Effect of Concomitant Tricuspid Valve Repair on Clinical and Echocardiographic Outcomes in Patients Undergoing Left Ventricular Assist Device Implantation
Abstract
1. Introduction
2. Patients and Methods
2.1. Ethical Statement
2.2. Study Design
2.3. Echocardiography
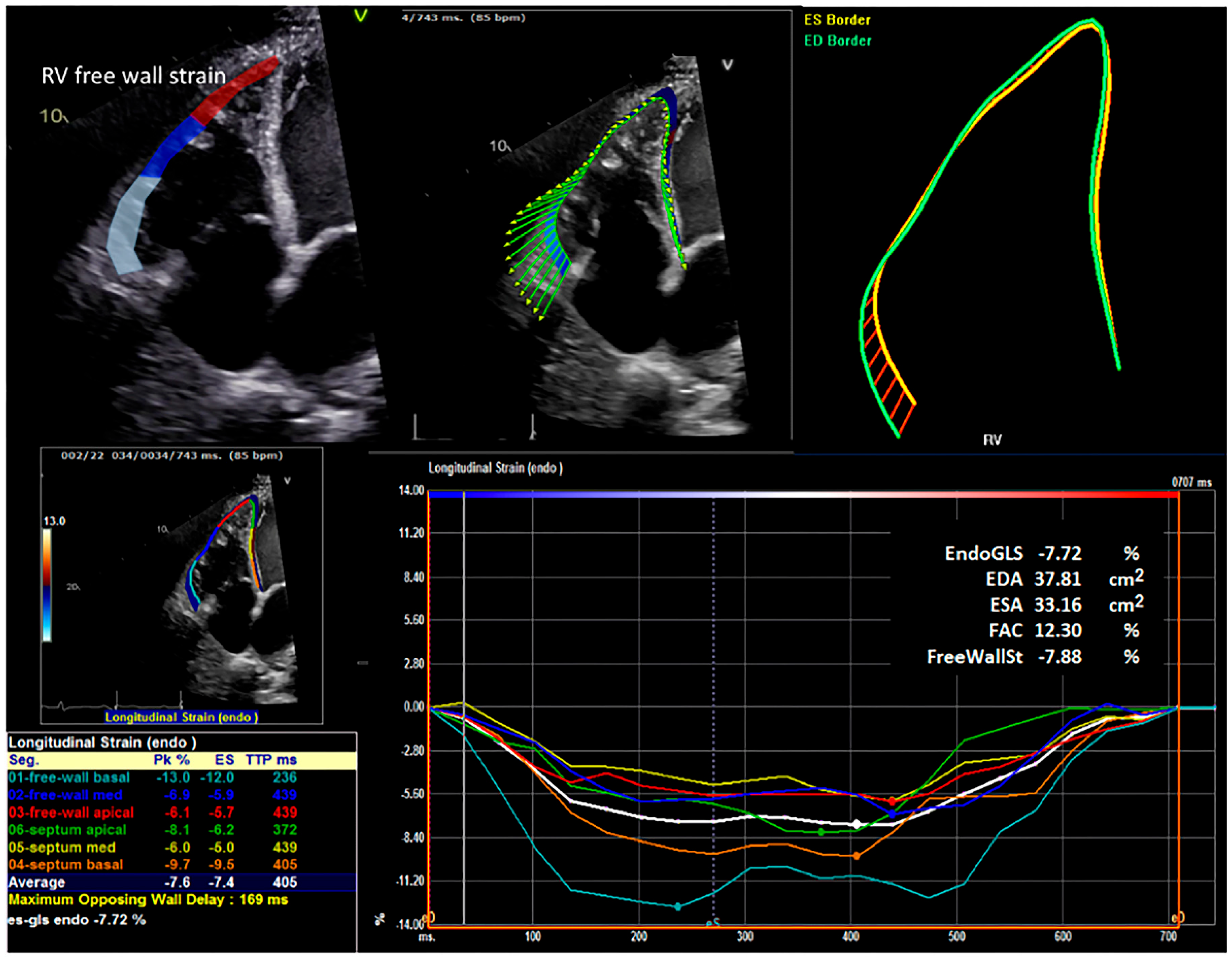
2.4. Strain Analysis
2.5. Hemodynamic Parameters
2.6. Clinical Variables and Operative Details
2.7. Endpoints
2.8. Statistical Analysis
3. Results
3.1. Preoperative Characteristics
3.2. Intraoperative Characteristics
3.3. Early Postoperative Outcomes
3.4. TR Trajectory and RV Structure/Function
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASE | American Society of Echocardiography |
| BIVAD | bi-ventricular assist device |
| FAC | fractional area change |
| FWS | free wall strain |
| LVAD | left ventricular assist device |
| RV | right ventricular |
| RVAD | right ventricular assist device |
| RHF | right heart failure |
| TR | tricuspid regurgitation |
| TVr | tricuspid valve repair |
| PVR | pulmonary vascular resistance |
| PFO | patent foramen ovale |
| sHR | subdistribution hazard ratio |
References
- Cersosimo, A.; Gavazzoni, M.; Inciardi, R.M.; Radulescu, C.I.; Adamo, M.; Arabia, G.; Metra, M.; Raddino, R.; Vizzardi, E. Right ventricle assessment before tricuspid valve interventions. J. Cardiovasc. Med. 2024, 25, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Piacentino, V., 3rd; Williams, M.L.; Depp, T.; Garcia-Huerta, K.; Blue, L.; Lodge, A.J.; Mackensen, G.B.; Swaminathan, M.; Rogers, J.G.; Milano, C.A. Impact of tricuspid valve regurgitation in patients treated with implantable left ventricular assist devices. Ann. Thorac. Surg. 2011, 91, 1342–1346; discussion 46–47. [Google Scholar] [CrossRef]
- Aymami, M.; Amsallem, M.; Adams, J.; Sallam, K.; Moneghetti, K.; Wheeler, M.; Hiesinger, W.; Teuteberg, J.; Weisshaar, D.; Verhoye, J.P.; et al. The Incremental Value of Right Ventricular Size and Strain in the Risk Assessment of Right Heart Failure Post—Left Ventricular Assist Device Implantation. J. Card. Fail. 2018, 24, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Veen, K.M.; Muslem, R.; Soliman, O.I.; Caliskan, K.; Kolff, M.E.A.; Dousma, D.; Manintveld, O.C.; Birim, O.; Bogers, A.J.; Takkenberg, J.J. Left ventricular assist device implantation with and without concomitant tricuspid valve surgery: A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2018, 54, 644–651. [Google Scholar] [CrossRef]
- Lee, S.; Kamdar, F.; Madlon-Kay, R.; Boyle, A.; Colvin-Adams, M.; Pritzker, M.; John, R. Effects of the HeartMate II continuous-flow left ventricular assist device on right ventricular function. J. Heart Lung Transplant. 2010, 29, 209–215. [Google Scholar] [CrossRef]
- Morgan, J.A.; Paone, G.; Nemeh, H.W.; Murthy, R.; Williams, C.T.; Lanfear, D.E.; Tita, C.; Brewer, R.J. Impact of continuous-flow left ventricular assist device support on right ventricular function. J. Heart Lung Transplant. 2013, 32, 398–403. [Google Scholar] [CrossRef]
- Hoopes, C. Tricuspid surgery at the time of LVAD implant: A critique. Front. Cardiovasc. Med. 2022, 9, 1056414. [Google Scholar] [CrossRef]
- Nakanishi, K.; Homma, S.; Han, J.; Takayama, H.; Colombo, P.C.; Yuzefpolskaya, M.; Garan, A.R.; Farr, M.A.; Kurlansky, P.; Di Tullio, M.R.; et al. Prevalence, Predictors, and Prognostic Value of Residual Tricuspid Regurgitation in Patients With Left Ventricular Assist Device. J. Am. Heart Assoc. 2018, 7, e008813. [Google Scholar] [CrossRef]
- Society of Thoracic Surgeons (STS). Guide to the STS Intermacs® Database, Version 6.1; STS: Chicago, IL, USA, 2023.
- Kislitsina, O.N.; DRich, J.; Wilcox, J.E.; Vorovich, E.; Wu, T.; Churyla, A.; SHarap, R.; Andrei, A.C.; McCarthy, P.M.; Yancy, C.W.; et al. Propensity Score-Matched Comparison of Right Ventricular Strain in Women and Men Before and After Left Ventricular Assist Device Implantation. Innovations 2022, 17, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Fortuni, F.; Dietz, M.F.; Prihadi, E.A.; van der Bijl, P.; De Ferrari, G.M.; Knuuti, J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Prognostic Implications of a Novel Algorithm to Grade Secondary Tricuspid Regurgitation. JACC Cardiovasc. Imaging 2021, 14, 1085–1095. [Google Scholar] [CrossRef]
- Cameli, M.; Lisi, M.; Righini, F.M.; Focardi, M.; Lunghetti, S.; Bernazzali, S.; Marchetti, L.; Biagioli, B.; Galderisi, M.; Maccherini, M.; et al. Speckle tracking echocardiography as a new technique to evaluate right ventricular function in patients with left ventricular assist device therapy. J. Heart Lung Transplant. 2013, 32, 424–430. [Google Scholar] [CrossRef]
- Kato, T.S.; Jiang, J.; Schulze, P.C.; Jorde, U.; Uriel, N.; Kitada, S.; Takayama, H.; Naka, Y.; Mancini, D.; Gillam, L.; et al. Serial echocardiography using tissue Doppler and speckle tracking imaging to monitor right ventricular failure before and after left ventricular assist device surgery. JACC Heart Fail. 2013, 1, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Raymer, D.S.; Moreno, J.D.; Sintek, M.A.; Nassif, M.E.; Sparrow, C.T.; Adamo, L.; Novak, E.L.; LaRue, S.J.; Vader, J.M. The Combination of Tricuspid Annular Plane Systolic Excursion and HeartMate Risk Score Predicts Right Ventricular Failure After Left Ventricular Assist Device Implantation. ASAIO J. 2019, 65, 247–251. [Google Scholar] [CrossRef]
- Gumus, F.; Durdu, M.S.; Cakici, M.; Kurklu, T.S.T.; Inan, M.B.; Dincer, I.; Sirlak, M.; Akar, A.R. Right ventricular free wall longitudinal strain and stroke work index for predicting right heart failure after left ventricular assist device therapy. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 674–682. [Google Scholar] [CrossRef]
- Oezpeker, C.; Zittermann, A.; Paluszkiewicz, L.; Piran, M.; Puehler, T.; Sayin, A.O.; Ensminger, S.; Milting, H.; Morshuis, M.; Gummert, J.F. Tricuspid valve repair in patients with left-ventricular assist device implants and tricuspid valve regurgitation: Propensity score-adjusted analysis of clinical outcome. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 741–747. [Google Scholar] [CrossRef]
- Fujita, T.; Kobayashi, J.; Hata, H.; Seguchi, O.; Murata, Y.; Yanase, M.; Nakatani, T. Right heart failure and benefits of adjuvant tricuspid valve repair in patients undergoing left ventricular assist device implantation. Eur. J. Cardiothorac. Surg. 2014, 46, 802–807. [Google Scholar] [CrossRef]
- Brewer, R.J.; Cabrera, R.; El-Atrache, M.; Zafar, A.; Hrobowski, T.N.; Nemeh, H.M.; Selektor, Y.; Paone, G.; Williams, C.T.; Velez, M.; et al. Relationship of tricuspid repair at the time of left ventricular assist device implantation and survival. Int. J. Artif. Organs 2014, 37, 834–838. [Google Scholar] [CrossRef]
- Saeed, D.; Kidambi, T.; Shalli, S.; Lapin, B.; Malaisrie, S.C.; Lee, R.; Cotts, W.G.; McGee, E.C., Jr. Tricuspid valve repair with left ventricular assist device implantation: Is it warranted? J. Heart Lung Transplant. 2011, 30, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Song, H.K.; Gelow, J.M.; Mudd, J.; Chien, C.; Tibayan, F.A.; Hollifield, K.; Naftel, D.; Kirklin, J. Limited Utility of Tricuspid Valve Repair. at the Time of Left Ventricular Assist Device Implantation. Ann. Thorac. Surg. 2016, 101, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.O.; Grau-Sepulveda, M.V.; Okada, S.; O’Brien, S.M.; Matthew Brennan, J.; Shah, A.S.; Itoh, A.; Damiano, R.J.; Prasad, S.; Silvestry, S.C. Concomitant tricuspid valve surgery during implantation of continuous-flow left ventricular assist devices: A Society of Thoracic Surgeons database analysis. J. Heart Lung Transplant. 2014, 33, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Boegershausen, N.; Zayat, R.; Aljalloud, A.; Musetti, G.; Goetzenich, A.; Tewarie, L.; Moza, A.; Amerini, A.; Autschbach, R.; Hatam, N. Risk factors for the development of right ventricular failure after left ventricular assist device implantation—A single-centre retrospective with focus on deformation imaging. Eur. J. Cardiothorac. Surg. 2017, 52, 1069–1076. [Google Scholar] [CrossRef] [PubMed]

| Variable | No Tricuspid Valve Repair (n = 56) | Tricuspid Valve Repair (n = 44) | p-Value | ||
|---|---|---|---|---|---|
| Age (years) | 54.2 | ±12.6 | 55.0 | ±16.6 | 0.78 |
| Body mass index (kg/m2) | 25.2 | ±4.6 | 29.0 | ±6.5 | <0.001 |
| CHA2DS2VASc score | 2.9 | ±1.3 | 3.4 | ±1.6 | 0.08 |
| Creatinine level (mg/dL) | 1.1 | (0.9, 1.5) | 1.5 | (1.0, 2.0) | 0.016 |
| Albumin level (g/dL) | 3.2 | (2.7, 3.5) | 3.5 | (3.3, 3.8) | 0.003 |
| Gender (female) | 22 | (39) | 10 | (23) | 0.08 |
| Diabetes | 10 | (18) | 24 | (55) | <0.001 |
| Dyslipidemia | 29 | (52) | 27 | (61) | 0.34 |
| Hypertension | 25 | (45) | 23 | (52) | 0.45 |
| Chronic lung disease | 20 | (36) | 17 | (39) | 0.76 |
| Prior stroke | 5 | (9) | 4 | (9) | 0.98 |
| Prior pacemaker | 21 | (38) | 7 | (16) | 0.017 |
| Coronary artery disease | 13 | (42) | 13 | (68) | 0.07 |
| Atrial fibrillation history | 19 | (34) | 28 | (64) | 0.003 |
| Bridge-to-transplant therapy | 19 | (34) | 34 | (81) | <0.001 |
| Preoperative Echocardiography Characteristics | |||||
| Ejection fraction (%) | 15.0 | (13.0, 20.0) | 15.0 | (10.5, 20.0) | 0.80 |
| Left atrial size (cm) | 4.4 | (3.9, 5.1) | 5.0 | (4.2, 5.5) | 0.005 |
| Left ventricular outflow tract cardiac index (L/min/m2) | 1.6 | (1.2, 2.2) | 1.6 | (1.4, 2.0) | 0.87 |
| Right ventricular outflow tract peak velocity (cm/s) | 51.0 | (40.0, 60.0) | 50.0 | (41.4, 60.4) | 0.69 |
| Tricuspid regurgitation peak gradient (cm/s) | 41.2 | (31.3, 51.7) | 34.2 | (26.3, 44.1) | 0.06 |
| Right ventricular free wall strain (%) | −5.1 | (−9.8, −3.9) | −6.7 | (−9.0, −4.5) | 0.40 |
| Right ventricular fractional area change (%) | 15.9 | (11.9, 21.9) | 19.6 | (12.6, 24.0) | 0.50 |
| Aortic insufficiency | 0.53 | ||||
| 0 = none/trivial | 33 | (7) | 27 | (82) | |
| 1 = mild | 8 | (18) | 5 | (15) | |
| 2 = moderate | 4 | (9) | 1 | (3) | |
| Mitral insufficiency | 0.30 | ||||
| 0 = none/trivial | 2 | (4) | 1 | (2) | |
| 1 = mild | 8 | (15) | 5 | (12) | |
| 2 = moderate | 10 | (19) | 16 | (37) | |
| 3 = mod/sev | 3 | (6) | 4 | (9) | |
| 4 = severe | 29 | (56) | 17 | (40) | |
| Tricuspid insufficiency | 0.002 | ||||
| 2 = moderate | 40 | (75) | 18 | (41) | |
| 3 = mod/sev | 3 | (6) | 7 | (16) | |
| 4 = severe | 10 | (19) | 19 | (43) | |
| Right ventricle dilation severity | 0.038 | ||||
| Moderate–severe/severe | 4 | (7) | 4 | (9) | |
| Mild–moderate/moderate | 13 | (23) | 21 | (48) | |
| Mild/none/normal | 21 | (38) | 13 | (30) | |
| Unknown/missing | 18 | (32) | 6 | (14) | |
| Reduced right ventricular systolic function | 50 | (89) | 42 | (95) | 0.19 |
| Left ventricular assist device placed as bridge-to-transplant | 38 | (68) | 15 | (34) | <0.001 |
| Intermacs class | 0.701 | ||||
| 1 | 13 | (23) | 10 | (23) | |
| 2 | 23 | (41) | 20 | (45) | |
| 3 | 8 | (14) | 9 | (20) | |
| 4 | 4 | (7) | 2 | (5) | |
| Unknown | 8 | (14) | 3 | (7) | |
| Variable | No Tricuspid Valve Repair (n = 56) | Tricuspid Valve Repair (n = 44) | p-Value | ||
|---|---|---|---|---|---|
| Right atrial mean pressure (mmHg) | 15.5 | (8.0, 19.0) | 17.0 | (10.0, 21.0) | 0.41 |
| Right ventricular systolic pressure (mmHg) | 56.5 | (47.0, 63.0) | 52.0 | (43.0, 63.0) | 0.19 |
| Pulmonary artery systolic pressure (mmHg) | 57.0 | (47.0, 66.0) | 50.5 | (44.5, 65.0) | 0.29 |
| Pulmonary artery diastolic pressure (mmHg) | 28.0 | (25.0, 34.0) | 28.0 | (23.0, 32.0) | 0.53 |
| Pulmonary artery mean pressure (mmHg) | 41.5 | (35.0, 47.0) | 37.5 | (32.0, 46.0) | 0.10 |
| Pulmonary capillary wedge mean pressure (mmHg) | 29.5 | (24.0, 37.0) | 27.0 | (23.0, 33.0) | 0.20 |
| Pulmonary artery pulsatility index (index value) | 2.0 | (1.3, 2.9) | 1.6 | (1.0, 2.2) | 0.109 |
| Pulmonary artery saturation (% O2) | 57.5 | (44.5, 62.0) | 53.0 | (47.0, 58.0) | 0.23 |
| Cardiac output (L/min) | 4.3 | (3.5, 5.7) | 3.9 | (3.5, 4.7) | 0.26 |
| Cardiac index (L/min/m2) | 2.1 | (1.8, 3.3) | 2.0 | (1.7, 2.3) | 0.12 |
| Systolic blood pressure (mmHg) | 105.0 | (97.5, 118.0) | 108.0 | (103.0, 121.0) | 0.15 |
| Diastolic blood pressure (mmHg) | 70.0 | (65.0, 78.0) | 74.0 | (69.0, 81.0) | 0.033 |
| Mean arterial pressure (mmHg) | 84.5 | (78.0, 94.0) | 87.3 | (82.0, 97.0) | 0.08 |
| PVR_preop, median (Q1, Q3) | 184.9 | (111.4, 252.1) | 201.2 | (134.9, 310.3) | |
| PVR_3mo, median (Q1, Q3) | 158.8 | (104.7, 233.7) | 162.0 | (132.1, 243.2) | |
| Papi_preop, median (Q1, Q3) | 2.0 | (1.3, 3.0) | 1.6 | (1.0, 2.3) | |
| Papi_3mo, median (Q1, Q3) | 2.0 | (1.6, 3.8) | 1.9 | (1.4, 3.1) | |
| RV dilation severity at preop, No. (%) | 0.038 | ||||
| Moderate–severe/severe | 4 | (7%) | 4 | (9%) | |
| Mild–moderate/moderate | 13 | (23%) | 21 | (48%) | |
| Mild/none/normal | 21 | (38%) | 13 | (30%) | |
| Variable | No Tricuspid Valve Repair (n = 56) | Tricuspid Valve Repair (n = 44) | p-Value | ||
|---|---|---|---|---|---|
| Perfusion Time (min), Median (Q1, Q3) | 81.0 | (58.5, 109.0) | 114.5 | (102.0, 130.5) | <0.001 |
| Cross-Clamp Time (min), Median (Q1, Q3) | 53.0 | (27.0, 69.0) | 51.0 | (21.0, 53.0) | 0.35 |
| TAVR, No. (%) | 0 | (0%) | 0 | (0%) | . |
| Aortic Valve Surgery, No. (%) | 3 | (5%) | 2 | (5%) | 0.85 |
| Mitral Valve Surgery, No. (%) | 0 | (0%) | 1 | (2%) | 0.26 |
| Pulmonic Valve Surgery, No. (%) | 0 | (0%) | 0 | (0%) | . |
| Other Non-Cardiac Surgery, No. (%) | 0 | (0%) | 1 | (2%) | 0.26 |
| LVA Repair, No. (%) | 0 | (0%) | 0 | (0%) | . |
| VSD Repair, No. (%) | 0 | (0%) | 0 | (0%) | . |
| ASD (PFO), No. (%) | 3 | (5%) | 9 | (20%) | 0.021 |
| Congenital Defect Repair, No. (%) | 0 | (0%) | 0 | (0%) | . |
| Laser Revascularization, No. (%) | 0 | (0%) | 0 | (0%) | . |
| Cardiac Trauma, No. (%) | 0 | (0%) | 0 | (0%) | . |
| AF Ablation Surgery, No. (%) | 0 | (0%) | 0 | (0%) | . |
| Surgery Type, No. (%) | 0.15 | ||||
| Elective | 5 | (9%) | 8 | (18%) | |
| Emergent | 3 | (5%) | 0 | (0%) | |
| Emergent Salvage | 0 | (0%) | 1 | (2%) | |
| Urgent | 48 | (86%) | 35 | (80%) | |
| Variable | No Tricuspid Valve Repair (n = 56) | Tricuspid Valve Repair (n = 44) | p-Value | ||
|---|---|---|---|---|---|
| Ambler score | 10.8 | ±7.2 | 14.1 | ±10.3 | 0.06 |
| Total intensive care unit hours | 143.7 | (95.6, 200.3) | 131.9 | (97.0, 311.9) | 0.78 |
| Total length of stay, days | 29.0 | (23.0, 41.0) | 28.5 | (19.5, 41.0) | 0.36 |
| Postoperative length of stay, days | 20.0 | (13.5, 28.5) | 17.5 | (13.0, 25.0) | 0.24 |
| Readmitted to the intensive care unit | 14 | (25) | 9 | (20) | 0.59 |
| Pre-discharge complications | 47 | (84) | 35 | (80) | 0.57 |
| Postop stroke > 24 h | 2 | (4) | 0 | (0) | 0.21 |
| Prolonged ventilation > 24 h | 35 | (63) | 24 | (55) | 0.42 |
| Pulmonary embolism | 1 | (2) | 0 | (0) | 0.37 |
| Pneumonia | 5 | (9) | 7 | (16) | 0.29 |
| Renal failure | 5 | (9) | 7 | (16) | 0.29 |
| Dialysis required | 5 | (9) | 5 | (11) | 0.69 |
| Cardiac arrest | 1 | (2) | 2 | (5) | 0.42 |
| Postoperative atrial fibrillation | 10 | (18) | 6 | (14) | 0.57 |
| Discharged to home | 38 | (75) | 21 | (51) | 0.021 |
| Readmission within 30 days | 9 | (18) | 17 | (44) | 0.007 |
| Operative mortality | 0 | (0) | 0 | (0) | 1 |
| Discharge mortality | 5 | (9) | 3 | (7) | 0.70 |
| 30-day mortality | 0 | (0) | 0 | (0) | 1 |
| Transplant | 26 | (46) | 16 | (36) | 0.311 |
| Variable | No Tricuspid Valve Repair (N = 56) | Tricuspid Valve Repair (N = 44) | p-Value | ||
|---|---|---|---|---|---|
| Pre-Discharge | |||||
| Left ventricular outflow tract cardiac index (L/min/m2) | 0.9 | (0.6, 1.4) | 1.7 | (1.1, 1.9) | 0.17 |
| Right ventricular outflow tract peak velocity (cm/s) | 59.4 | (43.6, 81.9) | 64.5 | (49.3, 86.5) | 0.37 |
| Tricuspid regurgitation peak gradient (cm/s) | 24.2 | (18.7, 29.4) | 20.1 | (15.2, 23.3) | 0.10 |
| Right ventricle dilated | 40 | (71) | 36 | (82) | 0.34 |
| Right ventricle dilation severity | 0.22 | ||||
| Moderate–severe/severe | 12 | (21) | 6 | (14) | |
| Mild–moderate/moderate | 15 | (27) | 13 | (30) | |
| Mild/none/normal | 6 | (11) | 11 | (25) | |
| Tricuspid regurgitation | <0.001 | ||||
| Moderate–severe/severe | 12 | (21) | 1 | (2) | |
| Mild–moderate/moderate | 12 | (21) | 5 | (11) | |
| Mild/none/normal | 23 | (41) | 35 | (80) | |
| Reduced right ventricle systolic function | 44 | (79) | 41 | (93) | 0.047 |
| Three-Month Follow-Up | |||||
| Right ventricular outflow tract peak velocity (cm/s) | 57.9 | (48.1, 63.4) | 56.1 | (47.4, 66.2) | 0.89 |
| Tricuspid regurgitation peak gradient (cm/s) | 22.0 | (18.7, 27.2) | 20.0 | (18.1, 24.8) | 0.25 |
| Right ventricular free wall strain (%) | −8.0 | (−10.2, −5.1) | −6.0 | (−8.6, −4.2) | 0.07 |
| Right ventricular fractional area change (%) | 21.5 | (16.3, 27.7) | 17.5 | (12.7, 24.8) | 0.08 |
| Improved right ventricular dilation severity | 11 | (20) | 2 | (5) | 0.005 |
| Reduced right ventricular systolic function | 33 | (59) | 31 | (70) | 0.41 |
| Tricuspid regurgitation worsened | 6 | (11) | 1 | (2) | 0.019 |
| One-Year Follow-Up | |||||
| Reduced right ventricular systolic function | 29 | (52) | 27 | (61) | 0.34 |
| Right ventricular systolic function worsened (compared to postoperative) | 3 | (5) | 7 | (16) | 0.18 |
| Tricuspid regurgitation worsened (compared to postoperative) | 2 | (4) | 1 | (2) | 0.021 |
| Variable | Coefficient (Log-Odds) | OR (95%CI) | p-Value | |
|---|---|---|---|---|
| Age, Tear | 1.00 | 0.95 | 1.07 | 0.89 |
| Female vs. Male | 0.37 | 0.02 | 6.03 | 0.48 |
| Concomitant TV Repaired | 3.58 | 0.25 | 50.26 | 0.34 |
| Right Ventricular Endocardial Global Longitudinal Strain at Preop | 0.62 | 0.33 | 1.14 | 0.12 |
| Right Ventricular Fractional Area Change at Preop | 0.83 | 0.65 | 1.07 | 0.15 |
| PVR at Preop | 1.01 | 1.00 | 1.013 | 0.11 |
| PAPI at Preop | 0.46 | 0.16 | 1.35 | 0.16 |
| RV Dilation Severity at Preop, No. (%) | Overall p-0.49 | |||
| Mild–Moderate/Moderate | 0.39 | −2.93 | 3.70 | 0.82 |
| Mild/None/Normal | −1.99 | −6.08 | 2.11 | 0.34 |
| Tricuspid Regurgitation at Preop | ||||
| Mild–Moderate/Moderate | −0.41 | −2.64 | 1.81 | 0.71 |
| ΔRV-FWS Model | ||
|---|---|---|
| Predictor | β (95% CI) | p-value |
| TVr (vs. No-TVr) | +1.45 (−0.18 to +3.09) | 0.08 |
| Preop RV-FWS (per 1%) | +0.28 (0.06–0.49) | 0.012 |
| ΔRV-FAC model | ||
| TVr (vs. No-TVr) | −2.45 (−5.84 to +0.95) | 0.16 |
| Preop RV-FAC (per 1%) | +0.35 (0.15–0.55) | <0.001 |
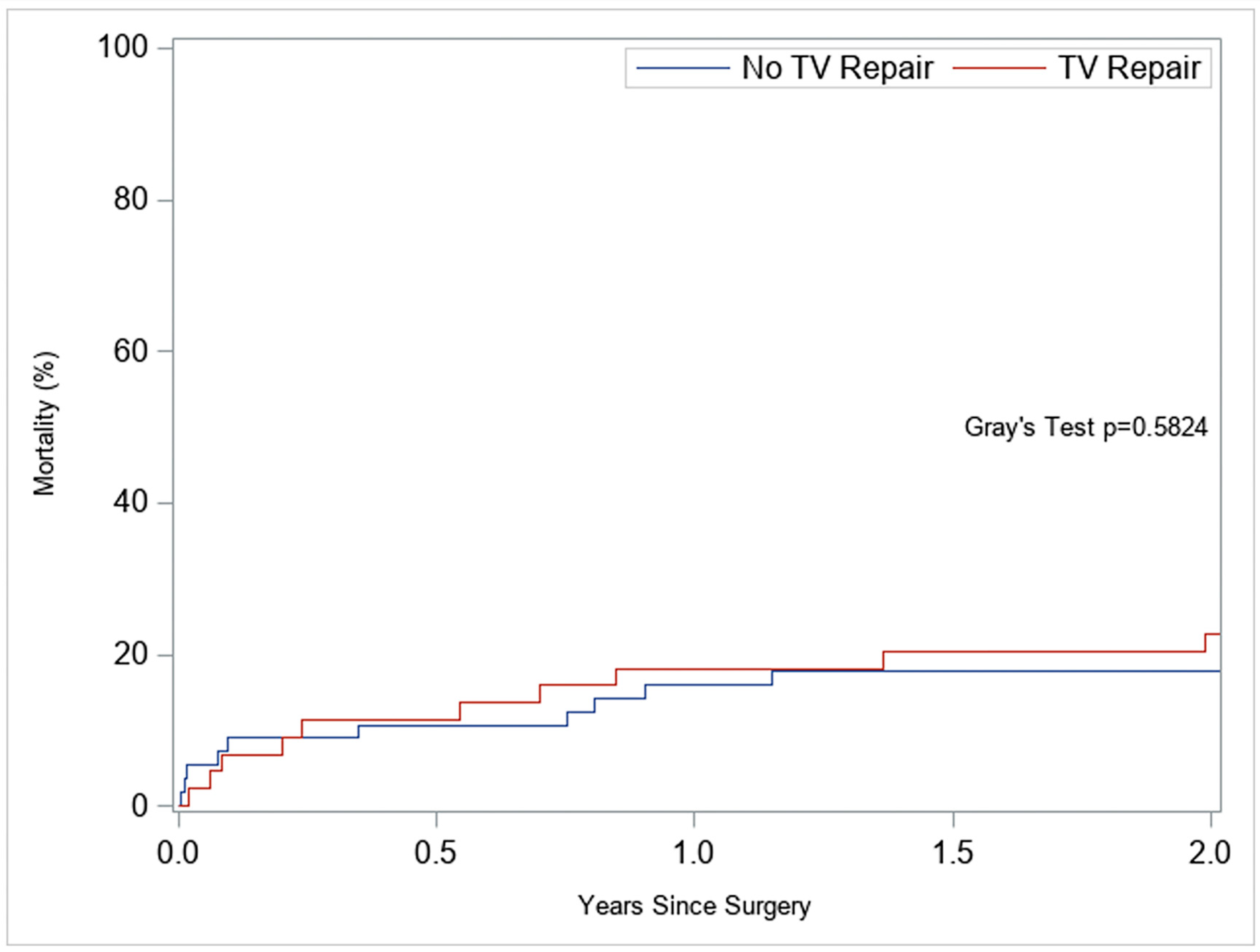
| Predictor | sHR (95% CI) | p-Value |
|---|---|---|
| Female | 0.30 (0.10–0.85) | 0.023 |
| TVr (vs. No-TVr) | 3.15 (0.96–10.33) | 0.058 |
| RV-FWS (preop) | 1.34 (1.12–1.60) | 0.002 |
| RV-FAC (preop) | 1.19 (1.06–1.33) | 0.003 |
| PVR (preop) | 0.998 (0.994–1.002) | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kislitsina, O.N.; Bharadwaj, S.N.; Wu, T.; Harap, R.; Kruse, J.; Vorovich, E.B.; Wilcox, J.E.; Yancy, C.W.; McCarthy, P.M.; Pham, D.T. Effect of Concomitant Tricuspid Valve Repair on Clinical and Echocardiographic Outcomes in Patients Undergoing Left Ventricular Assist Device Implantation. J. Clin. Med. 2025, 14, 7554. https://doi.org/10.3390/jcm14217554
Kislitsina ON, Bharadwaj SN, Wu T, Harap R, Kruse J, Vorovich EB, Wilcox JE, Yancy CW, McCarthy PM, Pham DT. Effect of Concomitant Tricuspid Valve Repair on Clinical and Echocardiographic Outcomes in Patients Undergoing Left Ventricular Assist Device Implantation. Journal of Clinical Medicine. 2025; 14(21):7554. https://doi.org/10.3390/jcm14217554
Chicago/Turabian StyleKislitsina, Olga N., Sandeep N. Bharadwaj, Tingqing Wu, Rebecca Harap, Jane Kruse, Esther B. Vorovich, Jane E. Wilcox, Clyde W. Yancy, Patrick M. McCarthy, and Duc T. Pham. 2025. "Effect of Concomitant Tricuspid Valve Repair on Clinical and Echocardiographic Outcomes in Patients Undergoing Left Ventricular Assist Device Implantation" Journal of Clinical Medicine 14, no. 21: 7554. https://doi.org/10.3390/jcm14217554
APA StyleKislitsina, O. N., Bharadwaj, S. N., Wu, T., Harap, R., Kruse, J., Vorovich, E. B., Wilcox, J. E., Yancy, C. W., McCarthy, P. M., & Pham, D. T. (2025). Effect of Concomitant Tricuspid Valve Repair on Clinical and Echocardiographic Outcomes in Patients Undergoing Left Ventricular Assist Device Implantation. Journal of Clinical Medicine, 14(21), 7554. https://doi.org/10.3390/jcm14217554







