Upadacitinib’s Effectiveness and Safety as a Second- or Third-Line Therapy in Patients with Ulcerative Colitis: Data from a Real-World Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Study Treatment
2.3. Clinical Assessment at Baseline and During the Follow-Up
2.4. Endoscopy Assessment at Baseline and During the Follow-Up
2.5. Outcomes
- -
- Clinical response at week eight and at the end of follow-up, defined as a decrease of at least two points in the PMS compared to baseline [25];
- -
- Steroid-free clinical remission at week 8 and at the end of follow-up, defined as a PMS ≤ 1 and the absence of steroid treatment [25];
- -
- Bowel urgency response, defined as the number of days from the start of UPA to a reduction in or the disappearance of bowel urgency;
- -
- Change in steroid use (defined as the rate of steroid use over the total number of patients in the time window considered) during follow-up;
- -
- UPA optimization, defined as an extension of induction to 16 weeks with UPA 45 mg once daily in case of a lack of clinical remission at week 8, or the chance to use an 8-week course again at 45 mg once daily during the follow-up to re-induce remission;
- -
- Mucosal healing (MH) at the end of follow-up (defined as a Mayo endoscopic subscore ≤ 1) [25];
- -
- Rate of colectomy during follow-up;
- -
- CRP, FC, and PMS variations during follow-up;
- -
- CRP, FC, and PMS short-term variations between 0 and 8 weeks according to the number of previous biological therapies performed;
- -
- Clinical response during follow-up.
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Primary Outcomes
3.3. Secondary Outcomes
3.4. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. Crohns Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohns Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef]
- Ben Horin, S.; Chowers, Y. Review article: Loss of response to anti-TNF treatment in Crohn’s disease. Aliment. Pharmacol. Ther. 2011, 33, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.O.; Armuzzi, A.; Gisbert, J.P.; Bokemeyer, B.; Peyrin-Biroulet, L.; Nguyen, G.C.; Smyth, M.; Patel, H. Indicator of suboptimal tumor necrosis factor antagonist therapy in inflammatory bowel disease. Dig. Liver Dis. 2017, 49, 1086–1091. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Heap, G.A.; Green, H.D.; Hamilton, B.; Bewshea, C.; Walker, G.J.; Thomas, A.; Nice, R.; Perry, M.H.; Bouri, S.; et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: A prospective, multicentre, cohort study. Lancet Gastroenterol. Hepatol. 2019, 4, 341–353. [Google Scholar] [CrossRef]
- Coskun, M.; Vermeire, S.; Nielsen, O.H. Novel Targeted Therapies for Inflammatory Bowel Disease. Trends Pharmacol. Sci. 2017, 38, 127–142. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Kontzias, A.; Yamaoka, K.; Tanaka, Y.; Laurence, A. Janus kinase inhibitors in autoimmune diseases. Ann. Rheum. Dis. 2013, 72 (Suppl. 2), ii111–ii115. [Google Scholar] [CrossRef]
- Nakase, H. Understanding the Efficacy of Individual Janus Kinase Inhibitors in the Treatment of Ulcerative Colitis for Future Positioning in Inflammatory Bowel Disease Treatment. Immunol. Med. 2023, 46, 121–130. [Google Scholar] [CrossRef]
- AbbVie. European Commission Approves RINVOQ® (Upadacitinib) for the Treatment of Adults with Moderate to Severe Ulcerative Colitis. 2022. Available online: https://news.abbvie.com/2022-07-26-European-Commission-Approves-RINVOQ-R-upadacitinib-for-the-Treatment-of-Adults-With-Moderate-to-Severe-Ulcerative-Colitis (accessed on 26 August 2025).
- Danese, S.; Vermeire, S.; Zhou, W.; Pangan, A.L.; Siffledeen, J.; Greenbloom, S.; Hébuterne, X.; D’Haens, G.; Nakase, H.; Panés, J.; et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: Results from three phase 3, multicentre, double-blind, randomised trials. Lancet 2022, 399, 2113–2128, Erratum in Lancet 2022, 400, 996. [Google Scholar] [CrossRef]
- Friedberg, S.; Choi, D.; Hunold, T.; Choi, N.K.; Garcia, N.M.; Picker, E.A.; Cohen, N.A.; Cohen, R.D.; Dalal, S.R.; Pekow, J.; et al. Upadacitinib Is Effective and Safe in Both Ulcerative Colitis and Crohn’s Disease: Prospective Real-World Experience. Clin. Gastroenterol. Hepatol. 2023, 21, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Dalal, R.S.; Kallumkal, G.; Cabral, H.J.; Bachour, S.; Barnes, E.L.; Allegretti, J.R. Clinical and Endoscopic Outcomes After Upadacitinib Induction for Ulcerative Colitis: A Multicenter Retrospective Cohort Study. Inflamm. Bowel Dis. 2024, 30, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Shimizu, H.; Fujii, T.; Kawamotoorcid, A.; Morikawaorcid, R.; Hibiyaorcid, S.; Takenakaorcid, K.; Nagahoriorcid, M.; Ohtsukaorcid, K.; Okamotoorcid, R. Comparative short-term efficacy of Upadacitinib versus Tofacitinib for ulcerative colitis: A 24-weekreal-world study in Japan. Intest. Res. 2025, 10, 180–186. [Google Scholar] [CrossRef]
- Levine, J.; McKibbin, J.; Ham, R.; Cohen-Mekelburg, S.; Bishu, S.; Tang, K.; Higgins, P.D.R.; Berinstein, J.A. Use of Upadacitinib in 16 Tofacitinib-refractory Ulcerative Colitis Patients: A Single-center Case 2 Series. Inflamm. Bowel Dis. 2024, 30, 2232–2235. [Google Scholar] [CrossRef]
- Hosomi, S.; Nishida, Y.; Fujiwara, Y. Efficacy of Upadacitinib As a Second-line JAK Inhibitor in Ulcerative Colitis: A Case Series. Intern. Med. 2024, 63, 1882–1885. [Google Scholar] [CrossRef]
- Dalal, R.S.; Kallumkal, G.; Cabral, H.J.; Bachour, S.; Barnes, E.L.; Allegretti, J.R. Comparative Effectiveness of Upadacitinib vs Ustekinumab for Ulcerative Colitis: A Multicenter Retrospective Cohort Study. Clin. Gastroenterol. Hepatol. 2024, 22, 666–668. [Google Scholar] [CrossRef]
- Kochhar, G.S.; Khataniar, H.; Jairath, V.; Farraye, F.A.; Desai, A. Comparative Effectiveness of Upadacitinib and Tofacitinib in Ulcerative Colitis: A US Propensity-Matched Cohort Study. Am. J. Gastroenterol. 2024, 119, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Boneschansker, L.; Ananthakrishnan, A.N.; Massachusetts General Hospital Crohn’s and Colitis Center Collaborators. Comparative effectiveness of upadacitinib and tofacitinib in inducing remission in ulcerative colitis: Real-world data. Clin. Gastroenterol. Hepatol. 2023, 21, 2427–2429.e1. [Google Scholar] [CrossRef]
- Dalal, R.S.; Kallumkal, G.; Cabral, H.J.; Barnes, E.L.; Allegretti, J.R. One-year comparative effectiveness of upadacitinib vs tofacitinib for ulcerative colitis: A multicenter cohort study. Am. J. Gastroenterol. 2024, 119, 1628–1631. [Google Scholar] [CrossRef]
- García, M.J.; Brenes, Y.; Vicuña, M.; Bermejo, F.; Sierra-Ausín, M.; Vicente, R.; Arroyo, M.T.; Montiel, P.M.; Villoria, A.; Ferrer, J.Á.; et al. Persistence, Effectiveness, and Safety of Upadacitinib in Crohn’s Disease and Ulcerative Colitis in Real Life: Results From a Spanish Nationwide Study (Ureal Study). Am. J. Gastroenterol. 2024, 120, 1593–1604. [Google Scholar] [CrossRef]
- Farkas, B.; Resal, T.; Lakatos, P.L.; Bessissow, T.; Limdi, J.K.; Armuzzi, A.; Bezzio, C.; Savarino, E.V.; Saibeni, S.; Michalopoulos, G.; et al. Upadacitinib is associate with better clinical and biochemical outcomes than tofacitinib in refractory, moderate-to-severe UC. Clin. Gastroenterol. Hepatol. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Agenzia Italiana del Farmaco. Regime di rimborsabilità e prezzo, a seguito di nuove indicazioni terapeutiche, del medicinale per uso umano “Rinvoq”. Gazz. Uff. Della Repubb. Ital. 2023, 58, 51–56. [Google Scholar]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J.F. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. N. Eng. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’hAens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Kishi, M.; Hirai, F.; Takatsu, N.; Hisabe, T.; Takada, Y.; Beppu, T.; Takeuchi, K.; Naganuma, M.; Ohtsuka, K.; Watanabe, K.; et al. A review on the current status and definitions of activity indices in inflammatory bowel disease: How to use indices for precise evaluation. J. Gastroenterol. 2022, 57, 246–266. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.; Hernandez-Rocha, C.; Duijvestein, M.; Faubion, W.; McGovern, D.; Vermeire, S.; Vetrano, S.; Casteele, N.V. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 323–333. [Google Scholar] [CrossRef]
- Shehab, M.; Alrashed, F.; Alsayegh, A.; Aldallal, U.; Ma, C.; Narula, N.; Jairath, V.; Singh, S.; Bessissow, T. Comparative Efficacy of Biologics and Small Molecule in Ulcerative Colitis: A Systematic Review and Network Meta-analysis. Clin. Gastroenterol. Hepatol. 2025, 23, 250–262. [Google Scholar] [CrossRef]
- Gilmore, R.; Fernandes, R.; Hartley, I.; Arzivian, A.; Leong, R.; Andrew, B.; Vasudevan, A.; Greeve, T.; Moore, G.T.; Kim, S.; et al. Upadacitinib induction is effective and safe in ulcerative colitis patients including those with prior exposure to Tofacitnib: A multicenter real-world cohort study. Intest. Res. 2024, 23, 347–357. [Google Scholar] [CrossRef]
- Taxonera, C.; García-Brenes, M.A.; Machín, M.; Olivares, D.; López-García, O.N.; Zapater, R.; Alba, C. Real-World Effectiveness and Safety of Upadacitinib in Patients with Ulcerative Colitis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 2232. [Google Scholar] [CrossRef]
- Farkas, B.; Bessisso, T.; Limdi, J.K.; Sethi-Arora, K.; Kagramanova, A.; Knyazev, O.; Bezzio, C.; Armuzzi, A.; Lukas, M.; Michalopoulos, G.; et al. Real-World Effectiveness and Safety of Selective JAK Inhibitors in Ulcerative Colitis and Crohn’s Disease: A Retrospective, Multicentre Study. J. Clin. Med. 2024, 13, 7804. [Google Scholar] [CrossRef]
- Akiyama, S.; Shimizu, H.; Tamura, A.; Yokoyama, K.; Sakurai, T.; Kobayashi, M.; Eizuka, M.; Yanai, S.; Nomura, K.; Shibuya, T.; et al. Comparative Efficacy and Safety of Three Janus Kinase Inhibitors in Ulcerative Colitis: A Real-World Multicentre Study in Japan. Aliment. Pharmacol. Ther. 2025, 61, 524–537. [Google Scholar] [CrossRef]
- Tursi, A.; Mocci, G.; Cingolani, L.; Savarino, E.; Pica, R.; Cocco, A.; Zippi, M.; Napolitano, D.; Schiavoni, E.; Pugliese, D.; et al. Use of tofacitinib as first or second-line therapy is associated with better outcomes in patients with ulcerative colitis: Data from a real-world study. Expert Opin. Pharmacother. 2023, 24, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Mocci, G.; Costa, F.; Ceccarelli, L.; Savarino, E.; De Barba, C.; Mucherino, C.; D’antonio, E.; Montesano, L.; Marzo, M.; et al. Filgotinib effectiveness and safety as second or third-line therapy in patients with ulcerative colitis: Data from a real-world study. Intest. Res. 2025, in press. [CrossRef] [PubMed]
- Lee, H.H.; Solitano, V.; Singh, S.; Ananthakrishnan, A.N.; Jairath, V.; Syal, G.; Boland, B.S.; Ghosh, P.; Chang, J.T.; Singh, S. Differential Efficacy of Advanced Therapies in Inducing Remission in Ulcerative Colitis Based on Prior Exposure to TNF Antagonists. Clin. Gastroenterol. Hepatol. 2024, 23, 2102–2114.e5. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V.; Colombel, J.F.; Takeuchi, K.; Gao, X.; Panaccione, R.; Danese, S.; Dubinsky, M.; Schreiber, S.; Ilo, D.; Finney-Hayward, T.; et al. Upadacitinib therapy reduces ulcerative colitis symptoms as early as day 1 of induction treatment. Clin. Gastroenterol. Hepatol. 2023, 21, 2347–2358. [Google Scholar] [CrossRef]
- Panaccione, R.; Lichtenstein, G.; Nakase, H.; Armuzzi, A.; Kucharzik, T.; Levy, G.; Palac, H.; Kujawski, M.; Klaff, J.; Cheon, J.H. Safety of upadacitinib in ulcerative colitis: Long-term data from the phase 3 open-label extension study (U-ACTIVATE). Gastroenterology 2023, 164, S-1100. [Google Scholar] [CrossRef]



| Male sex | 119 (58.9) |
| Median (IQR) age at diagnosis, years | 42.7 (29.8–51.7) |
| Median (IQR) disease duration prior to upadacinitib use, years | 8 (4 to 15) |
| Current smokers | 33 (16.3) |
| Presence of comorbidities | 96 (47.5) |
| Appendectomy | 7 (3.5) |
| Previous surgery | 8 (4.0) |
| Median (IQR) Mayo score | 6 (5–7) |
| Median (IQR) Mayo endoscopic score | 3 (2–3) |
| Extent of disease: | |
| Proctitis (E1) | 7 (3.5) |
| Left-sided colitis (distal colitis included, E2) | 75 (37.1) |
| Pancolitis (E3) | 120 (59.4) |
| Previous conventional therapy | |
| Mesalazine | 200 (99.2) |
| Steroids | 192 (95.1) |
| Tiopurine | 63 (31.2) |
| Indications for therapy with upadacinitib: | |
| Steroid-dependent/-resistant (first line) | 31 (15.3) |
| Failure of anti-TNFα treatment (second line) | 43 (21.3) |
| Failure of anti-TNFα plus anti-integrin or anti-IL12/23 (third line) | 128 (63.4) |
| Prior advanced drug therapy | |
| Infliximab | 161 (79.7%) |
| Adalimumab/Golimumab | 60 (29.7%)/18 (8.9%) |
| Vedolizumab | 82 (40.6%) |
| Ustekinumab | 46 (22.8%) |
| Tofacitinib | 47 (23.3%) |
| Filgotinib | 6 (3%) |
| Concomitant therapy | |
| Mesalazine | 197 (97.5) |
| Steroids | 40 (19.8) |
| Thiopurine | 2 (1.1) |
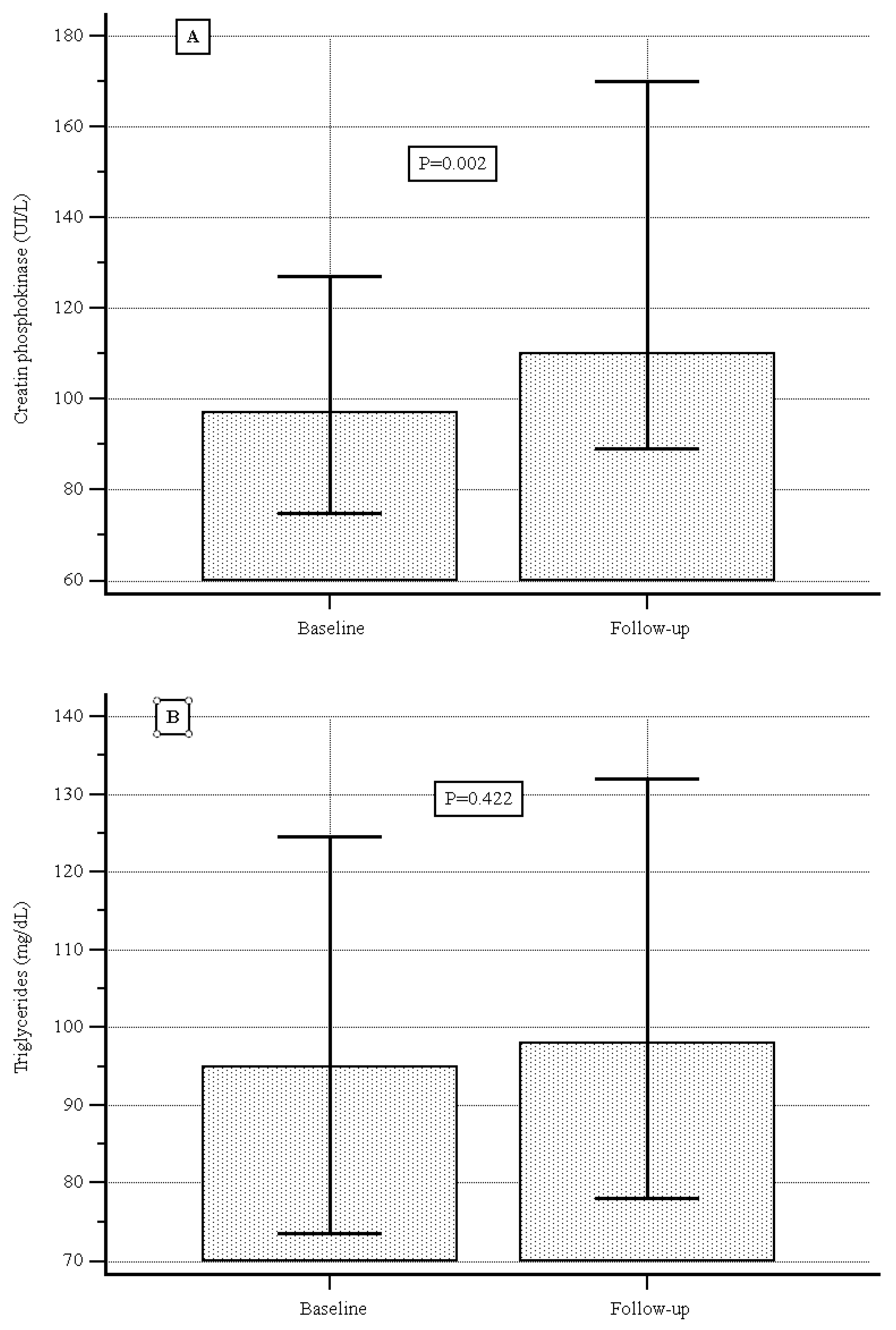
| Median (IQR) creatine phosphokinase level (UI/L) | 97.0 (74.7–127.0) |
| Median (IQR) cholesterol level (mg/dL) | 177.0 (153.0–199.5) |
| Median (IQR) LDL level (mg/dL) | 99.0 (67.0–111.5) |
| Median (IQR) triglyceride level (mg/dL) | 95.0 (73.5–124.5) |
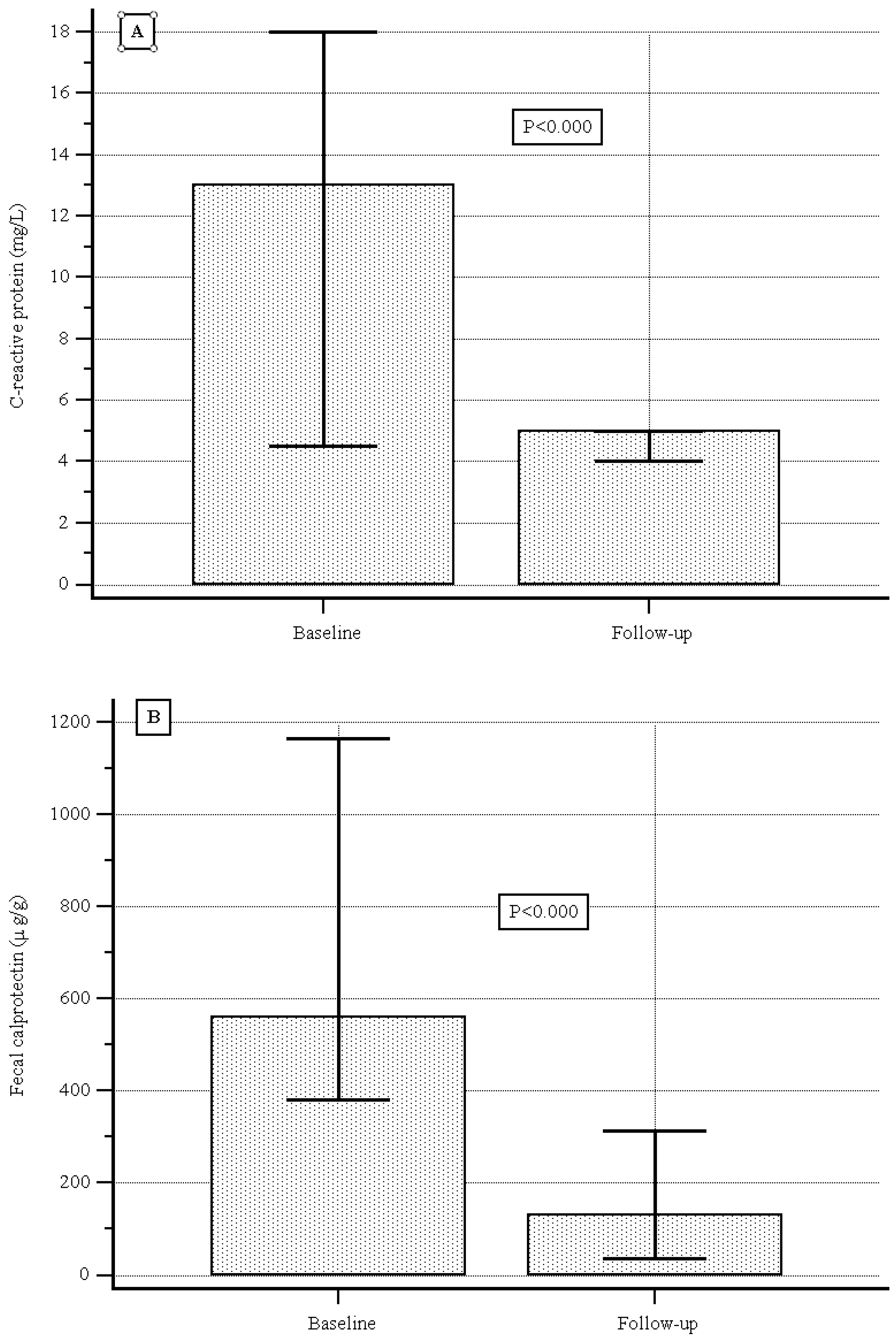
| Median (IQR) CRP (mg/L) | 13.0 (4.5–18.0) |
| Median (IQR) calprotectin (µg/g) | 560 (380.0–1166.0) |
| Remission | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Variables | Yes | No | HR (95% CI) | p | HR (95% CI) | p |
| Sex | ||||||
| Male | 71 (59.7) | 48 (40.3) | Ref. | . | ||
| Female | 51 (61.5) | 32 (38.5) | 0.97 (0.68–1.39) | 0.842 | 0.98 (0.68–1.42) | 0.921 |
| Age | ||||||
| ≤40 yrs | 55 (60.9) | 36 (39.1) | Ref. | - | ||
| >40 yrs | 66 (60.0) | 44 (40.0) | 1.02 (0.72–1.46) | 0.881 | 1.04 (0.69–1.57) | 0.843 |
| Disease duration | ||||||
| ≤8 yrs | 62 (57.4) | 46 (42.6) | Ref. | |||
| >8 yrs | 60 (63.8) | 34 (36.2) | 0.98 (0.69–1.40) | 0.901 | 0.89 (0.59–1.35) | 0.578 |
| Smoking | ||||||
| No | 101 (59.8) | 68 (40.2) | Ref. | - | ||
| Yes | 21 (63.6) | 12 (36.4) | 0.86(0.53–1.42) | 0.476 | 1.16 (0.71–1.90) | 0.540 |
| Comorbidities | ||||||
| No | 66 (62.3) | 40 (37.7) | Ref. | - | ||
| Yes | 56 (58.3) | 40 (41.7) | 1.22 (0.86–1.74) | 0.185 | 0.84 (0.58–1.22) | 0.358 |
| Extent of the disease | ||||||
| Proctitis/left-side colitis (E1/E2) | 46 (56.1) | 36 (43.9) | Ref. | |||
| Pancolitis (E3) | 76 (63.3) | 44 (36.7) | 1.03 (0.71–1.48) | 0.863 | 0.96 (0.64–1.42) | 0.837 |
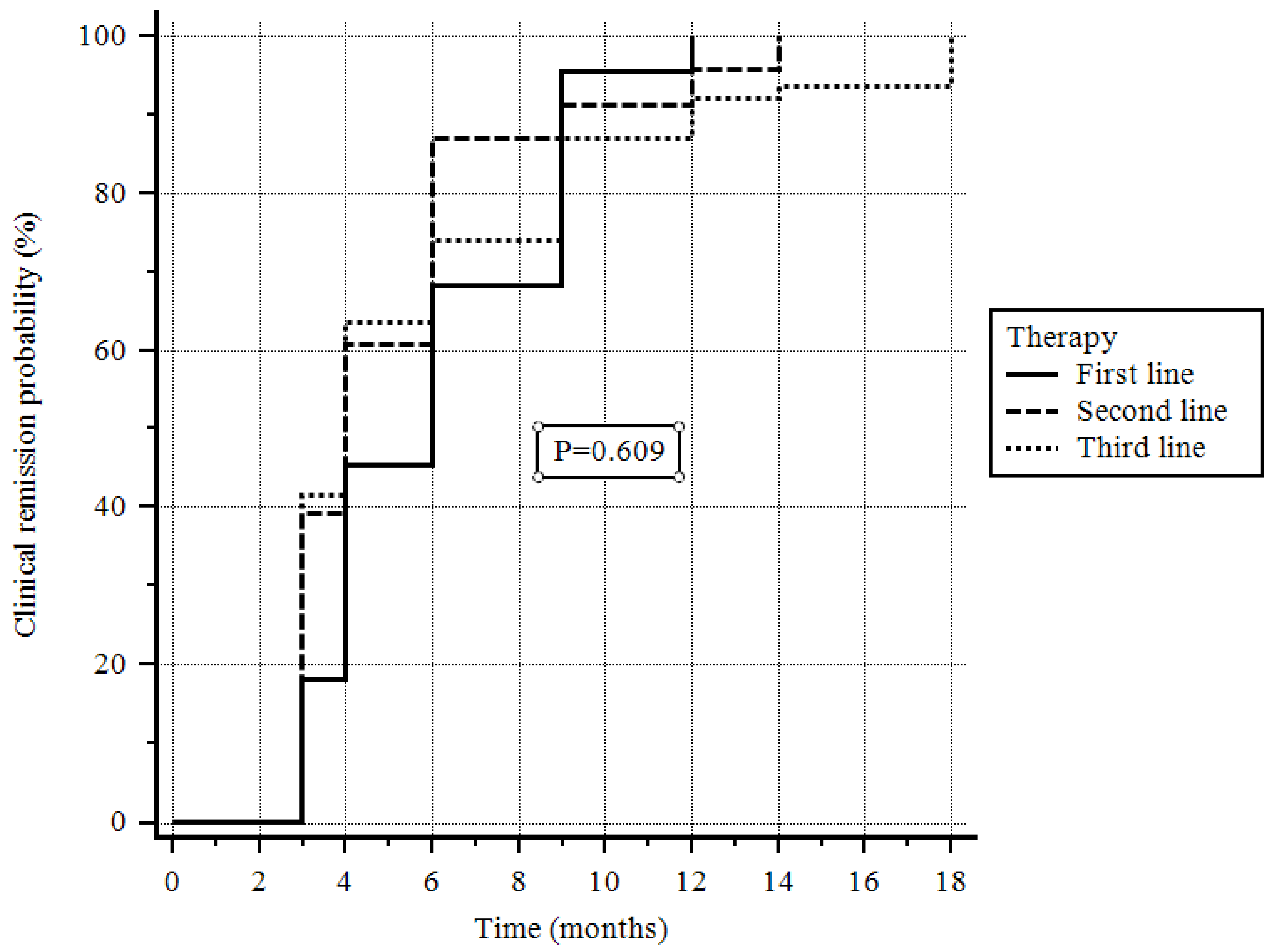
| Line of treatment | ||||||
| First line | 45 (60.8) | 29 (38.1) | Ref. | - | ||
| Second/Third line | 77 (60.2) | 51 (39.8) | 1.06 (0.73–1.54) | 0.707 | 0.98 (0.64–1.50) | 0.926 |
| Partial Mayo score (PMS) | ||||||
| ≤6 | 79 (71.8) | 31 (28.2) | Ref. | - | ||
| >6 | 43 (46.7) | 49 (53.3) | 0.54 (0.38–0.77) | 0.000 | 0.57 (0.38–0.87) | 0.008 |
| Mayo endoscopic subscore | ||||||
| ≤2 | 68 (68.7) | 31 (31.3) | Ref. | - | ||
| >2 | 54 (52.4) | 49 (47.2) | 0.71 (0.50–1.02) | 0.026 | 0.88 (0.60 to 1.32) | 0.558 |
| Clinical remission at 2 months | ||||||
| No | 38 (34.5) | 72 (65.5) | Ref. | - | ||
| Yes | 84 (91.3) | 8 (8.7) | 2.91 (2.04–4.17) | 0.000 | 2.95 (1.96–4.47) | 0.000 |
| Total N (%) | |
|---|---|
| Total AEs | 6 (2.5) |
| Mild–Moderate AE | 4 (80) |
| 1 |
| 1 |
| 1 |
| 1 |
| Severe AE | 2 (20) |
| 1 |
| 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mocci, G.; Tursi, A.; Scaldaferri, F.; Napolitano, D.; Pugliese, D.; Maconi, G.; Cataletti, G.; Pica, R.; Cassieri, C.; Savarino, E.V.; et al. Upadacitinib’s Effectiveness and Safety as a Second- or Third-Line Therapy in Patients with Ulcerative Colitis: Data from a Real-World Study. J. Clin. Med. 2025, 14, 7801. https://doi.org/10.3390/jcm14217801
Mocci G, Tursi A, Scaldaferri F, Napolitano D, Pugliese D, Maconi G, Cataletti G, Pica R, Cassieri C, Savarino EV, et al. Upadacitinib’s Effectiveness and Safety as a Second- or Third-Line Therapy in Patients with Ulcerative Colitis: Data from a Real-World Study. Journal of Clinical Medicine. 2025; 14(21):7801. https://doi.org/10.3390/jcm14217801
Chicago/Turabian StyleMocci, Giammarco, Antonio Tursi, Franco Scaldaferri, Daniele Napolitano, Daniela Pugliese, Giovanni Maconi, Giovanni Cataletti, Roberta Pica, Claudio Cassieri, Edoardo Vincenzo Savarino, and et al. 2025. "Upadacitinib’s Effectiveness and Safety as a Second- or Third-Line Therapy in Patients with Ulcerative Colitis: Data from a Real-World Study" Journal of Clinical Medicine 14, no. 21: 7801. https://doi.org/10.3390/jcm14217801
APA StyleMocci, G., Tursi, A., Scaldaferri, F., Napolitano, D., Pugliese, D., Maconi, G., Cataletti, G., Pica, R., Cassieri, C., Savarino, E. V., De Barba, C., Costa, F., Ceccarelli, L., Marzo, M., Elisei, W., Monterubbianesi, R., Faggiani, R., Lombardi, G., Patturelli, M., ... Papa, A. (2025). Upadacitinib’s Effectiveness and Safety as a Second- or Third-Line Therapy in Patients with Ulcerative Colitis: Data from a Real-World Study. Journal of Clinical Medicine, 14(21), 7801. https://doi.org/10.3390/jcm14217801










