Predicting Blood Pressure and Blood Pressure Variability in Spontaneous Intracerebral Hemorrhage in the Emergency Department Using Machine Learning
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Selection
2.2. Outcome Measures
2.3. Blood Pressure Variability
2.4. Data Collection and Management
2.5. Statistical Analysis
2.6. Machine Learning
3. Results
3.1. Patient Characteristics
3.2. Primary Outcome: Systolic Blood Pressure at ED Discharge
3.3. Secondary Outcomes: Blood Pressure Variability
3.3.1. Successive Variation in SBP (SBPSV)
3.3.2. Standard Deviation of SBP (SBPSD)
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. List of Variables Included in Random Forest and XGBoost Models (In the Order as They Appeared in Table 1)
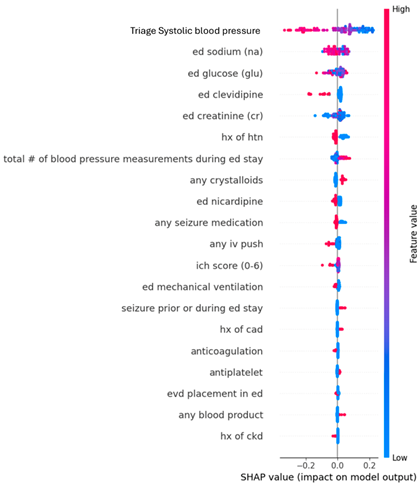
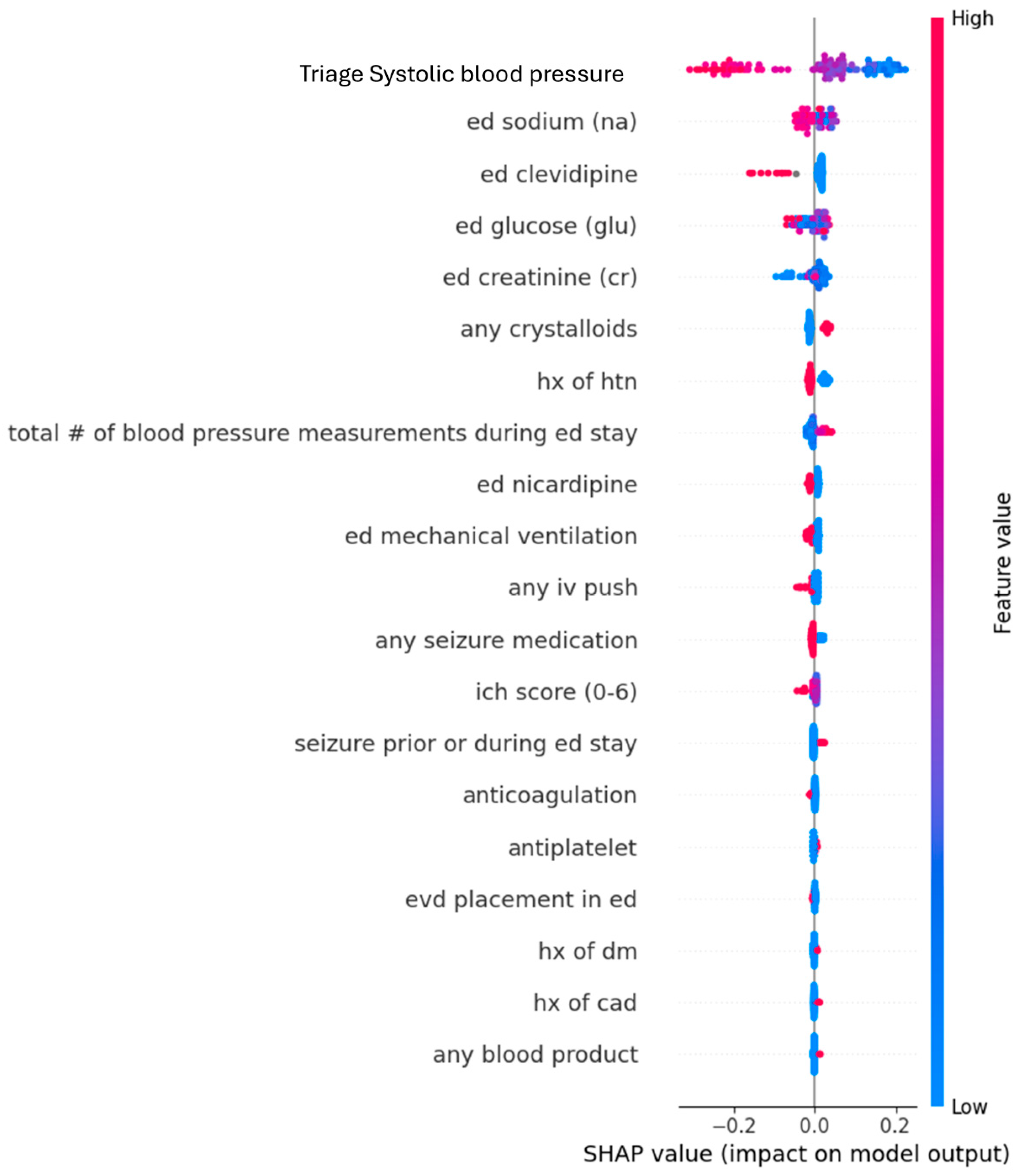
Appendix B. Dot Plots from XGBoost Analysis for Features Predicting SBP ≤ 160 mmHg upon Leaving Emergency Departments
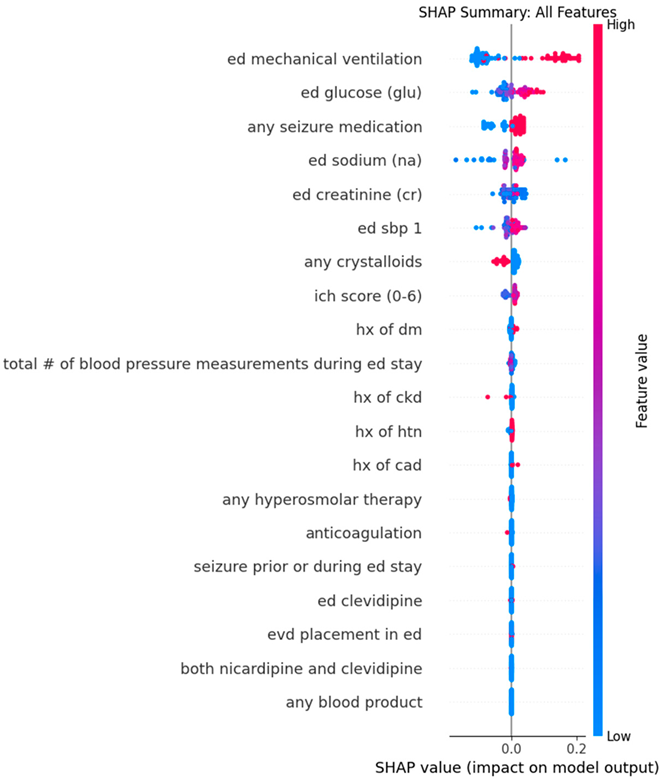
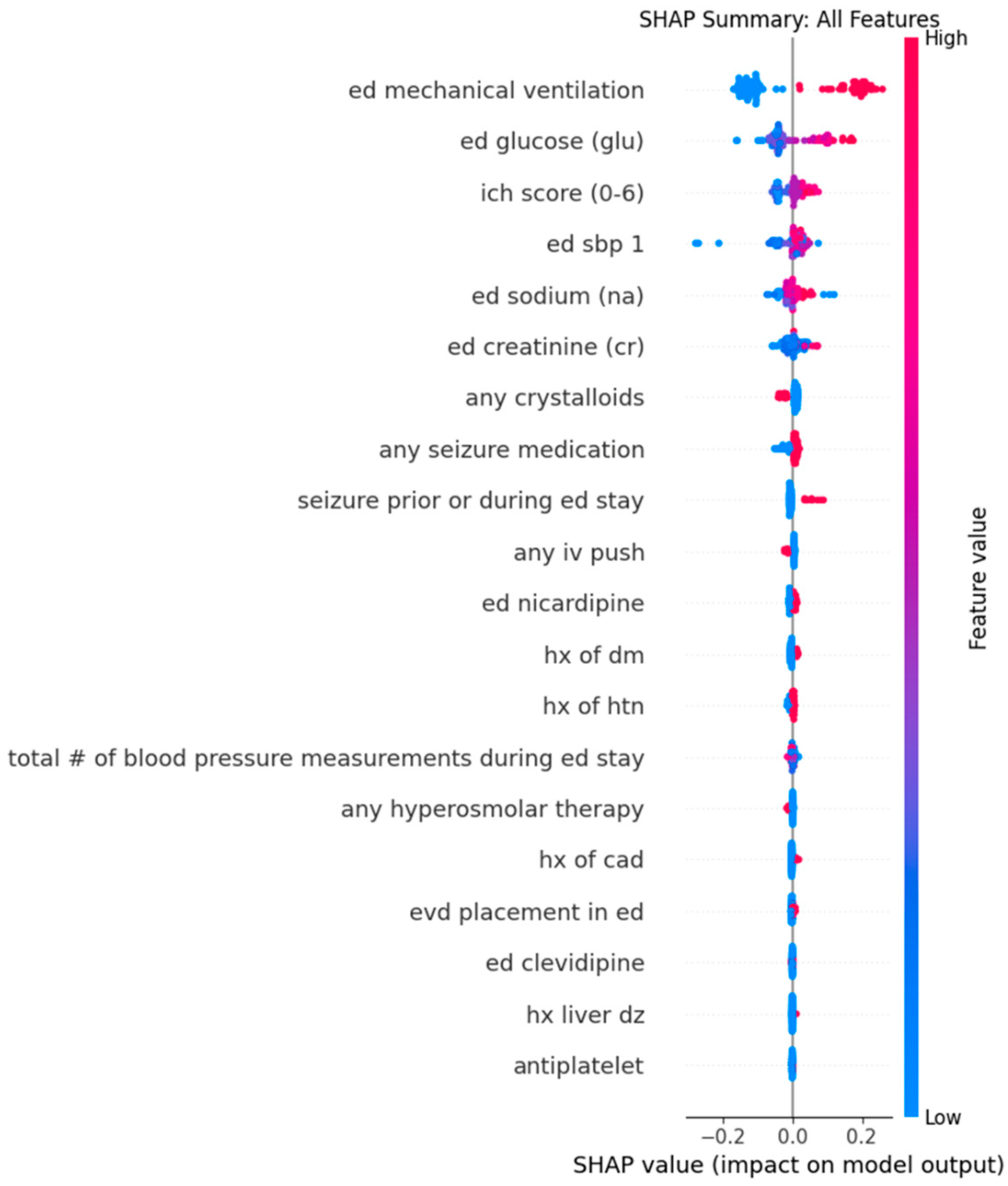
Appendix C. Dot Plots from XGBoost Analysis for Features Predicting Successive Variation in Systolic Blood Pressure During Emergency Department Stay
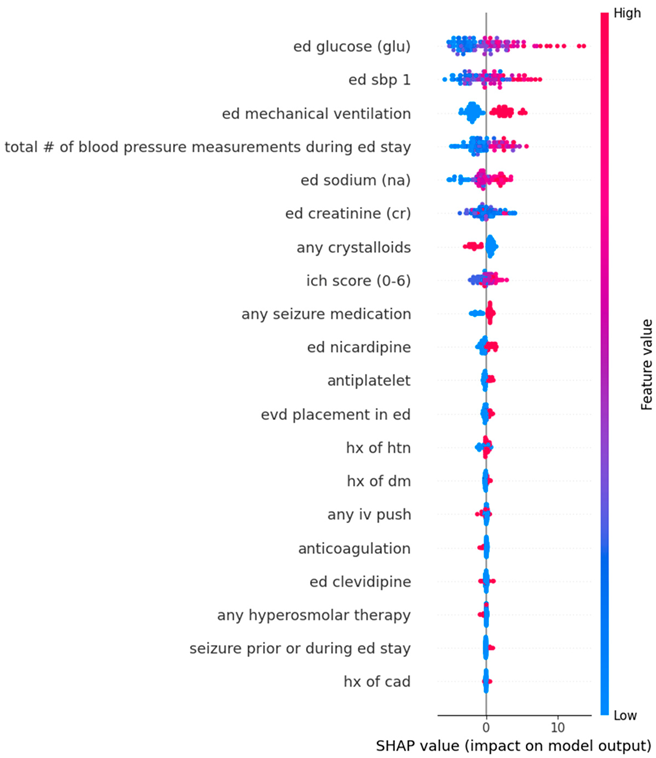
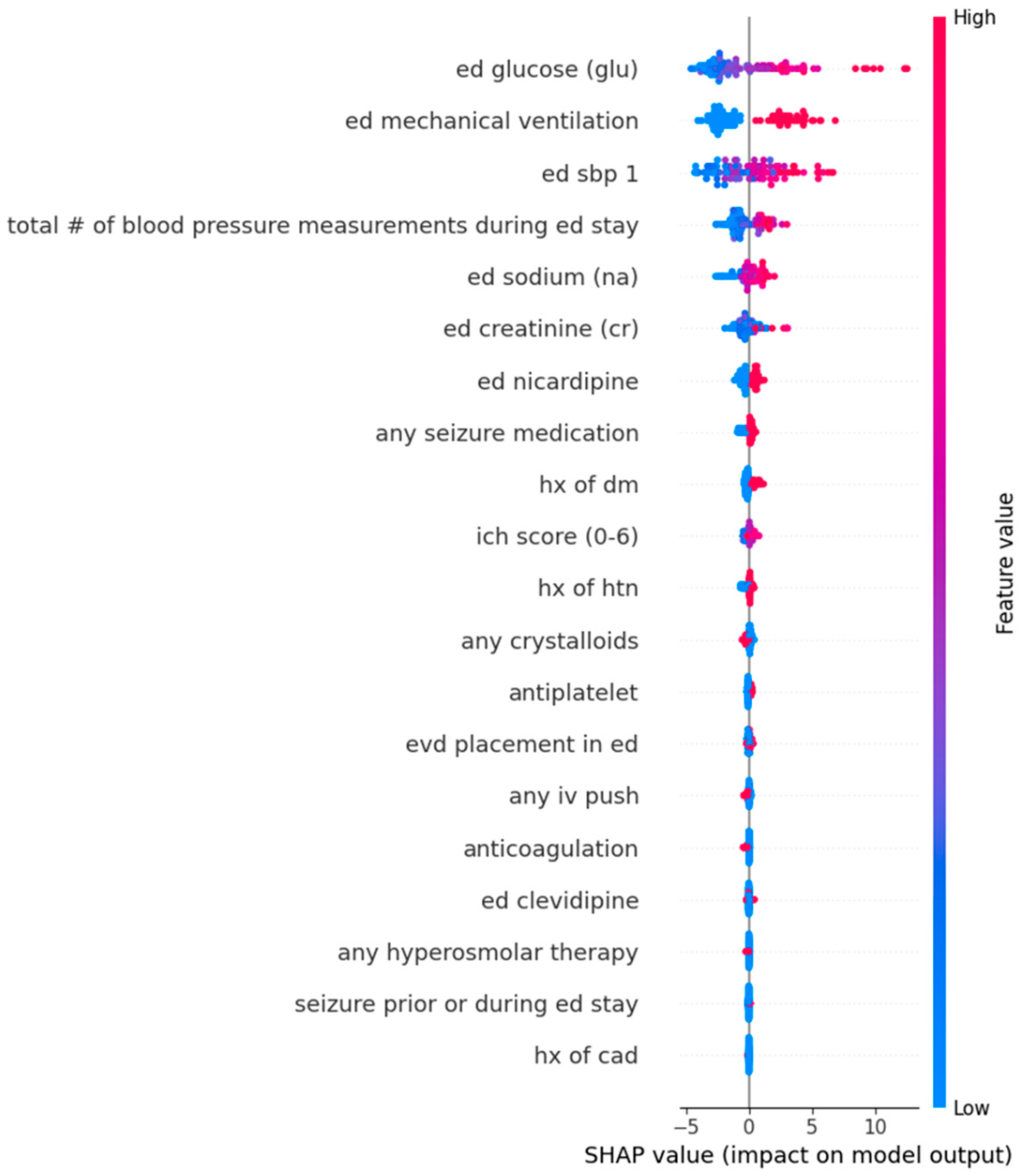
Appendix D. Dot Plots from Random Forest Analysis for Features Predicting Standard Deviation in Systolic Blood Pressure During Emergency Department Stay
Appendix F. Anderson–Darling’s Test
| Anderson–Darling’s Test Value | p-Value | |
| Age | 0.613 | 0.109 |
| Initial ED SBP | 0.626 | 0.101 |
| Initial ED heart rate | 2 | 0.05 |
| Sodium | 1.9 | 0.05 |
| ICH score | 3.6 | 0.05 |
| SBP upon leaving ED | 0.95 | 0.16 |
References
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.C.; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. 2022 Guideline for the Management of Patients with Spontaneous Intracerebral Hemorrhage: A Guideline from the American Heart Association/American Stroke Association. Stroke 2022, 53, E282–E361. [Google Scholar] [CrossRef] [PubMed]
- Broderick, J.P.; Adams, H.P.; Barsan, W.; Feinberg, W.; Feldmann, E.; Grotta, J.; Kase, C.; Krieger, D.; Mayberg, M.; Tilley, B.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage. Stroke 1999, 30, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Poomalai, G.; Prabhakar, S.; Jagadesh, N.S. Functional Ability and Health Problems of Stroke Survivors: An Explorative Study. Cureus 2023, 15, e33375. [Google Scholar] [CrossRef]
- Haupenthal, D.; Kuramatsu, J.B.; Volbers, B.; Sembill, J.A.; Mrochen, A.; Balk, S.; Hoelter, P.; Lücking, H.; Engelhorn, T.; Dörfler, A.; et al. Disability-Adjusted Life-Years Associated with Intracerebral Hemorrhage and Secondary Injury. JAMA Netw. Open 2021, 4, e2115859. [Google Scholar] [CrossRef]
- Sheth, K.N. Spontaneous Intracerebral Hemorrhage. N. Engl. J. Med. 2022, 387, 1589–1596. [Google Scholar] [CrossRef]
- Anderson, C.S.; Huang, Y.; Wang, J.G.; Arima, H.; Neal, B.; Peng, B.; Heeley, E.; Skulina, C.; Parsons, M.W.; Kim, J.S.; et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): A randomised pilot trial. Lancet Neurol. 2008, 7, 391–399. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Palesch, Y.Y.; Barsan, W.G.; Hanley, D.F.; Hsu, C.Y.; Martin, R.L.; Moy, C.S.; Silbergleit, R.; Steiner, T.; Suarez, J.I.; et al. Intensive Blood-Pressure Lowering in Patients with Acute Cerebral Hemorrhage. N. Engl. J. Med. 2016, 375, 1033–1043. [Google Scholar] [CrossRef]
- Tuteja, G.; Uppal, A.; Strong, J.; Nguyen, T.; Pope, K.; Jenkins, R.; Al Rebh, H.; Gatz, D.; Chang, W.-T.; Tran, Q.K. Interventions affecting blood pressure variability and outcomes after intubating patients with spontaneous intracranial hemorrhage. Am. J. Emerg. Med. 2019, 37, 1665–1671. [Google Scholar] [CrossRef]
- Tran, Q.K.; Nguyen, T.; Pope, K.; Capobianco, P.; Cao-Pham, M.; Hassan, S.; Kole, M.J.; O’COnnell, C.; Wessell, A.; Strong, J. Sedation patterns and hyperosmolar therapy in emergency departments were associated with blood pressure variability and outcomes in patients with spontaneous intracranial hemorrhage. J. Emergencies Trauma Shock 2020, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Alahmadi, H.; Vachhrajani, S.; Cusimano, M.D. The natural history of brain contusion: An analysis of radiological and clinical progression: Clinical article. J. Neurosurg. 2010, 112, 1139–1145. [Google Scholar] [CrossRef]
- Cepeda, S.; Gómez, P.A.; Castaño-Leon, A.M.; Martínez-Pérez, R.; Munarriz, P.M.; Lagares, A. Traumatic Intracerebral Hemorrhage: Risk Factors Associated with Progression. J. Neurotrauma 2015, 32, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, L.; Haase, D.J.; Boswell, K.; Dietrich, M.E.; Najafali, D.; Olexa, J.; Rea, J.; Sapru, M.; Scalea, T.; Tran, Q.K. Care intensity of spontaneous intracranial hemorrhage: Effectiveness of the critical care resuscitation unit. Am. J. Emerg. Med. 2021, 46, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.D.; Muir, K.W. INTERACT-2: Should Blood Pressure Be Aggressively Lowered Acutely After Intracerebral Hemorrhage? Stroke 2013, 44, 2951–2952. [Google Scholar] [CrossRef]
- Wang, X.; Ren, X.; Li, Q.; Ouyang, M.; Chen, C.; Delcourt, C.; Chen, X.; Wang, J.; Robinson, T.; Arima, H.; et al. Effects of blood pressure lowering in relation to time in acute intracerebral haemorrhage: A pooled analysis of the four INTERACT trials. Lancet Neurol. 2025, 24, 571–579. [Google Scholar] [CrossRef]
- Allison, T.A.; Bowman, S.; Gulbis, B.; Hartman, H.; Schepcoff, S.; Lee, K. Comparison of Clevidipine and Nicardipine for Acute Blood Pressure Reduction in Patients with Stroke. J. Intensive Care Med. 2019, 34, 990–995. [Google Scholar] [CrossRef]
- Seifi, A.; Jafari, A.A.; Mirmoeeni, S.; Shah, M.; Jafari, M.A.; Nazari, S.; Asgarzadeh, S.; Godoy, D.A. Comparison between clevidipine and nicardipine in cerebrovascular diseases: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2023, 227, 107644. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Baskett, W.I.; Lodhi, A.; Gomez, F.; Arora, N.; Chandrasekaran, P.N.; Siddiq, F.; Gomez, C.R.; Shyu, C.-R. Assessment of Blood Pressure and Heart Rate Related Variables in Acute Stroke Patients Receiving Intravenous Antihypertensive Medication Infusions. Neurocritical Care 2024, 41, 434–444. [Google Scholar] [CrossRef]
- Cole, N.I.; Suckling, R.J.; Swift, P.A.; He, F.J.; MacGregor, G.A.; Hinton, W.; van Vlymen, J.; Hayward, N.; Jones, S.; de Lusignan, S. The association between serum sodium concentration, hypertension and primary cardiovascular events: A retrospective cohort study. J. Hum. Hypertens. 2019, 33, 69–77. [Google Scholar] [CrossRef]
- Chamarthi, B.; Williams, J.S.; Williams, G.H. A mechanism for salt-sensitive hypertension: Abnormal dietary sodium-mediated vascular response to angiotensin-II. J. Hypertens. 2010, 28, 1020–1026. [Google Scholar] [CrossRef]
- Libianto, R.; Batu, D.; MacIsaac, R.J.; Cooper, M.E.; Ekinci, E.I. Pathophysiological Links Between Diabetes and Blood Pressure. Can. J. Cardiol. 2018, 34, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Panchapakesan, U.; Pollock, C.; Saad, S. Renal epidermal growth factor receptor: Its role in sodium and water homeostasis in diabetic nephropathy. Clin. Exp. Pharmacol. Physiol. 2011, 38, 84–88. [Google Scholar] [CrossRef]
- Harita, N.; Hayashi, T.; Sato, K.K.; Nakamura, Y.; Yoneda, T.; Endo, G.; Kambe, H. Lower Serum Creatinine Is a New Risk Factor of Type 2 Diabetes. Diabetes Care 2009, 32, 424–426. [Google Scholar] [CrossRef]
- Moullaali, T.J.; Wang, X.; Martin, R.H.; Shipes, V.B.; Robinson, T.G.; Chalmers, J.; I Suarez, J.; I Qureshi, A.; Palesch, Y.Y.; Anderson, C.S. Blood pressure control and clinical outcomes in acute intracerebral haemorrhage: A preplanned pooled analysis of individual participant data. Lancet Neurol. 2019, 18, 857–864. [Google Scholar] [CrossRef]
- Hou, D.; Liu, B.; Zhang, J.; Wang, Q.; Zheng, W. Evaluation of the Efficacy and Safety of Short-Course Deep Sedation Therapy for the Treatment of Intracerebral Hemorrhage After Surgery: A Non-Randomized Control Study. Med. Sci. Monit. 2016, 22, 2670–2678. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Li, F.; Li, B.; Chen, Q.; Huang, X.; Zhang, J.; Huang, Q.; Zhang, Z.; Cao, Y.; Yang, M.; et al. Effects of an Early Intensive Blood Pressure–lowering Strategy Using Remifentanil and Dexmedetomidine in Patients with Spontaneous Intracerebral Hemorrhage: A Multicenter, Prospective, Superiority, Randomized Controlled Trial. Anesthesiology 2024, 141, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Rouxinol-Dias, A.L.; Gonçalves, M.L.; Ramalho, D.; Silva, J.; Barbosa, L.; Polónia, J. Comparison of Blood Pressure Variability between 24 h Ambulatory Monitoring and Office Blood Pressure in Diabetics and Nondiabetic Patients: A Cross-Sectional Study. Int. J. Hypertens. 2022, 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bakkar, N.-M.Z.; Dwaib, H.S.; Fares, S.; Eid, A.H.; Al-Dhaheri, Y.; El-Yazbi, A.F. Cardiac Autonomic Neuropathy: A Progressive Consequence of Chronic Low-Grade Inflammation in Type 2 Diabetes and Related Metabolic Disorders. Int. J. Mol. Sci. 2020, 21, 9005. [Google Scholar] [CrossRef]
- Sun, S.; Fei, X.; Gong, K.; Huang, X. Dynamic sodium dysregulation predicts mortality in intracerebral hemorrhage: A retrospective cohort study of temporal variability and absolute thresholds. Neurosurg. Rev. 2025, 48, 615. [Google Scholar] [CrossRef]
- Tsaousi, G.; Stazi, E.; Cinicola, M.; Bilotta, F. Cardiac output changes after osmotic therapy in neurosurgical and neurocritical care patients: A systematic review of the clinical literature. Br. J. Clin. Pharmacol. 2018, 84, 636–648. [Google Scholar] [CrossRef]
- Orešković, D.; Maraković, J.; Varda, R.; Radoš, M.; Jurjević, I.; Klarica, M. New Insight into the Mechanism of Mannitol Effects on Cerebrospinal Fluid Pressure Decrease and Craniospinal Fluid Redistribution. Neuroscience 2018, 392, 164–171. [Google Scholar] [CrossRef]
- Stocchetti, N.; Maas, A.I. Traumatic Intracranial Hypertension. N. Engl. J. Med. 2014, 370, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, Q.; Wang, J.; Wang, D.W.; Ding, H.; Wang, W. Prognostic value of elevated cardiac troponin I in patients with intracerebral hemorrhage. Clin. Cardiol. 2019, 43, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Oras, J.; Grivans, C.; Bartley, A.; Rydenhag, B.; Ricksten, S.-E.; Seeman-Lodding, H. Elevated high-sensitive troponin T on admission is an indicator of poor long-term outcome in patients with subarachnoid haemorrhage: A prospective observational study. Crit. Care 2015, 20, 11. [Google Scholar] [CrossRef] [PubMed]
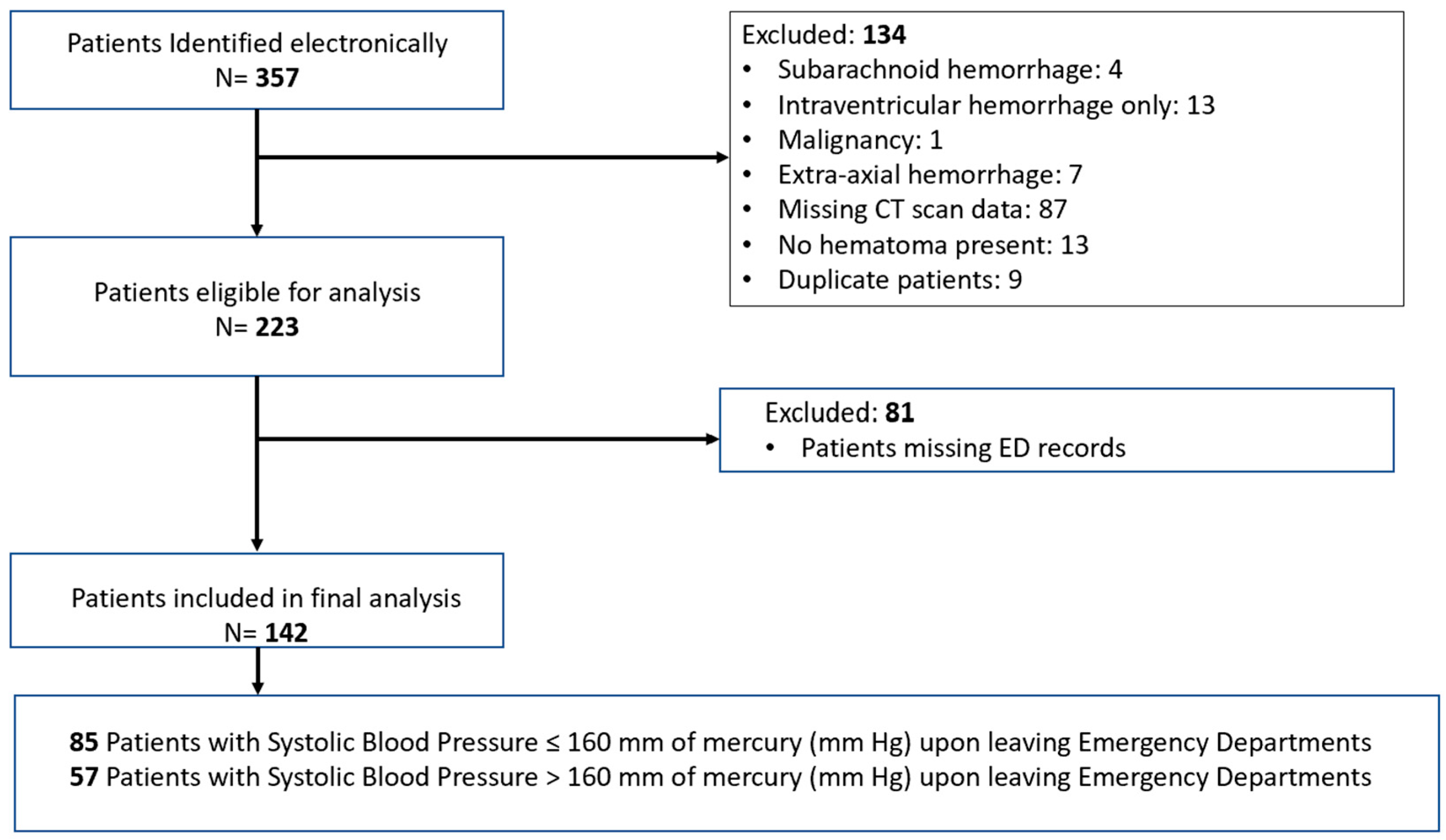
| Parameters | All Patients | SBP ≤ 160 mmHg | SBP > 160 mmHg | Difference Between Group | 95% CI | p |
|---|---|---|---|---|---|---|
| N = 142 | N = 85 (60%) | N = 57 (40%) | ||||
| Demographics | ||||||
| Age, years, mean (SD) | 62.6 (14.8) | 62.5 (16.1) | 62.7 (12.7) | −0.24 | (−5.04, 4.57) | 0.92 |
| Age ≥ 80 years, N (%) | 19 (13.4) | 13 (15.3) | 6 (10.5) | 0.05 | (−0.06, 0.16) | 0.4 |
| Past medical history, N (%) | ||||||
| Coronary Artery Disease | 15 (10.6) | 11 (12.9) | 4 (7) | 0.06 | (−0.04, 0.16) | 0.4 |
| Diabetes Mellitus | 37 (26.1) | 22 (25.9) | 15 (26.3) | −0.004 | (−0.15, 0.14) | 0.95 |
| Hypertension | 102 (71.8) | 53 (62.4) | 49 (86) | −0.24 | (−0.37, −0.10) | 0.001 |
| Chronic Kidney Disease | 13 (9.2) | 9 (10.6) | 4 (7) | 0.04 | (−0.06, 0.13) | 0.56 |
| Any liver disease | 2 (1.4) | 2 (2.4) | 0 (0) | 0.02 | (−0.01, 0.06) | 0.52 |
| Past medications, N (%) | ||||||
| Any antiplatelet | 39 (27.5) | 22 (25.9) | 17 (29.8) | −0.04 | (−0.19, 0.11) | 0.61 |
| Any anticoagulation | 22 (15.5) | 14 (16.5) | 8 (14) | 0.02 | (−0.10, 0.14) | 0.69 |
| Clinical characteristics upon ED admission | ||||||
| Initial ED SBP (mmHg), mean (SD) | 173.6 (37.5) | 161.6 (33.1) | 191.4 (36.7) | −30 | (−41.83, −17.89) | <0.001 |
| Initial ED heart rate (bpm), mean (SD) | 85.8 (22.3) | 86.9 (21.9) | 83.9 (23.3) | 3 | (−5.28, 11.25) | 0.48 |
| Sodium (mEq/L), mean (SD) | 139.1 (3.6) | 139.1 (3.6) | 139.2 (3.7) | −0.06 | (−1.29, 1.17) | 0.93 |
| Creatinine (mg/dL), median [IQR] | 1 [0.8–1.2] | 0.9 [0.8–1.2] | 1 [0.8–1.3] | −0.1 | (−0.20, 0.02) | 0.13 |
| Glucose (mg/dL), median [IQR] | 139.5 [118–177.3] | 140 [119–177] | 137 [115–177.5] | 1 | (−14, 15) | 0.92 |
| Other clinical characteristics | ||||||
| GCS on ED admission, median [IQR] | 13 [8.8–15] | 13 [9.5–15] | 13 [7–15] | 0 | (0, 2) | 0.15 |
| Presenting parenchymal hematoma volume (mL), median [IQR] | 19 [7.3–35.9] | 16 [7.5–34] | 21.3 [7–39.4] | −1.71 | (−7.82, 3.25) | 0.5 |
| ICH volume ≥ 30 mL, N (%) | 50 (35.2) | 28 (32.9) | 22 (38.6) | −0.06 | (−0.22, 0.10) | 0.49 |
| Intraventricular hemorrhage, N (%) | 83 (58.5) | 42 (49.4) | 41 (71.9) | −0.23 | (−0.38, −0.07) | 0.01 |
| Infratentorial bleed, N (%) | 27 (19) | 15 (17.6) | 12 (21.1) | −0.03 | (−0.17, 0.10) | 0.62 |
| ICH score, mean (SD) | 1.8 (1.1) | 1.7 (1.0) | 2.1 (1.2) | −0.38 | (−0.76, −0.002) | 0.05 |
| Clinical seizure prior or during ED stay, N (%) | 15 (10.6) | 11 (12.9) | 4 (7) | 0.06 | (−0.04, 0.16) | 0.4 |
| Mechanical ventilation, N (%) | 57 (40.1) | 31 (36.5) | 26 (45.6) | −0.09 | (−0.26, 0.07) | 0.28 |
| Interval of triage to ED mechanical ventilation (minutes), median [IQR] | 63 [36.5–116.5] | 67 [33–149] | 57.5 [41.5–94] | 14 | (−13, 48) | 0.26 |
| EVD placement in ED, N (%) | 44 (31) | 25 (29.4) | 19 (33.3) | −0.04 | (−0.20, 0.12) | 0.62 |
| ED Length of stay (minutes), median [IQR] | 174.5 [129.8–269.8] | 207 [150–286] | 146 [103.5–220.5] | 57 | (26, 88) | <0.001 |
| SBP at Leaving ED (mmHg), mean (SD) | 153.9 (31.2) | 133.2 (16.1) | 184.7 (21.3) | −51.5 | (−58, −45) | <0.001 |
| Mortality (Hospice/Death), N (%) | 31 (21.8) | 18 (21.2) | 13 (22.8) | −0.02 | (−0.16, 0.12) | 0.82 |
| Medical therapy | ||||||
| Any crystalloids, N (%) | 39 (27.5) | 30 (35.3) | 9 (15.8) | 0.2 | (0.06, 0.33) | 0.01 |
| Any Nicardipine, N (%) | 63 (44.4) | 32 (37.6) | 31 (54.4) | −0.17 | (−0.33, −0.002) | 0.05 |
| Interval of triage to ED Nicardipine infusion (minutes), median [IQR] | 68 [33–133.5] | 104 [36–144.5] | 56 [22–99.5] | 27 | (−1, 66) | 0.06 |
| Any Clevidipine, N (%) | 17 (12) | 4 (4.7) | 13 (22.8) | −0.18 | (−0.30, −0.06) | 0.003 |
| Interval of triage to ED Clevidipine infusion (minutes), median [IQR] | 45 [27.5–76.5] | 59.5 [42.8–198.5] | 32 [22–69.5] | 22 | (−18, 182) | 0.23 |
| Both Nicardipine and Clevidipine, N (%) | 1 (0.7) | 1 (1.2) | 0 (0) | 0.01 | (−0.01, 0.03) | 0.99 |
| Any IV push antihypertensive, N (%) | 31 (21.8) | 16 (18.8) | 15 (26.3) | −0.07 | (−0.22, 0.07) | 0.3 |
| >1 IV push, N (%) | 4 (2.8) | 2 (2.4) | 2 (3.5) | −0.01 | (−0.07, 0.05) | 0.99 |
| Interval of triage to first ED IV push (minutes), median [IQR] | 66 [33–109] | 74 [36.8–104.5] | 58 [25–120] | 6 | (−38, 47) | 0.76 |
| Interval of triage to second ED IV push (minutes), median [IQR] | 278 [199.3–1431.5] | 1064 [329–1799] | 208.5 [190–227] | 856 | (102, 1609) | 0.25 |
| Any seizure medication, N (%) | 99 (69.7) | 57 (67.1) | 42 (73.7) | −0.07 | (−0.22, 0.09) | 0.39 |
| Any Phenytoin, N (%) | 4 (2.8) | 4 (4.7) | 0 (0) | 0.05 | (0.002, 0.09) | 0.15 |
| Any Levetiracetam/Keppra, N (%) | 96 (67.6) | 54 (63.5) | 42 (73.7) | −0.1 | (−0.25, 0.05) | 0.2 |
| >1 seizure medication, N (%) | 1 (0.7) | 1 (1.2) | 0 (0) | 0.01 | (−0.01, 0.03) | 0.99 |
| Any hyperosmolar therapy, N (%) | 21 (14.8) | 13 (15.3) | 8 (14) | 0.01 | (−0.11, 0.13) | 0.84 |
| 3% saline, N (%) | 2 (1.4) | 2 (2.4) | 0 (0) | 0.02 | (−0.01, 0.06) | 0.52 |
| Mannitol, N (%) | 19 (13.4) | 11 (12.9) | 8 (14) | −0.01 | (−0.13, 0.10) | 0.85 |
| Any blood product, N (%) | 12 (8.5) | 7 (8.2) | 5 (8.8) | −0.01 | (−0.10, 0.09) | 0.91 |
| Fresh frozen plasma, N (%) | 2 (1.4) | 1 (1.2) | 1 (1.8) | −0.01 | (−0.05, 0.04) | 0.99 |
| Platelets, N (%) | 3 (2.1) | 1 (1.2) | 2 (3.5) | −0.02 | (−0.08, 0.03) | 0.56 |
| PCC, N (%) | 9 (6.3) | 6 (7.1) | 3 (5.3) | 0.02 | (−0.06, 0.10) | 0.74 |
| Blood pressure variability | ||||||
| SBP standard deviation, median [IQR] | 18.7 [12.1–26] | 16.3 [11–25.6] | 20.2 [13.9–27.9] | −3.31 | (−6.74, 0.08) | 0.05 |
| SBP successive variation, median [IQR] | 18.4 [12.5–25.9] | 18.1 [11.2–23.5] | 21 [14.4–29.9] | −3.18 | (−6.60, 0.04) | 0.05 |
| Heart rate variability | N = 132 | N = 80 | N = 52 | |||
| Heart rate standard deviation, median [IQR] | 7.9 [4.7–12.2] | 7.7 [4.1–10.6] | 8.6 [5.4–12.7] | −1.37 | (−3.35, 0.43) | 0.15 |
| Heart rate successive variation, median [IQR] | 8.5 [4.6–12.9] | 7.8 [4.4–11.7] | 10.2 [5.1–13.5] | −1.32 | (−3.33, 0.84) | 0.19 |
| Hematoma progression | ||||||
| Hematoma Volume Change Score ≥ 30%, N (%) | 40 (28.2) | 20 (23.5) | 20 (35.1) | −0.12 | (−0.27, 0.04) | 0.14 |
| Absolute Hematoma Change Score ≥ 12.5 mL, N (%) | 20 (14.1) | 11 (12.9) | 9 (15.8) | −0.03 | (−0.15, 0.09) | 0.64 |
| Outcome: SBP < 160 at Leaving ED | ||
|---|---|---|
| Random Forest Classification | XGBoost Classification | |
| Accuracy | 72.40% | 79.30% |
| F1 score | 0.76 | 0.79 |
| Feature 1 (mean absolute SHAP value) | Triage SBP (0.14) | Triage SBP (0.13) |
| Feature 2 (mean absolute SHAP value) | Serum sodium (0.028) | Serum Sodium (0.034) |
| Feature 3 (mean absolute SHAP value) | Clevidipine (0.026) | Serum Glucose (0.029) |
| Feature 4 (mean absolute SHAP value) | Serum Glucose (0.022) | Clevidipine (0.027) |
| Feature 5 (mean absolute SHAP value) | Serum Creatinine (0.021) | Serum Creatinine (0.026) |
| Outcome: Successive Variation in Systolic Blood Pressure During ED | ||
|---|---|---|
| Random Forest | XGBoost | |
| Root mean squared error (RMSE) * | 24.21 | 24.94 |
| Feature 1 (mean absolute SHAP value) | Mechanical ventilation in ED (0.15) | Mechanical ventilation in ED (0.11) |
| Feature 2 (mean absolute SHAP value) | Serum Glucose (0.064) | Serum Sodium (0.030) |
| Feature 3 (mean absolute SHAP value) | ICH score (0.029) | Any seizure medication (0.029) |
| Feature 4 (mean absolute SHAP value) | Triage SBP (0.027) | Serum Sodium (0.028) |
| Feature 5 (mean absolute SHAP value) | Serum Sodium (0.019) | Serum Creatinine (0.017) |
| Outcome: Standard Deviation in Systolic Blood Pressure During ED | ||
|---|---|---|
| Random Forest | XGBoost | |
| Root mean squared error (RMSE) * | 13.66 | 14.46 |
| Feature 1 (mean absolute SHAP value) | Serum Glucose (2.79) | Serum Glucose (2.84) |
| Feature 2 (mean absolute SHAP value) | Mechanical ventilation in ED (2.60) | Mechanical ventilation in ED (2.56) |
| Feature 3 (mean absolute SHAP value) | Triage SBP (2.05) | Triage SBP (2.36) |
| Feature 4 (mean absolute SHAP value) | Total number of BP Measurements (1.06) | Total number of BP Measurements (1.12) |
| Feature 5 (mean absolute SHAP value) | Serum Sodium (0.66) | Serum Sodium (0.64) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leggett, E.; Kim, A.; Jaddu, S.; Patel, P.; Seyoum, N.Y.; Zahid, M.; Chan, A.; Syed, H.; Shapsay, M.; Dreizin, D.; et al. Predicting Blood Pressure and Blood Pressure Variability in Spontaneous Intracerebral Hemorrhage in the Emergency Department Using Machine Learning. J. Clin. Med. 2025, 14, 7800. https://doi.org/10.3390/jcm14217800
Leggett E, Kim A, Jaddu S, Patel P, Seyoum NY, Zahid M, Chan A, Syed H, Shapsay M, Dreizin D, et al. Predicting Blood Pressure and Blood Pressure Variability in Spontaneous Intracerebral Hemorrhage in the Emergency Department Using Machine Learning. Journal of Clinical Medicine. 2025; 14(21):7800. https://doi.org/10.3390/jcm14217800
Chicago/Turabian StyleLeggett, Emmeline, Abigail Kim, Shriya Jaddu, Priya Patel, Nahom Y. Seyoum, Manahel Zahid, Angie Chan, Hassan Syed, Milana Shapsay, David Dreizin, and et al. 2025. "Predicting Blood Pressure and Blood Pressure Variability in Spontaneous Intracerebral Hemorrhage in the Emergency Department Using Machine Learning" Journal of Clinical Medicine 14, no. 21: 7800. https://doi.org/10.3390/jcm14217800
APA StyleLeggett, E., Kim, A., Jaddu, S., Patel, P., Seyoum, N. Y., Zahid, M., Chan, A., Syed, H., Shapsay, M., Dreizin, D., Olexa, J., Walker, J. A., Cardona, S., & Tran, Q. K. (2025). Predicting Blood Pressure and Blood Pressure Variability in Spontaneous Intracerebral Hemorrhage in the Emergency Department Using Machine Learning. Journal of Clinical Medicine, 14(21), 7800. https://doi.org/10.3390/jcm14217800






