SGLT2-is in Acute Heart Failure
Abstract
1. Upfront Utilization of SGLT2 Inhibitors in Acute Heart Failure
2. Early Benefit from Trials on SGLT2is in Chronic HF
3. Studies on the Use of SGLT2is in Acute Heart Failure
4. Empagliflozin
5. Sotagliflozin
6. Dapagliflozin
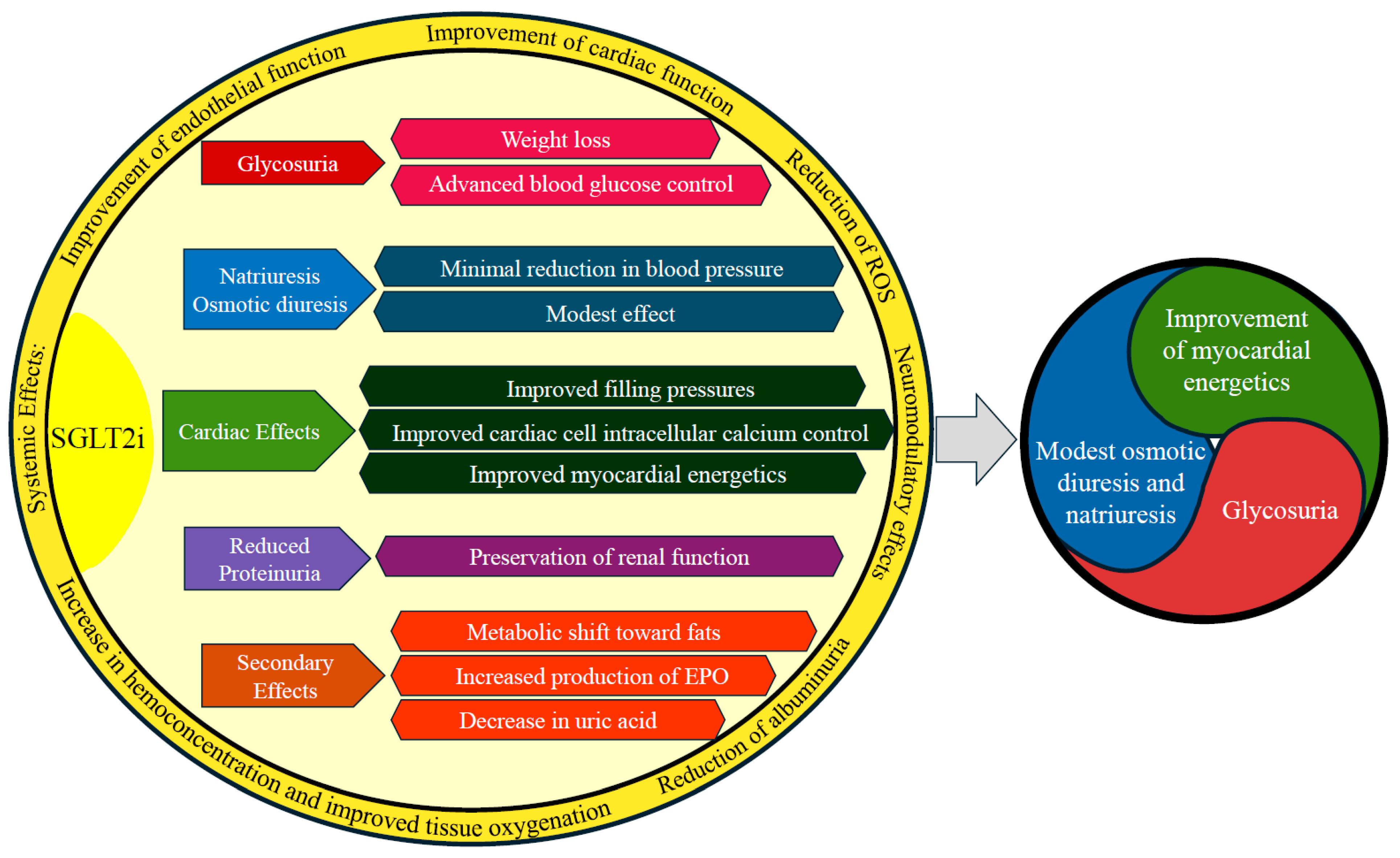
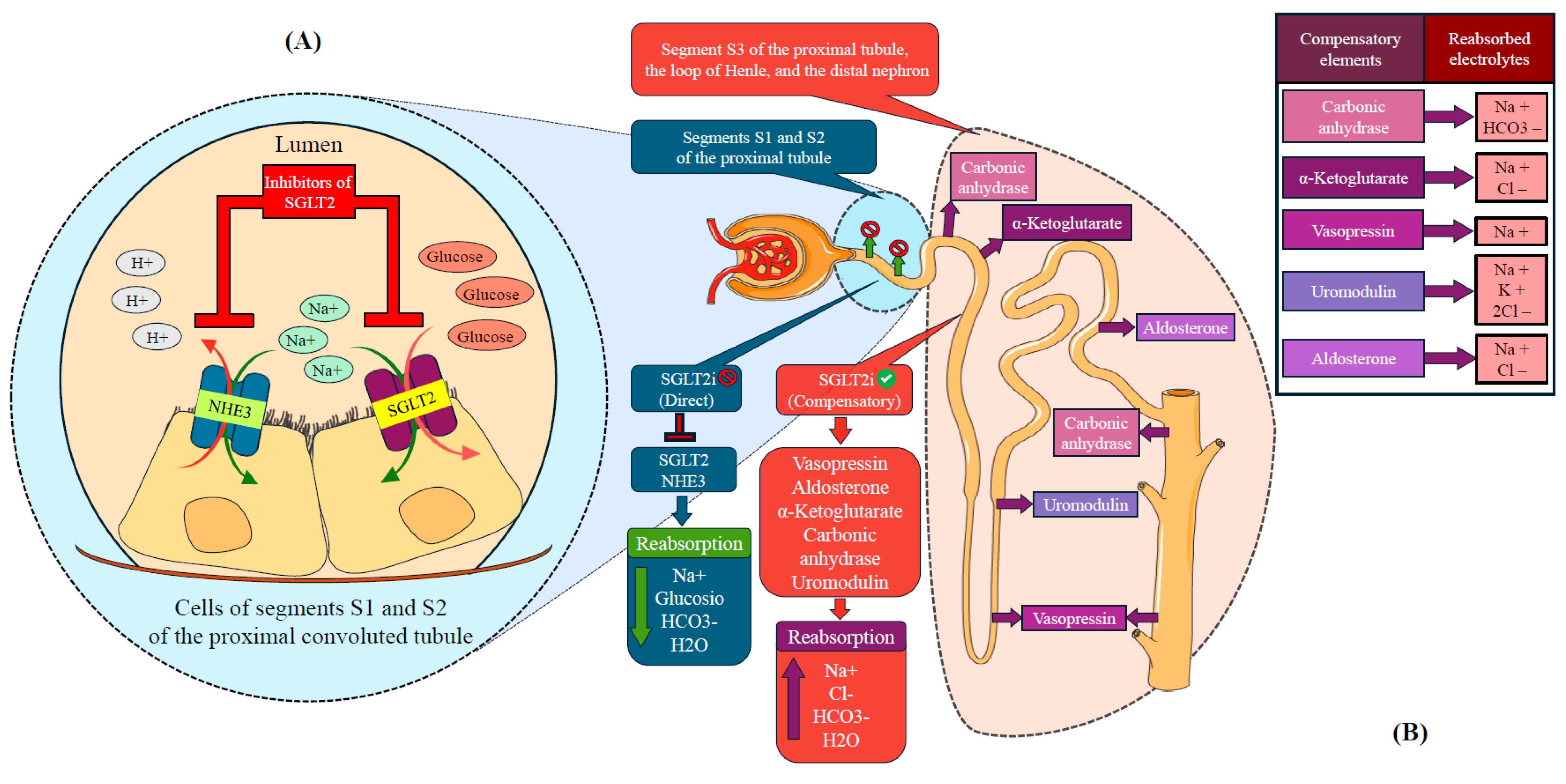
7. Mechanisms of SGLT2is in Acute Heart Failure
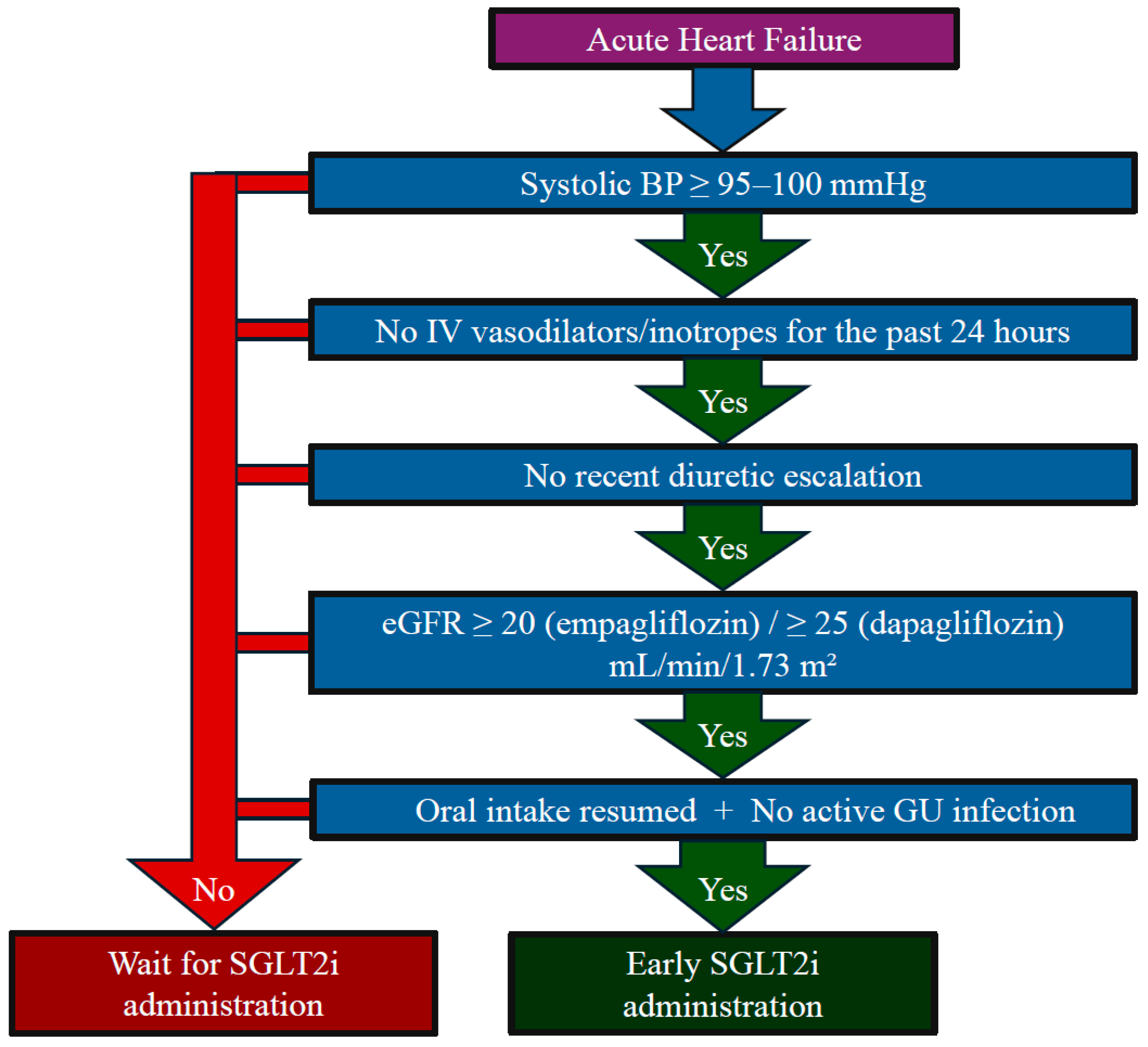
8. Red Flags for Deferred Use
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 16. Diabetes Care in the Hospital: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S267–S278. [Google Scholar] [CrossRef] [PubMed]
- Korytkowski, M.T.; Muniyappa, R.; Antinori-Lent, K.; Donihi, A.C.; Drincic, A.T.; Hirsch, I.B.; Luger, A.; McDonnell, M.E.; Murad, M.H.; Nielsen, C.; et al. Management of Hyperglycemia in Hospitalized Adult Patients in Non-Critical Care Settings: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2022, 107, 2101–2128. [Google Scholar] [CrossRef]
- Pasquel, F.J.; Lansang, M.C.; Dhatariya, K.; Umpierrez, G.E. Management of diabetes and hyperglycaemia in the hospital. Lancet Diabetes Endocrinol. 2021, 9, 174–188. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, S.M.; Stevenson, L.W.; Ahmad, T.; Bozkurt, B.; Butler, J.; Davis, L.L.; Drazner, M.H.; Kirkpatrick, J.N.; Morris, A.A.; Page, R.L., 2nd; et al. 2024 ACC Expert Consensus Decision Pathway on Clinical Assessment, Management, and Trajectory of Patients Hospitalized with Heart Failure Focused Update: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2024, 84, 1241–1267. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Straus, S.E.; Farkouh, M.E.; Austin, P.C.; Taljaard, M.; Chong, A.; Fahim, C.; Poon, S.; Cram, P.; Smith, S.; et al. Trial of an Intervention to Improve Acute Heart Failure Outcomes. N. Engl. J. Med. 2023, 388, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): A multinational, open-label, randomised, trial. Lancet Lond. Engl. 2022, 400, 1938–1952. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Solomon, S.D.; Vaduganathan, M.; Claggett, B.L.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Cunningham, J.W.; Vaduganathan, M.; Claggett, B.L.; Kulac, I.J.; Desai, A.S.; Jhund, P.S.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; et al. Dapagliflozin in Patients Recently Hospitalized with Heart Failure and Mildly Reduced or Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2022, 80, 1302–1310. [Google Scholar] [CrossRef]
- Pitt, B.; Bhatt, D.L.; Szarek, M.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Effect of Sotagliflozin on Early Mortality and Heart Failure-Related Events: A Post Hoc Analysis of SOLOIST-WHF. JACC Heart Fail. 2023, 11 Pt 1, 879–889. [Google Scholar] [CrossRef]
- Damman, K.; Beusekamp, J.C.; Boorsma, E.M.; Swart, H.P.; Smilde, T.D.J.; Elvan, A.; van Eck, J.W.M.; Heerspink, H.J.L.; Voors, A.A. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur. J. Heart Fail. 2020, 22, 713–722. [Google Scholar] [CrossRef]
- Damman, K.; Beusekamp, J.C.; Boorsma, E.M.; Swart, H.P.; Smilde, T.D.J.; Elvan, A.; van Eck, J.W.M.; Heerspink, H.J.L.; Voors, A.A. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Emara, A.N.; Wadie, M.; Mansour, N.O.; Shams, M.E.E. The clinical outcomes of dapagliflozin in patients with acute heart failure: A randomized controlled trial (DAPA-RESPONSE-AHF). Eur. J. Pharmacol. 2023, 961, 176179. [Google Scholar] [CrossRef]
- Yeoh, S.E.; Osmanska, J.; Petrie, M.C.; Brooksbank, K.J.M.; Clark, A.L.; Docherty, K.F.; Foley, P.W.X.; Guha, K.; Halliday, C.A.; Jhund, P.S.; et al. Dapagliflozin vs. metolazone in heart failure resistant to loop diuretics. Eur. Heart J. 2023, 44, 2966–2977. [Google Scholar] [CrossRef] [PubMed]
- Cox, Z.L.; Collins, S.P.; Hernandez, G.A.; McRae, A.T., 3rd; Davidson, B.T.; Adams, K.; Aaron, M.; Cunningham, L.; Jenkins, C.A.; Lindsell, C.J.; et al. Efficacy and Safety of Dapagliflozin in Patients With Acute Heart Failure. J. Am. Coll. Cardiol. 2024, 83, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Ghaleb, R.; Mansour, H.; Hanafy, A.; Mahmoud, N.M.; Abdelfatah Elsharef, M.; Kamal Salama, M.; Elsaughier, S.M.; Abdel-Wahid, L.; Embarek Mohamed, M.; et al. Safety and Efficacy of Adding Dapagliflozin to Furosemide in Type 2 Diabetic Patients With Decompensated Heart Failure and Reduced Ejection Fraction. Front. Cardiovasc. Med. 2020, 7, 602251. [Google Scholar] [CrossRef]
- Berg, D.D.; Patel, S.M.; Haller, P.M.; Cange, A.L.; Palazzolo, M.G.; Bellavia, A.; Kuder, J.F.; Desai, A.S.; Inzucchi, S.E.; McMurray, J.J.V.; et al. Dapagliflozin in Patients Hospitalized for Heart Failure: Primary Results of the DAPA ACT HF-TIMI 68 Randomized Clinical Trial and Meta-Analysis of Sodium-Glucose Cotransporter-2 Inhibitors in Patients Hospitalized for Heart Failure. Circulation 2025. [Google Scholar] [CrossRef] [PubMed]
- Schulze, P.C.; Bogoviku, J.; Westphal, J.; Aftanski, P.; Haertel, F.; Grund, S.; von Haehling, S.; Schumacher, U.; Möbius-Winkler, S.; Busch, M. Effects of Early Empagliflozin Initiation on Diuresis and Kidney Function in Patients with Acute Decompensated Heart Failure (EMPAG-HF). Circulation 2022, 146, 289–298. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Morillas, H.; Galcerá, E.; Alania, E.; Seller, J.; Larumbe, A.; Núñez, J.; Valle, A. Sodium-glucose Co-transporter 2 Inhibitors in Acute Heart Failure: A Review of the Available Evidence and Practical Guidance on Clinical Use. Rev. Cardiovasc. Med. 2022, 23, 139. [Google Scholar] [CrossRef]
- Carvalho, P.E.P.; Veiga, T.M.A.; Simões ESilva, A.C.; Gewehr, D.M.; Dagostin, C.S.; Fernandes, A.; Nasi, G.; Cardoso, R. Cardiovascular and renal effects of SGLT2 inhibitor initiation in acute heart failure: A meta-analysis of randomized controlled trials. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2023, 112, 1044–1055. [Google Scholar] [CrossRef]
- Cox, Z.L.; Nandkeolyar, S.; Johnson, A.J.; Lindenfeld, J.; Rali, A.S. In-hospital Initiation and Up-titration of Guideline-directed Medical Therapies for Heart Failure with Reduced Ejection Fraction. Card. Fail. Rev. 2022, 8, e21. [Google Scholar] [CrossRef]
- Boorsma, E.M.; Beusekamp, J.C.; Ter Maaten, J.M.; Figarska, S.M.; Danser, A.H.J.; van Veldhuisen, D.J.; van der Meer, P.; Heerspink, H.J.L.; Damman, K.; Voors, A.A. Effects of empagliflozin on renal sodium and glucose handling in patients with acute heart failure. Eur. J. Heart Fail. 2021, 23, 68–78. [Google Scholar] [CrossRef]
- Packer, M.; Wilcox, C.S.; Testani, J.M. Critical Analysis of the Effects of SGLT2 Inhibitors on Renal Tubular Sodium, Water and Chloride Homeostasis and Their Role in Influencing Heart Failure Outcomes. Circulation 2023, 148, 354–372. [Google Scholar] [CrossRef]
- Marton, A.; Saffari, S.E.; Rauh, M.; Sun, R.N.; Nagel, A.M.; Linz, P.; Lim, T.T.; Takase-Minegishi, K.; Pajarillaga, A.; Saw, S.; et al. Water Conservation Overrides Osmotic Diuresis During SGLT2 Inhibition in Patients with Heart Failure. J. Am. Coll. Cardiol. 2024, 83, 1386–1398. [Google Scholar] [CrossRef]
- Omar, M.; Jensen, J.; Burkhoff, D.; Frederiksen, P.H.; Kistorp, C.; Videbæk, L.; Poulsen, M.K.; Gustafsson, F.; Køber, L.; Borlaug, B.A.; et al. Effect of Empagliflozin on Blood Volume Redistribution in Patients with Chronic Heart Failure and Reduced Ejection Fraction: An Analysis from the Empire HF Randomized Clinical Trial. Circ. Heart Fail. 2022, 15, e009156. [Google Scholar] [CrossRef] [PubMed]
- Biegus, J.; Voors, A.A.; Collins, S.P.; Kosiborod, M.N.; Teerlink, J.R.; Angermann, C.E.; Tromp, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; et al. Impact of empagliflozin on decongestion in acute heart failure: The EMPULSE trial. Eur. Heart J. 2023, 44, 41–50. [Google Scholar] [CrossRef]
- Hallow, K.M.; Helmlinger, G.; Greasley, P.J.; McMurray, J.J.V.; Boulton, D.W. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes. Metab. 2018, 20, 479–487. [Google Scholar] [CrossRef]
- Itkin, M.; Rockson, S.G.; Burkhoff, D. Pathophysiology of the Lymphatic System in Patients with Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S.; Shen, W.; Boulton, D.W.; Leslie, B.R.; Griffen, S.C. Interaction Between the Sodium-Glucose-Linked Transporter 2 Inhibitor Dapagliflozin and the Loop Diuretic Bumetanide in Normal Human Subjects. J. Am. Heart Assoc. 2018, 7, e007046. [Google Scholar] [CrossRef]
- Ferrannini, G.; Hach, T.; Crowe, S.; Sanghvi, A.; Hall, K.D.; Ferrannini, E. Energy Balance After Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care 2015, 38, 1730–1735. [Google Scholar] [CrossRef]
- Nakagaito, M.; Imamura, T.; Joho, S.; Ushijima, R.; Nakamura, M.; Kinugawa, K. Efficacy of Continuing SGLT2 Inhibitors on Outcomes in Patients with Acute Decompensated Heart Failure. Int. Heart J. 2021, 62, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Butler, J.; Zeller, C.; Pocock, S.J.; Brueckmann, M.; Ferreira, J.P.; Filippatos, G.; Usman, M.S.; Zannad, F.; Anker, S.D. Blinded Withdrawal of Long-Term Randomized Treatment with Empagliflozin or Placebo in Patients with Heart Failure. Circulation 2023, 148, 1011–1022. [Google Scholar] [CrossRef] [PubMed]

| Study | Type of SGLT Inhibition | Comparison Groups | Main Eligibility Criteria | Method of Initiating SGLTis | Follow-Up | Primary Outcomes | Overall Treatment Effect | Relevant Data |
|---|---|---|---|---|---|---|---|---|
| SOLOIST-WHF [14] | SGLT1 and SGLT2 | Sotagliflozin 200 mg once daily (titrated up to 400 mg) vs. placebo (n = 1222) | Reduced and preserved LVEF Type 2 diabetes eGFR ≥ 30 mL/min/m2 | Before discharge (48.8%) Right after discharge (median 2 days, 51.2%) | 9 months | Total number of CV deaths and hospitalizations and urgent visits for heart failure | 51.0 vs. 76.3 events per 100 patient-years Hazard ratio 0.67 (95% CI 0.52–0.85) | Early termination of the trial due to loss of funding by the sponsor Benefit driven by a reduction in hospitalizations and visits for heart failure Benefit consistent across subgroups and treatment initiation methods Higher incidence of diarrhea and severe hypoglycemia in the sotagliflozin group |
| EMPA-RESPONSE-AHF [15] | SGLT2 | Empagliflozin 10 mg once daily vs. placebo for 30 days (n = 80) | Clinical signs of congestion NT-proBNP ≥1400 pg/mL Need for initiation of loop diuretics | Within 24 h of hospitalization | 60 days | Change in dyspnea VAS score, NT-proBNP, diuretic response, and length of hospital stay | Mean combined difference in various secondary endpoints −0.019 (95% CI −0.306–0.269) | No significant difference in any primary outcomes Reduction in a combined secondary endpoint of in-hospital HF worsening, HF hospitalization, or death at 60 days vs. placebo Increased urine output by day 4 Safety and tolerability. No adverse effects on blood pressure or renal function |
| EMPULSE [16] | SGLT2 | Empagliflozin 10 mg once daily vs. placebo (n = 530) | NT-proBNP ≥1600 pg/mL Furosemide dose ≥40 mg IV eGFR ≥ 20 mL/min/m2 | From the first to the fifth day after hospitalization | 90 days | Combination of death, number of HF episodes, time to first HF event, and change in KCCQ-TSS | Clinical benefit 53.9% vs. 39.7% Win ratio 1.36 (95% CI 1.09–1.68) | Significant numerical reduction in deaths and HF events with empagliflozin Benefit also demonstrated with standard survival analysis Early and sustained weight loss of 1.5 kg Serious adverse events were more frequent in the placebo group No cases of ketoacidosis |
| DAPA-RESPONSE-AHF [17] | SGLT2 | Dapagliflozin 10 mg once daily vs. placebo (n = 87) | Age ≥ 18 Acute HF with dyspnea at rest or minimal exertion At least one additional sign of congestion History of type 2 diabetes Newly diagnosed HbA1c ≥ 6.5% at hospitalization | Within 24 h of hospitalization up to 30 days | From day 4 to 60 days post-hospitalization (30 days post-treatment) | Difference between groups in AUC of VAS dyspnea score during the first 4 days | Dapagliflozin significantly reduced the AUC of VAS dyspnea score vs. placebo (3192.2 ± 1631.9 mm × h vs. 4713.1 ± 1714.9 mm × h, p < 0.001) | Greater relative NT-proBNP reduction from baseline with dapagliflozin (−34.89% vs. −10.085%, p = 0.001) Higher cumulative urine volume after 4 days of therapy (18,600 mL with dapagliflozin vs. 13,700 mL with placebo, p = 0.031) Dapagliflozin reduced the number of re-hospitalizations within 30 days of discharge Dapagliflozin did not affect urinary Na+ concentration, HF worsening incidence, or mortality |
| DAPA-RESIST [18] | SGLT2 | Dapagliflozin 10 mg once daily vs. metolazone 5–10 mg once daily for 3 days (n = 61) | Patients hospitalized for HF Resistance to IV furosemide therapy BNP ≥ 100 pg/mL or NT-proBNP ≥ 400 pg/mL Persistent congestion Expected hospital stay >3 days | For up to three consecutive days after admission | 5 days | Diuretic effect assessed by weight change (kg), pulmonary congestion changes (lung ultrasound), loop diuretic efficiency (weight change per 40 mg furosemide), and congestion assessment score | Mean weight reduction at 96 h: 3.0 kg with dapagliflozin vs. 3.6 kg with metolazone [mean difference 0.65, 95% CI −0.12, 1.41 kg; p = 0.11] | Loop diuretic efficiency was lower with dapagliflozin vs. metolazone Pulmonary congestion changes were similar between treatments Plasma sodium and potassium decreases and urea and creatinine increases were smaller with dapagliflozin than with metolazone Serious adverse events were similar between treatments Dapagliflozin patients received more furosemide but had fewer biochemical alterations than metolazone patients Dapagliflozin was not more effective than metolazone in alleviating congestion |
| DICTATE-AHF [19] | SGLT2 | Dapagliflozin 10 mg once daily vs. protocolized diuretic titration until day 5 or hospital discharge (n = 240) | Adults with type 2 diabetes eGFR ≥ 30 mL/min/1.73 m2 Hospitalized with AHF Planned or ongoing IV loop diuretics Hypervolemia and randomization within 24 h of ER presentation or direct admission to hospital | For up to five consecutive days of admission or until hospital discharge | 30 days post-discharge (telephone follow-up for outcome assessment) | Concentration of natriuretic peptides, weight, and congestion assessment using edema scale at day 5 or discharge | Dapagliflozin is not associated with greater body weight reduction after 5 days of treatment (−0.42 kg/40 mg furosemide vs. −0.31 kg/40 mg furosemide, CI 0.41–1.02; p = 0.06) | No difference was found between the diuretic efficacy of dapagliflozin and that of usual therapy Dapagliflozin was associated with reduced loop diuretic doses Early dapagliflozin initiation did not increase diabetic, renal, or cardiovascular adverse events. Dapagliflozin improved mean 24 h natriuresis and urine production, accelerating hospital discharge |
| Ibrahim et al. 2020 [20] | SGLT2 | Dapagliflozin 10 mg once daily alone or with insulin (as needed) and furosemide (n = 100) | Age > 18, Type 2 diabetic patients with HF history Indication for AHF hospitalization ≥1 symptom: respiratory distress or orthopnea ≥1 clinical sign of congestion (peripheral edema, jugular venous distension, or pulmonary congestion signs) Chronic furosemide therapy ≥1 month pre-admission LVEF ≤ 40% | From admission to hospital discharge | During hospital stay | 24 h urine volume and until discharge, diuretic efficiency, body weight change from admission to discharge, renal function changes, serum electrolyte changes, and dyspnea improvement during hospitalization | Dapagliflozin enhanced loop diuretic action and diuretic efficiency (34.8 ± 2.21 mL/mg loop diuretic) vs. controls (19.5 ± 1.23 mL/mg loop diuretic) No significant effect on serum potassium (4.11 ± 0.42 mEq/L) vs. controls (3.83 ± 0.50 mEq/L) or renal function [mean serum creatinine (1.39 ± 0.23 mg/dL) vs. controls (1.53 ± 0.34 mg/dL)] | In the study group, urine output, fluid loss per diuretic dose, and mean serum potassium were higher, while fluid/diuretic balance, mean total furosemide dose, furosemide/day dose, mean weight, and total daily insulin dose were significantly lower A significant increase in serum creatinine, a statistically significant reduction in serum sodium, and significant improvements in dyspnea were observed in both groups at discharge. |
| DAPA ACT HF-TIMI 68 [21] | SGLT2 | Dapagliflozin 10 mg vs. Placebo | Age ≥18 Currently hospitalized for acute HF defined as 1. Presentation with worsening symptoms of HF 2. Objective signs or diagnostic testing consistent with volume overload 3. Intensification of acute HF therapy during admission Elevated NT-proBNP or BNP during the current hospitalization | Eligible patients randomized no earlier than 24 h and up to 14 d after presentation while still hospitalized once they have been stabilized: 1. No increase in iv diuretics in the 12 h before randomization 2. No use of intravenous vasodilators or inotropes during the 24 h before randomization | Primary endpoint window: 2 months | Primary efficacy endpoint of the trial is the time to first occurrence of cardiovascular death or a worsening HF event defined as: 1. Worsening HF during the index hospitalization requiring inotropes o mechanical/ventilatory support. 2. Re-admission to the hospital for worsening HF 3. Urgent ambulatory visit with iv diuretics | No statistically significant reduction in cardiovascular mortality or worsening HF (10.9% vs. 12.7%; (HR 0.86; 95% CI 0.68–1.08; p = 0.20) Higher rates of symptomatic hypotension (3.6% vs. 2.2%) and renal function decline (5.9% vs. 4.7%) without significant increase in study drug discontinuation. | Admission to randomization time 3.6 (IQR 2.1–5.4) days. Safety of dapagliflozin both in patients hospitalized for de novo or worsening HF Lack off efficacy in reducing primary endpoint probably related to short follow-up and lower-than-expected rate of events |
| What We Know About SGLT2i Therapy | What We Do Not Know About SGLT2i Therapy |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianco, M.; Di Nora, C.; De Maria, R.; Mousavi, A.H.; Carigi, S.; De Gennaro, L.; Manca, P.; Matassini, M.V.; Rizzello, V.; Tinti, M.D.; et al. SGLT2-is in Acute Heart Failure. J. Clin. Med. 2025, 14, 7799. https://doi.org/10.3390/jcm14217799
Bianco M, Di Nora C, De Maria R, Mousavi AH, Carigi S, De Gennaro L, Manca P, Matassini MV, Rizzello V, Tinti MD, et al. SGLT2-is in Acute Heart Failure. Journal of Clinical Medicine. 2025; 14(21):7799. https://doi.org/10.3390/jcm14217799
Chicago/Turabian StyleBianco, Matteo, Concetta Di Nora, Renata De Maria, Amir Hassan Mousavi, Samuela Carigi, Luisa De Gennaro, Paolo Manca, Maria Vittoria Matassini, Vittoria Rizzello, Maria Denitza Tinti, and et al. 2025. "SGLT2-is in Acute Heart Failure" Journal of Clinical Medicine 14, no. 21: 7799. https://doi.org/10.3390/jcm14217799
APA StyleBianco, M., Di Nora, C., De Maria, R., Mousavi, A. H., Carigi, S., De Gennaro, L., Manca, P., Matassini, M. V., Rizzello, V., Tinti, M. D., Geraci, G., Iacovoni, A., Colivicchi, F., Grimaldi, M., & Oliva, F. (2025). SGLT2-is in Acute Heart Failure. Journal of Clinical Medicine, 14(21), 7799. https://doi.org/10.3390/jcm14217799







