: 12% overall. DPPS group: perforations, sepsis. LAMS group: 1 sepsis. |
| Chen et al. [108] | Multicenter retrospective, n = 189 (LAMS = 102, DPPS = 87) | Clinical success (WON ≤3 cm within 6 months without PD or surgery) | Higher clinical success with LAMS (80.4% vs. 57.5%, p = 0.001). Surgery more frequent with DPPS (16.1% vs. 5.6%, p = 0.02). Recurrence lower with LAMS (5.6% vs. 22.9%, p = 0.04). | Overall AE rates comparable (9.8% LAMS vs. 10.3% DPPS). Severe AEs rare. Stent dysfunction similar (migration 2.9% vs. 6.9%, occlusion 20.6% vs. 12.6%). |
| Study | Design & Patients | Primary endpoint | Main results | Safety/AEs |
| Bang et al. [105] | RCT, n = 60 (LAMS = 31, DPPS = 29) | No. of procedures to achieve treatment success (clinical + CT at 6 months) | No significant difference (2 vs. 3, p = 0.192). Procedure time shorter with LAMS (15 vs. 40 min, p < 0.001). Higher costs with LAMS (USD 12,155 vs. 6609). | Stent-related AEs more frequent with LAMS (32.3% vs. 6.9%, p = 0.01): buried stents, severe bleeding, biliary strictures. |
| Boxhoorn et al. [106] | Multicenter prospective cohort vs. historical RCT cohort (n = 53 LAMS, n = 51 DPPS) | Requirement for ETN | ETN needed in 64% LAMS vs. 53% DPPS (NS). No differences in mortality, major AEs, LOS, or costs. | Bleeding: 9% LAMS vs. 22% DPPS (NS). |
| Karstensen et al. [107] | Single-center RCT, (n = 22 DPPS, n = 20 LAMS) | No. of necrosectomies to achieve treatment success (clinical + CT) | No significant differences: necrosectomies (2.2 vs. 3.2, p = 0.42), LOS (43 vs. 58 days, p = 0.71), mortality 4.8%. | AEs: 12% overall. DPPS group: perforations, sepsis. LAMS group: 1 sepsis. |
| Chen et al. [108] | Multicenter retrospective, n = 189 (LAMS = 102, DPPS = 87) | Clinical success (WON ≤3 cm within 6 months without PD or surgery) | Higher clinical success with LAMS (80.4% vs. 57.5%, p = 0.001). Surgery more frequent with DPPS (16.1% vs. 5.6%, p = 0.02). Recurrence lower with LAMS (5.6% vs. 22.9%, p = 0.04). | Overall AE rates comparable (9.8% LAMS vs. 10.3% DPPS). Severe AEs rare. Stent dysfunction similar (migration 2.9% vs. 6.9%, occlusion 20.6% vs. 12.6%). |
| Study | Design & Population | Comparison | Key Outcomes | Main Findings | Adverse Events (AEs) |
|---|---|---|---|---|---|
| Wan et al. [140] | 8-year prospective cohort, n = 147 (62 EUS-TD, 67 PD) | EUS-TD vs. PD | Clinical success, reintervention, mortality | PP: EUS-TD higher initial success (94.9% vs. 65%, p = 0.003); fewer reinterventions (2.6% vs. 35%, p = 0.004). WON: initial success higher with EUS-TD (65.2% vs. 17%, p < 0.001). | PD had higher AEs (85.1% vs. 26.1%). Residual necrosis only in PD group. Mortality lower with EUS-TD (3 vs. 6 deaths). |
| Keane et al. [141] | 14-year cohort, n = 164 (109 EUS-TD, 55 PD) | EUS-TD vs. PD | Treatment success, LOS, reintervention, residual collections | Treatment success higher with EUS-TD (70% vs. 31%, p < 0.001). PD needed more interventions (3.3 vs. 1.8), more residual collections (67% vs. 21%), more surgical rescue (11% vs. 4%). LOS shorter with EUS-TD (median 4 days). | Procedural AEs slightly higher in EUS-TD (10% vs. 1%) but outweighed by long-term benefits. |
| Samanta et al. [142] | Real-world cohort, India, n = 218 WON | EUS-TD vs. PD | Clinical success, impact of solid component | Clinical success higher with EUS-TD (92.1% vs. 64.6%, p < 0.0001). PD outcomes worse when >40% solid debris; EUS-TD best when <40%. | AE rates not detailed; safety of EUS-TD emphasized. |
| Khizar et al. [143] | Meta-analysis, 17 studies, n = 1170 (543 EUS-TD, 627 PD) | Pooled EUS-TD vs. PD | Clinical success, technical success, reintervention, LOS, mortality | Technical success similar. Clinical success favored EUS-TD (OR 2.23), especially WON (OR 2.74). LOS shorter (–15 days). Reinterventions fewer (OR 0.25). Mortality lower (OR 0.24). | AE rates lower with EUS-TD across pooled studies. |
| Bakker et al. [144] | RCT, n = 22 | Endoscopic transgastric necrosectomy vs. surgical necrosectomy | IL-6, major complications/death | Composite endpoint lower with endoscopy (20% vs. 80%). Lower systemic inflammation. | Endoscopy prevented new-onset organ failure (0% vs. 50%) and reduced fistulas (10% vs. 70%). |
| Bang et al. [145] | RCT, single center, n = 66 | ESU vs. minimally invasive SSU | Major complications/death, QoL, costs | ESU superior (11.8% vs. 40.6%, p = 0.007). Better QoL, lower costs. | Fewer complications with endoscopy; no fistulas vs. 28.1% in surgery. |
| TENSION trial [146] | RCT, multicenter, n = 98 | ESU vs. SSU | Death/major complications (6 mo) | No difference in primary endpoint (43% vs. 45%). Endoscopy: shorter LOS, fewer ICU days, fewer fistulas. | Pancreatic fistulas fewer with endoscopy; mortality similar. |
| ExTENSION study [137] | Long-term follow-up of TENSION survivors, n = 83, 7-year follow-up | ESU vs. SSU | Death/major complications, reinterventions, pancreatic function | Primary endpoint similar (53% vs. 57%). Endoscopy: fewer fistulas (8% vs. 34%), fewer late reinterventions (7% vs. 24%), lower recurrent pancreatitis (19% vs. 37%). | Pancreatic insufficiency similar (≈40–60%). Fewer long-term complications with endoscopy. |
| Tan et al. [147] | Network meta-analysis, 21 studies, n = 1850 | Endoscopic necrosectomy vs. step-up vs. surgical | Mortality, complications, organ failure, cost-effectiveness | Endoscopic necrosectomy ranked best across most outcomes; upfront open necrosectomy worst. Recovery faster (–34.1 days). | Lowest complication and fistula rates with endoscopy; economic benefit favored endoscopy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Anna, G.; Lavalle, S.; Biamonte, P.; Fanizza, J.; Masiello, E.; Bruni, A.; Mandarino, F.V.; Preatoni, P.; Azzolini, F.; Dhar, J.; et al. Clinical, Radiological, and Endoscopic Features of Pancreatic Pseudocyst and Walled-Off Necrosis: How to Diagnose and How to Drain Them. J. Clin. Med. 2025, 14, 7818. https://doi.org/10.3390/jcm14217818
Dell’Anna G, Lavalle S, Biamonte P, Fanizza J, Masiello E, Bruni A, Mandarino FV, Preatoni P, Azzolini F, Dhar J, et al. Clinical, Radiological, and Endoscopic Features of Pancreatic Pseudocyst and Walled-Off Necrosis: How to Diagnose and How to Drain Them. Journal of Clinical Medicine. 2025; 14(21):7818. https://doi.org/10.3390/jcm14217818
Chicago/Turabian StyleDell’Anna, Giuseppe, Salvatore Lavalle, Paolo Biamonte, Jacopo Fanizza, Edoardo Masiello, Angelo Bruni, Francesco Vito Mandarino, Paoletta Preatoni, Francesco Azzolini, Jahnvi Dhar, and et al. 2025. "Clinical, Radiological, and Endoscopic Features of Pancreatic Pseudocyst and Walled-Off Necrosis: How to Diagnose and How to Drain Them" Journal of Clinical Medicine 14, no. 21: 7818. https://doi.org/10.3390/jcm14217818
APA StyleDell’Anna, G., Lavalle, S., Biamonte, P., Fanizza, J., Masiello, E., Bruni, A., Mandarino, F. V., Preatoni, P., Azzolini, F., Dhar, J., Samanta, J., Facciorusso, A., Stasi, E., Brigida, M., Dell’Anna, A., Spampinato, M., Maida, M., Massironi, S., Annese, V., ... Danese, S. (2025). Clinical, Radiological, and Endoscopic Features of Pancreatic Pseudocyst and Walled-Off Necrosis: How to Diagnose and How to Drain Them. Journal of Clinical Medicine, 14(21), 7818. https://doi.org/10.3390/jcm14217818










