Routine Chest X-Rays in Critical Bronchiolitis Do Not Improve Outcomes
Abstract
1. Introduction
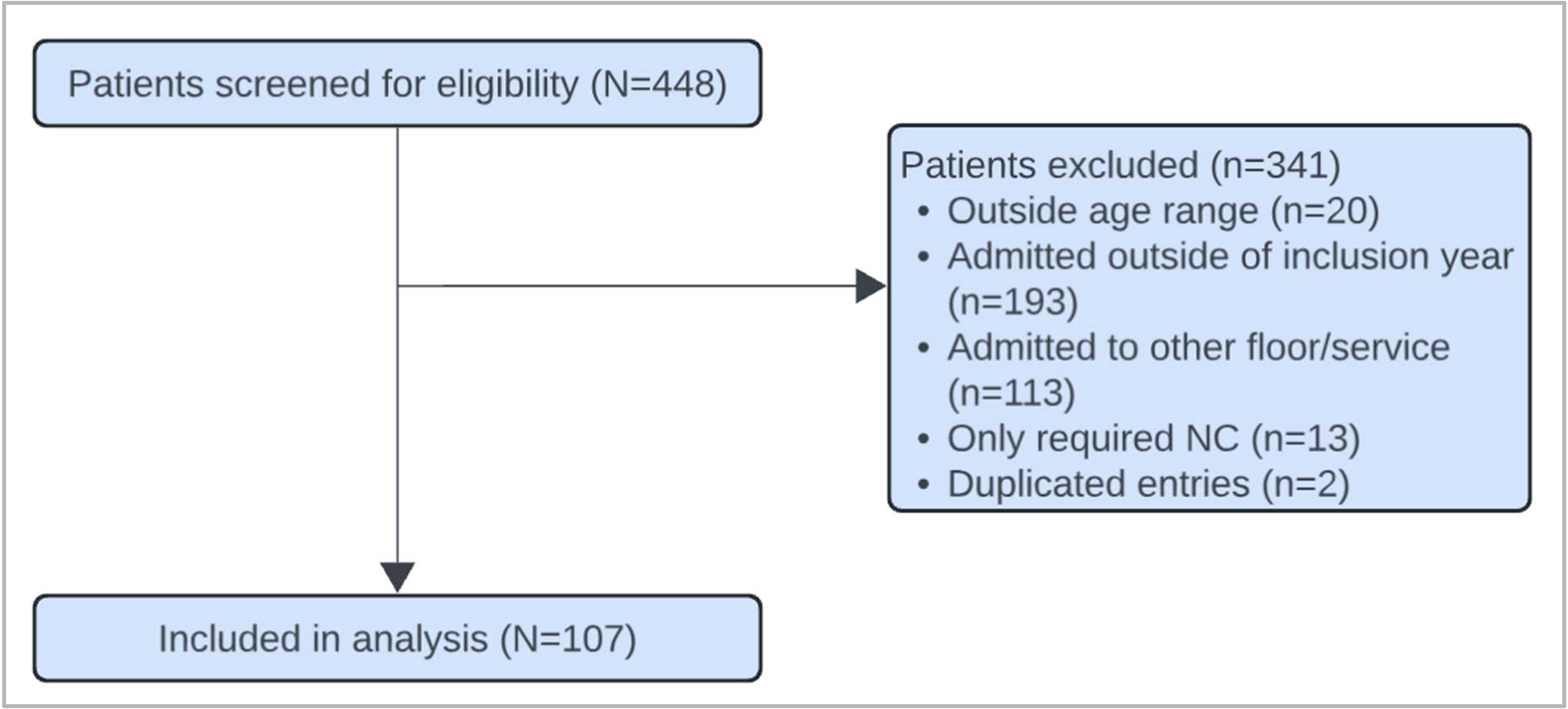
2. Materials and Methods
2.1. Study Overview
2.2. Outcome Measure
2.3. Data Collection
2.4. Statistical Analysis
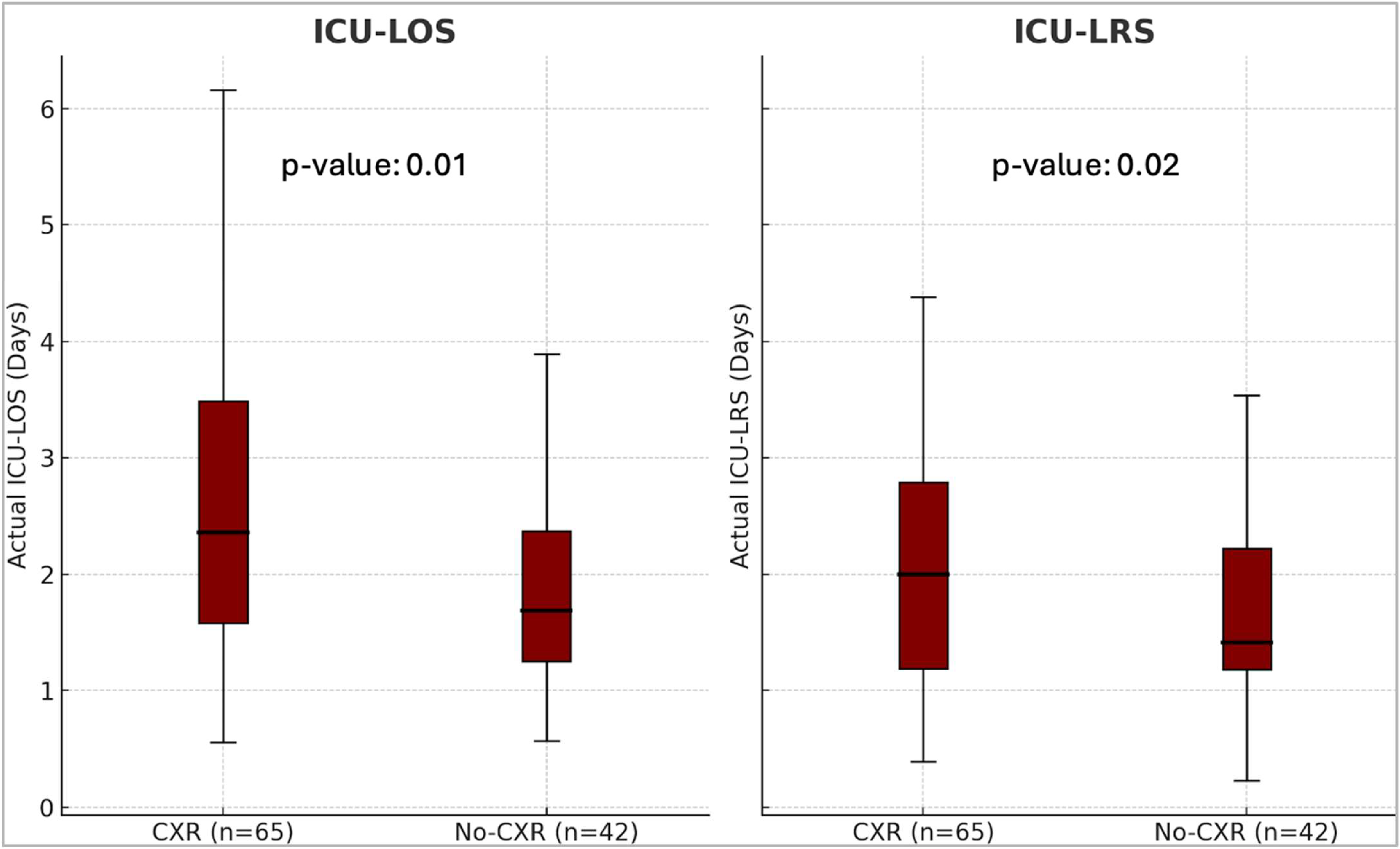
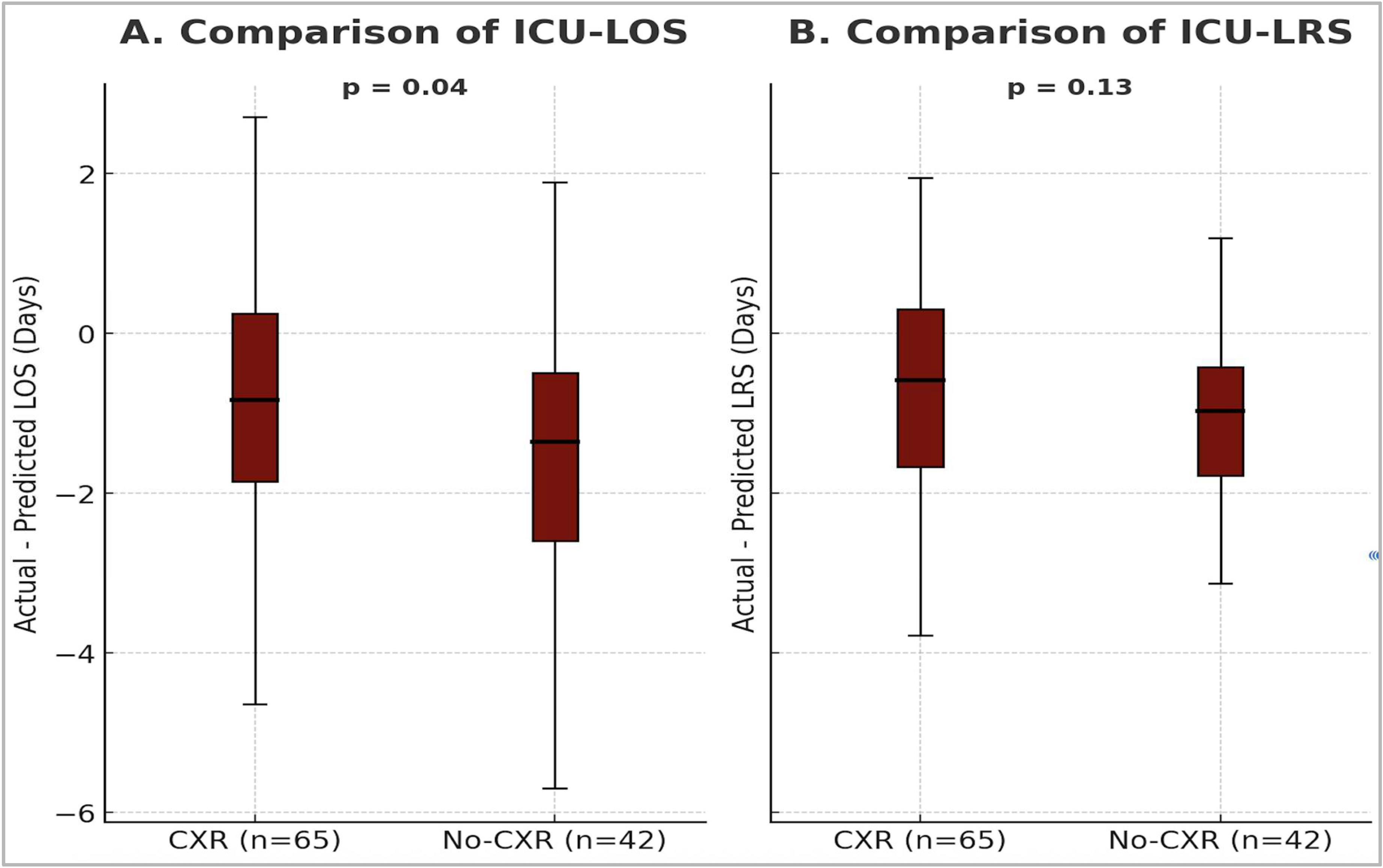
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PICU | Pediatric intensive care unit |
| SDICU | Step-down intensive care unit |
| PCTICU | Pediatric cardiac intensive care unit |
| ICU | Intensive care unit |
| LOS | Length of stay |
| CBS | Critical Bronchiolitis Score |
| LRS | Level of respiratory support |
| HR | Heart rate |
| RR | Respiratory rate |
| GCS | Glasgow Coma Scale |
| pCO2 | Partial pressure of carbon dioxide |
| SBP | Systolic blood pressure |
| BUN | Blood urea nitrogen |
| HFNC | High-flow nasal cannula |
| NIMV | Non-invasive mechanical ventilation |
| IMV | Invasive mechanical ventilation |
| CXR | Chest X-ray |
| IQR | Interquartile range |
| AAP | American Academy of Pediatrics |
Appendix A
| Variable | Units | Concept |
|---|---|---|
| Demographic data | - | Patient characteristics |
| Past medical history | - | Medical conditions prior to admission |
| Past surgical history | - | Surgical procedures prior to admission |
| Past family history of atopy in a first-degree relative | - | Family predisposition to atopic conditions |
| Home-oxygen use | Yes/No | Pre-admission oxygen therapy at home |
| Day-of-illness on presentation | Days | Duration of illness before presentation |
| Month and year of presentation | Month/Year | Temporal factors of admission |
| Presence of viral infection | Names of virus identified | Confirmed viral etiology |
| ICU-LOS | Days | Length of ICU stay |
| Hospital LOS | Days | Length of hospital stay |
| HFNC use | Yes/No, L/Kg, Days | Duration of respiratory support |
| NIMV use (including nCPAP) | Yes/No, Highest PIP/PEEP/RR/FiO2, Days | Duration of respiratory support |
| IMV use (including HFOV) | Yes/No, Highest PIP/PEEP/MAP/RR/FiO2, Days | Duration of respiratory support |
| CXR use | Yes/No | Diagnostic modality |
| Other imaging- US, Echocardiography, CT | Yes/No | Diagnostics |
| Antibiotic use | Yes/No | Clinical management |
| Nebulizations—Albuterol, 3%, racemic epinephrine | Yes/No | Adjunctive therapies |
| Highest RR | Breaths per minute | Vital signs |
| Highest HR | Beats per minute | Vital signs |
| Highest temperature | °C or °F | Vital signs |
| Worst GCS | GCS score | Clinical decision tool |
| Lowest serum bicarbonate | mmol/L | Measure of dehydration |
| Lowest pH/highest pCO2 ratio | pH, mmHg | Measure of respiratory acidosis |
| Highest BUN | mg/dL | Measure of dehydration |
| Lowest SBP | mmHg | Vital signs |
References
- Florin, T.A.; Plint, A.C.; Zorc, J.J. Viral bronchiolitis. Lancet 2017, 389, 211–224. [Google Scholar] [CrossRef]
- Manti, S.; Staiano, A.; Orfeo, L.; Midulla, F.; Marseglia, G.L.; Ghizzi, C.; Zampogna, S.; Carnielli, V.P.; Favilli, S.; Ruggieri, M.; et al. UPDATE-2022 Italian guidelines on the management of bronchiolitis in infants. Ital. J. Pediatr. 2023, 49, 19. [Google Scholar] [CrossRef] [PubMed]
- Borland, M.L.; Loveys, K.; Babl, F.E.; Cotterell, E.; Haskell, L.; O’Brien, S.; Oakley, E.; Wilson, C.L.; Alsweiler, J.; Armstrong, D.; et al. Australasian Bronchiolitis Guideline: 2025 Update. J. Paediatr. Child Health 2025, 61, 1197–1215. [Google Scholar] [CrossRef] [PubMed]
- Zurca, A.D.; González-Dambrauskas, S.; Colleti, J.; Vasquez-Hoyos, P.; Prata-Barbosa, A.; Boothe, D.; Combs, B.E.; Lee, J.H.; Franklin, D.; Pon, S.; et al. Intensivists’ Reported Management of Critical Bronchiolitis: More Data and New Guidelines Needed. Hosp. Pediatr. 2023, 13, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Almadani, A.; Noël, K.C.; Aljassim, N.; Maratta, C.; Tam, I.; Papenburg, J.; Quach, C.; Thampi, N.; McNally, J.D.; Lefebvre, M.A.; et al. Bronchiolitis Management and Unnecessary Antibiotic Use Across 3 Canadian PICUs. Hosp. Pediatr. 2022, 12, 369–382. [Google Scholar] [CrossRef]
- Yong, J.H.E.; Schuh, S.; Rashidi, R.; Vanderby, S.; Lau, R.; Laporte, A.; Nauenberg, E.; Ungar, W.J. A cost effectiveness analysis of omitting radiography in diagnosis of acute bronchiolitis. Pediatr. Pulmonol. 2009, 44, 122–127. [Google Scholar] [CrossRef]
- Hasegawa, K.; Mansbach, J.M.; Bochkov, Y.A.; Gern, J.E.; Piedra, P.A.; Bauer, C.S.; Teach, S.J.; Wu, S.; Sullivan, A.F.; Camargo, C.A.; et al. Association of Rhinovirus C Bronchiolitis and Immunoglobulin E Sensitization During Infancy with Development of Recurrent Wheeze. JAMA Pediatr. 2019, 173, 544. [Google Scholar] [CrossRef]
- Ralston, S.L.; Lieberthal, A.S.; Meissner, H.C.; Alverson, B.K.; Baley, J.E.; Gadomski, A.M.; Johnson, D.W.; Light, M.J.; Maraqa, N.F.; Mendonca, E.A.; et al. Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis. Pediatrics 2014, 134, e1474–e1502. [Google Scholar] [CrossRef]
- Reyes, M.A.; Etinger, V.; Hronek, C.; Hall, M.; Davidson, A.; Mangione-Smith, R.; Kaiser, S.V.; Parikh, K. Pediatric Respiratory Illnesses: An Update on Achievable Benchmarks of Care. Pediatrics 2023, 152, e2022058389. [Google Scholar] [CrossRef]
- Quinonez, R.A.; Garber, M.D.; Schroeder, A.R.; Alverson, B.K.; Nickel, W.; Goldstein, J.; Bennett, J.S.; Fine, B.R.; Hartzog, T.H.; McLean, H.S.; et al. Choosing wisely in pediatric hospital medicine: Five opportunities for improved healthcare value. J. Hosp. Med. 2013, 8, 479–485. [Google Scholar] [CrossRef]
- Lawrence, J.; Hiscock, H.; Voskoboynik, A.; Walpola, R.; Sharma, A. Reducing unwarranted chest x-rays in bronchiolitis: Importance of a robust analysis. J. Paediatr. Child Health 2024, 60, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Dalziel, S.R.; Haskell, L.; O’Brien, S.; Borland, M.L.; Plint, A.C.; Babl, F.E.; Oakley, E. Bronchiolitis. Lancet 2022, 400, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Mount, M.C.; Ji, X.; Kattan, M.W.; Slain, K.N.; Clayton, J.A.; Rotta, A.T.; Shein, S.L. Derivation and Validation of the Critical Bronchiolitis Score for the PICU. Pediatr. Crit. Care Med. 2022, 23, e45–e54. [Google Scholar] [CrossRef] [PubMed]
- Wrotek, A.; Czajkowska, M.; Jackowska, T. Chest Radiography in Children Hospitalized with Bronchiolitis. Adv. Exp. Med. Biol. 2019, 1222, 55–62. [Google Scholar] [CrossRef]
- Nazif, J.M.; Taragin, B.H.; Azzarone, G.; Rinke, M.L.; Liewehr, S.; Choi, J.; Esteban-Cruciani, N. Clinical Factors Associated with Chest Imaging Findings in Hospitalized Infants with Bronchiolitis. Clin. Pediatr. 2017, 56, 1054–1059. [Google Scholar] [CrossRef]
- Christakis, D.A.; Cowan, C.A.; Garrison, M.M.; Molteni, R.; Marcuse, E.; Zerr, D.M. Variation in Inpatient Diagnostic Testing and Management of Bronchiolitis. Pediatrics 2005, 115, 878–884. [Google Scholar] [CrossRef]
- Ortmann, L.A.; Nabower, A.; Cullimore, M.L.; Kerns, E. Antibiotic use in nonintubated children with bronchiolitis in the intensive care unit. Pediatr. Pulmonol. 2023, 58, 804–810. [Google Scholar] [CrossRef]
- Friedman, J.N.; Rieder, M.J.; Walton, J.M.; Canadian Paediatric Society; Acute Care Committee; Drug Therapy and Hazardous Substances Committee. Bronchiolitis: Recommendations for diagnosis, monitoring and management of children one to 24 months of age. Paediatr. Child Health 2014, 19, 485–498. [Google Scholar] [CrossRef]
- Hunter, B.M.; Castiglioni, C.; Nellis, A.B.; Wood, A.R.; Giblin, B.; Malakooti, M.; Stephen, R.J. Improving Length of Stay by Reducing High-Flow Nasal Cannula Duration in Respiratory Illnesses. Hosp. Pediatr. 2025, 15, 195–203. [Google Scholar] [CrossRef]
- Byrd, C.; Noelck, M.; Kerns, E.; Bryan, M.; Hamline, M.; Garber, M.; Ostrow, O.; Riss, V.; Shadman, K.; Shein, S.; et al. Multicenter Quality Collaborative to Reduce Overuse of High-Flow Nasal Cannula in Bronchiolitis. Pediatrics 2024, 153, e2023063509. [Google Scholar] [CrossRef]
- Chao, J.H.; Lin, R.C.-J.; Marneni, S.; Pandya, S.; Alhajri, S.; Sinert, R. Predictors of Airspace Disease on Chest X-ray in Emergency Department Patients with Clinical Bronchiolitis: A Systematic Review and Meta-analysis. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2016, 23, 1107–1118. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef]
| Demographics | Group | CXR | No CXR | p-Value 1 |
|---|---|---|---|---|
| N | 65 | 42 | ||
| Gender | F | 26 (40%) | 17 (40%) | 0.96 |
| M | 39 (60%) | 25 (60%) | ||
| Age on Admission | 12.1 months | 12.0 months | N/A | |
| Ethnicity | Asian | 4 (6%) | 2 (5%) | 0.61 |
| AA | 6 (14%) | 2 (5%) | ||
| Caucasian | 15 (23%) | 15 (36%) | ||
| Hispanic | 40 (62%) | 22 (52%) | ||
| Other | 1 (2%) | 1 (2%) | ||
| PMHx | Prematurity | 21 (32%) | 10 (24%) | 0.51 |
| BPD | 1 (2%) | 1 (2%) | 1 | |
| Atopy | 9 (14%) | 10 (24%) | 0.3 | |
| CHD | 8 (12%) | 4 (10%) | 0.9 | |
| Day of Illness | <3 days | 38 (58%) | 24 (57%) | 1 |
| >3 days | 27 (42%) | 18 (43%) | ||
| Location of Admission | PICU | 39 (60%) | 29 (69%) | 0.9 |
| SDICU | 16 (25%) | 7 (17%) | 0.8 | |
| CTICU | 10 (15%) | 6 (14%) | 1 |
| LOS | LRS | |||||
|---|---|---|---|---|---|---|
| CXR Y | CXR N | p-value 1 | CXR Y | CXR N | p-value 1 | |
| Actual | 2.3 (1.6–3.5) | 1.7 (1.2–2.4) | 0.01 | 2.0 (1.2–2.8) | 1.4 (1.2–2.2) | 0.018 |
| Predicted | 3.5 (2.7–4.2) | 3.3 (2.5–4.1) | 0.43 | 2.8 (2.2–3.3) | 2.6 (2.0–3.2) | 0.31 |
| n (%) | Antibiotic (n, %) | No Antibiotic (n, %) | |
|---|---|---|---|
| CXR | 65 (60.7%) | 25 (38.5%) * | 40 (61.5%) |
| CXR—Actionable | 8 (7.5%) | 8 (100%) | 0 (0%) |
| CXR—Questionable | 16 (15%) | 7 (43.8%) | C9 (56.3%) |
| CXR—Expected | 41 (38.3%) | 10 (24.4%) | 31 (75.6%) |
| No CXR | 42 (39.3%) | 3 (7.1%) * | 39 (92.9%) |
| Total (N) | 107 (100%) | 28 (26.2%) | 79 (73.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunderajan, T.; Choi, D.-E.; LaFerla, C.; Guglielmo, R.D.; Chandnani, H.K.; Mount, M.C.; Chawla, H.S.; Giang, M.E. Routine Chest X-Rays in Critical Bronchiolitis Do Not Improve Outcomes. J. Clin. Med. 2025, 14, 7810. https://doi.org/10.3390/jcm14217810
Sunderajan T, Choi D-E, LaFerla C, Guglielmo RD, Chandnani HK, Mount MC, Chawla HS, Giang ME. Routine Chest X-Rays in Critical Bronchiolitis Do Not Improve Outcomes. Journal of Clinical Medicine. 2025; 14(21):7810. https://doi.org/10.3390/jcm14217810
Chicago/Turabian StyleSunderajan, Trisha, Da-Eun (Shira) Choi, Caroline LaFerla, Robert D. Guglielmo, Harsha K. Chandnani, Michael C. Mount, Harmanpreet S. Chawla, and Michael E. Giang. 2025. "Routine Chest X-Rays in Critical Bronchiolitis Do Not Improve Outcomes" Journal of Clinical Medicine 14, no. 21: 7810. https://doi.org/10.3390/jcm14217810
APA StyleSunderajan, T., Choi, D.-E., LaFerla, C., Guglielmo, R. D., Chandnani, H. K., Mount, M. C., Chawla, H. S., & Giang, M. E. (2025). Routine Chest X-Rays in Critical Bronchiolitis Do Not Improve Outcomes. Journal of Clinical Medicine, 14(21), 7810. https://doi.org/10.3390/jcm14217810






