The Impact of Immune-Related Adverse Events on the Survival of Patients Treated with Immune Checkpoint Inhibitors: The Distinct Role of Cardiac Toxicities
Abstract
1. Introduction
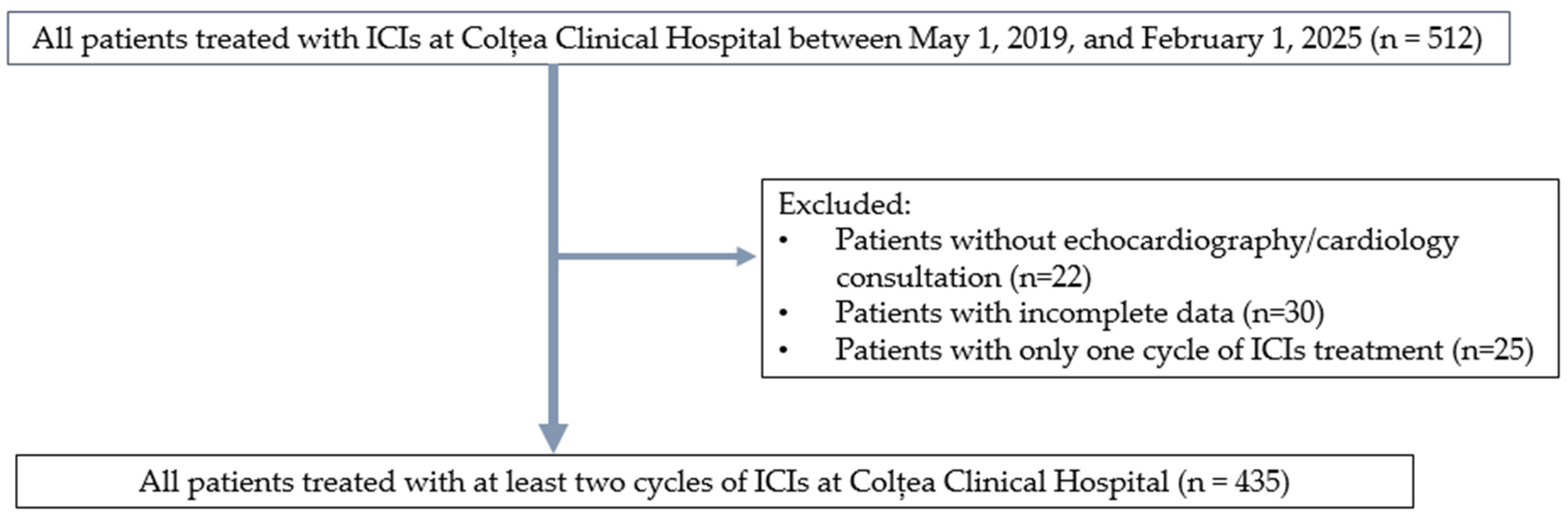
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analysis
3. Results
3.1. Demographic Analysis
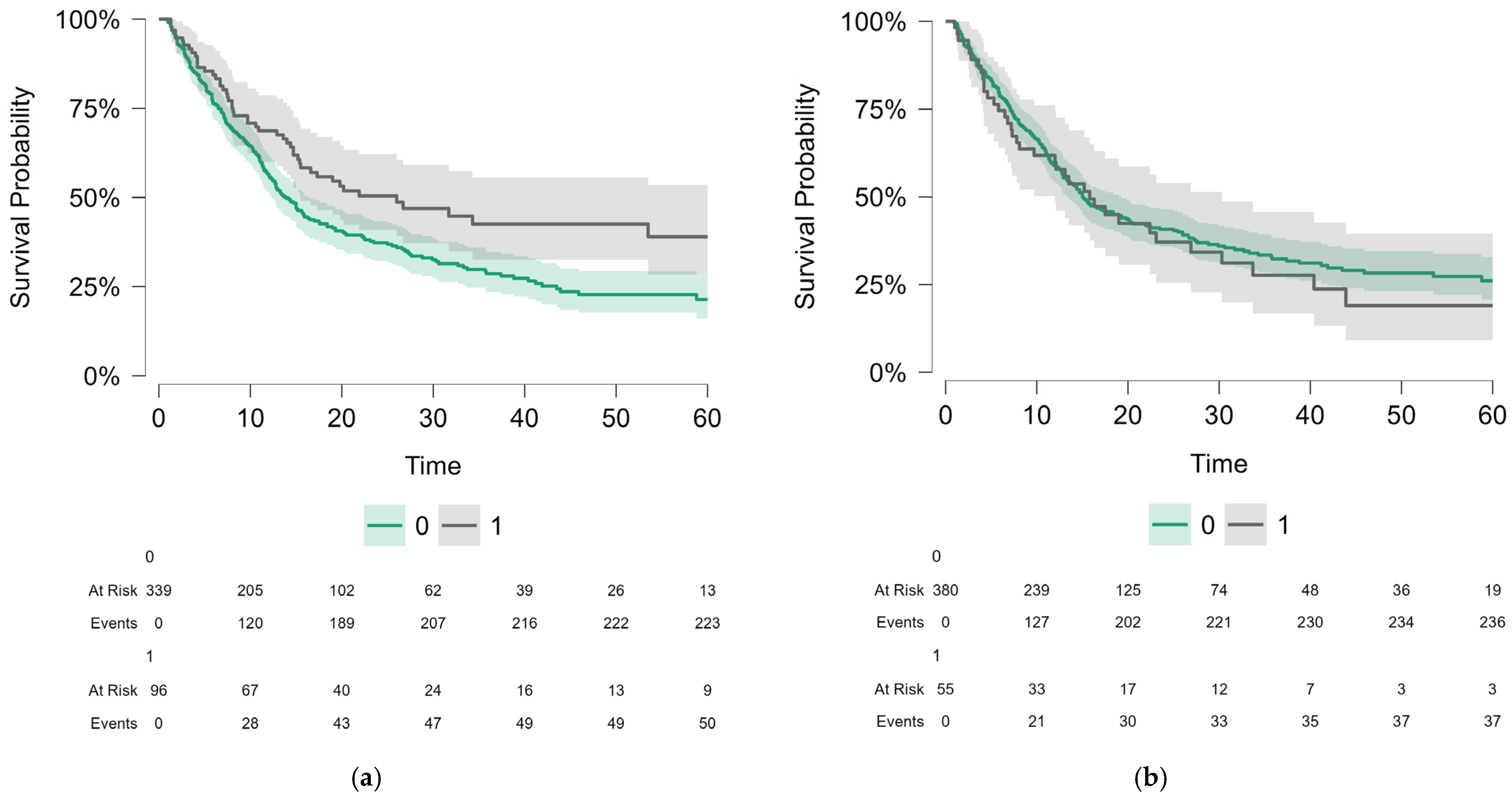
3.2. Survival Analysis for the Whole Population
3.3. Survival Analysis for Cancer Subgroups
4. Discussion
4.1. General Considerations
4.2. Non-Cardiac irAEs as Prognostic Markers
4.2.1. Dermatologic Toxicities
4.2.2. Endocrine Toxicities
4.2.3. Gastrointestinal and Hepatic Toxicities
4.2.4. Pulmonary Toxicities
4.2.5. Renal Toxicities (Nephritis)
4.3. Cardiac irAEs: Neutral or Negative Impact
4.4. Mechanisms of ICI-Associated Cardiotoxicity
4.5. Clinical Implications
4.6. Relationship Between PFS and OS
4.7. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nardin, S.; Ruffilli, B.; Costantini, P.; Mollace, R.; Taglialatela, I.; Pagnesi, M.; Chiarito, M.; Soldato, D.; Cao, D.; Conte, B.; et al. Navigating Cardiotoxicity in Immune Checkpoint Inhibitors: From Diagnosis to Long-Term Management. J. Cardiovasc. Dev. Dis. 2025, 12, 270. [Google Scholar] [CrossRef]
- Heemelaar, J.C.; Louisa, M.; Neilan, T.G. Treatment of Immune Checkpoint Inhibitor-Associated Myocarditis. J. Cardiovasc. Pharmacol. 2024, 83, 384–391. [Google Scholar] [CrossRef]
- Cook, S.; Samuel, V.; Meyers, D.E.; Stukalin, I.; Litt, I.; Sangha, R.; Morris, D.G.; Heng, D.Y.C.; Pabani, A.; Dean, M.; et al. Immune-Related Adverse Events and Survival Among Patients With Metastatic NSCLC Treated With Immune Checkpoint Inhibitors. JAMA Netw. Open 2024, 7, e2352302. [Google Scholar] [CrossRef]
- Lin, L.; Liu, Y.; Chen, C.; Wei, A.; Li, W. Association between Immune-Related Adverse Events and Immunotherapy Efficacy in Non-Small-Cell Lung Cancer: A Meta-Analysis. Front. Pharmacol. 2023, 14, 1190001. [Google Scholar] [CrossRef]
- Hussaini, S.; Chehade, R.; Boldt, R.G.; Raphael, J.; Blanchette, P.; Maleki Vareki, S.; Fernandes, R. Association between Immune-Related Side Effects and Efficacy and Benefit of Immune Checkpoint Inhibitors—A Systematic Review and Meta-Analysis. Cancer Treat. Rev. 2021, 92, 102134. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.L.; Juhl, C.B.; Nielsen, O.H.; Chen, I.M.; Herrmann, J. Immune Checkpoint Inhibitor–Induced Cardiotoxicity. JAMA Oncol. 2024, 10, 1390. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Xiong, L.; Shen, Y.; Zhou, Z.; Wang, S.; Xu, X. A Review of Immune Checkpoint Inhibitor-Associated Myocarditis: Epidemiology, Pathogenesis, and Biomarkers. Hum. Vaccin. Immunother. 2025, 21, 2512645. [Google Scholar] [CrossRef] [PubMed]
- Itzhaki Ben Zadok, O.; Levi, A.; Divakaran, S.; Nohria, A. Severe vs. Nonsevere Immune Checkpoint Inhibitor-Induced Myocarditis. Cardio Oncol. 2023, 5, 732–744. [Google Scholar] [CrossRef]
- Ozaki, A.F.; Sayer, M.; Hamano, H.; Nagasaka, M.; Lee, B.J.; Doh, J.; Naqvi, A.; Nowrouzi, N.; Zamami, Y.; Patel, P.M. Incidence and Survival Outcomes of Myocarditis and Pericardial Diseases Associated with Immune Checkpoint Inhibitor Therapy. Cardio-Oncology 2025, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xie, W.; Huang, H.; Wang, Y.; Li, G.; Geng, Y.; Hao, Y.; Zhang, Z. Association of Immune Related Adverse Events With Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Cancers: A Systemic Review and Meta-Analysis. Front. Oncol. 2021, 11, 633032. [Google Scholar] [CrossRef]
- Schadendorf, D.; Wolchok, J.D.; Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Chesney, J.; et al. Efficacy and Safety Outcomes in Patients With Advanced Melanoma Who Discontinued Treatment With Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase II and III Trials. J. Clin. Oncol. 2017, 35, 3807–3814. [Google Scholar] [CrossRef]
- Braghieri, L.; Gharaibeh, A.; Nkashama, L.; Abushouk, A.; Abushawer, O.; Mehdizadeh-Shrifi, A.; Honnekeri, B.; Calabrese, C.; Menon, V.; Funchain, P.; et al. Long-term Cardiovascular Outcomes of Immune Checkpoint Inhibitor-related Myocarditis: A Large Single–centre Analysis. ESC Heart Fail. 2025, 12, 1237–1245. [Google Scholar] [CrossRef]
- Gong, J.; Drobni, Z.D.; Zafar, A.; Quinaglia, T.; Hartmann, S.; Gilman, H.K.; Raghu, V.K.; Gongora, C.; Sise, M.E.; Alvi, R.M.; et al. Pericardial Disease in Patients Treated with Immune Checkpoint Inhibitors. J. Immunother. Cancer 2021, 9, e002771. [Google Scholar] [CrossRef]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 Inhibitors as a Form of Cancer Immunotherapy: A Comprehensive Review of Registration Trials and Future Considerations. J. Immunother. Cancer 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Han, S.; Zhang, P.; Mi, L.; Wang, Y.; Nie, J.; Dai, L.; Hu, W.; Zhang, J.; Chen, X.; et al. Immune Checkpoint Inhibitor-Related Myocarditis in Patients with Lung Cancer. BMC Cancer 2025, 25, 685. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chen, Y.; Zhou, X.; Gong, Y.; Xu, Y.; Zou, B.; Peng, F.; Huang, M.; Lu, Y.; Liu, Y. Combining Immune Checkpoint Inhibitors with Thoracic Radiotherapy Enhances Outcomes in Advanced Non-Small-Cell Lung Cancer: A Real-World Study. Front. Oncol. 2025, 15, 1611528. [Google Scholar] [CrossRef]
- Haratani, K.; Hayashi, H.; Chiba, Y.; Kudo, K.; Yonesaka, K.; Kato, R.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; Takeda, M.; et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non–Small-Cell Lung Cancer. JAMA Oncol. 2018, 4, 374. [Google Scholar] [CrossRef]
- Ricciuti, B.; Genova, C.; De Giglio, A.; Bassanelli, M.; Dal Bello, M.G.; Metro, G.; Brambilla, M.; Baglivo, S.; Grossi, F.; Chiari, R. Impact of Immune-Related Adverse Events on Survival in Patients with Advanced Non-Small Cell Lung Cancer Treated with Nivolumab: Long-Term Outcomes from a Multi-Institutional Analysis. J. Cancer Res. Clin. Oncol. 2019, 145, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Freeman-Keller, M.; Kim, Y.; Cronin, H.; Richards, A.; Gibney, G.; Weber, J.S. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin. Cancer Res. 2016, 22, 886–894. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Ali, F.S.; Wang, Y. Immune-Checkpoint Inhibitors Induced Diarrhea and Colitis. Curr. Opin. Gastroenterol. 2020, 36, 25–32. [Google Scholar] [CrossRef]
- Naidoo, J.; Wang, X.; Woo, K.M.; Iyriboz, T.; Halpenny, D.; Cunningham, J.; Chaft, J.E.; Segal, N.H.; Callahan, M.K.; Lesokhin, A.M.; et al. Pneumonitis in Patients Treated With Anti–Programmed Death-1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2017, 35, 709–717. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.-E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors. JAMA Oncol. 2018, 4, 1721. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.-E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.-P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular Toxicities Associated with Immune Checkpoint Inhibitors: An Observational, Retrospective, Pharmacovigilance Study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-R.; Florido, R.; Lipson, E.J.; Naidoo, J.; Ardehali, R.; Tocchetti, C.G.; Lyon, A.R.; Padera, R.F.; Johnson, D.B.; Moslehi, J. Cardiovascular Toxicities Associated with Immune Checkpoint Inhibitors. Cardiovasc. Res. 2019, 115, 854–868. [Google Scholar] [CrossRef]
- Salem, J.-E.; Allenbach, Y.; Vozy, A.; Brechot, N.; Johnson, D.B.; Moslehi, J.J.; Kerneis, M. Abatacept for Severe Immune Checkpoint Inhibitor–Associated Myocarditis. N. Engl. J. Med. 2019, 380, 2377–2379. [Google Scholar] [CrossRef]
- Agostinetto, E.; Eiger, D.; Lambertini, M.; Ceppi, M.; Bruzzone, M.; Pondé, N.; Plummer, C.; Awada, A.H.; Santoro, A.; Piccart-Gebhart, M.; et al. Cardiotoxicity of Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. Eur. J. Cancer 2021, 148, 76–91. [Google Scholar] [CrossRef]
- Michel, L.; Rassaf, T.; Totzeck, M. Cardiotoxicity from Immune Checkpoint Inhibitors. IJC Heart Vasc. 2019, 25, 100420. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Olenchock, B.A.; Salem, J.-E.; Wiviott, S.D.; Ederhy, S.; Cohen, A.; Stewart, G.C.; Choueiri, T.K.; Di Carli, M.; Allenbach, Y.; et al. Myocarditis in the Setting of Cancer Therapeutics. Circulation 2019, 140, 80–91. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Zeng, Z.; Liu, A. Arrhythmic Events Associated with Immune Checkpoint Inhibitors Therapy: A Real-world Study Based on the Food and Drug Administration Adverse Event Reporting System Database. Cancer Med. 2023, 12, 6637–6648. [Google Scholar] [CrossRef] [PubMed]
- Palaskas, N.; Lopez-Mattei, J.; Durand, J.B.; Iliescu, C.; Deswal, A. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J. Am. Heart Assoc. 2020, 9, e013757. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y.-P.; Du, X.-J.; Liu, J.-Q.; Huang, C.-L.; Chen, L.; Zhou, G.-Q.; Li, W.-F.; Mao, Y.-P.; Hsu, C.; et al. Comparative Safety of Immune Checkpoint Inhibitors in Cancer: Systematic Review and Network Meta-Analysis. BMJ 2018, 363, k4226. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.; Wu, Y.; Yang, K. PD-1/PD-L1 in Cardiovascular Disease. Clin. Chim. Acta 2020, 505, 26–30. [Google Scholar] [CrossRef]
- Buehning, F.; Lerchner, T.; Vogel, J.; Hendgen-Cotta, U.B.; Totzeck, M.; Rassaf, T.; Michel, L. Preclinical Models of Cardiotoxicity from Immune Checkpoint Inhibitor Therapy. Basic Res. Cardiol. 2024, 120, 171–185. [Google Scholar] [CrossRef]
- Raschi, E.; Rossi, S.; De Giglio, A.; Fusaroli, M.; Burgazzi, F.; Rinaldi, R.; Potena, L. Cardiovascular Toxicity of Immune Checkpoint Inhibitors: A Guide for Clinicians. Drug Saf. 2023, 46, 819–833. [Google Scholar] [CrossRef]
- Baik, A.H.; Oluwole, O.O.; Johnson, D.B.; Shah, N.; Salem, J.-E.; Tsai, K.K.; Moslehi, J.J. Mechanisms of Cardiovascular Toxicities Associated With Immunotherapies. Circ. Res. 2021, 128, 1780–1801. [Google Scholar] [CrossRef]
- Mensah, G.A.; Arnold, N.; Prabhu, S.D.; Ridker, P.M.; Welty, F.K. Inflammation and Cardiovascular Disease: 2025 ACC Scientific Statement. JACC, 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Su, Z.; Lian, X. Risk Factors of Immune Checkpoint Inhibitor-Related Cardiotoxicity: A Scoping Review. Oncologist 2025, 30, oyaf187. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yu, H.; Dai, S.; He, M.; Ma, L.; Xu, Z.; Luo, F.; Wang, L. Immune Checkpoint Inhibitors Break Whose Heart? Perspectives from Cardio-Immuno-Oncology. Genes Dis. 2024, 11, 807–818. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Zhang, L.; Zafar, A.; Drobni, Z.D.; Mahmood, S.S.; Cabral, M.; Awadalla, M.; Nohria, A.; Zlotoff, D.A.; Thuny, F.; et al. Myocardial T1 and T2 Mapping by Magnetic Resonance in Patients with Immune Checkpoint Inhibitor–Associated Myocarditis. J. Am. Coll. Cardiol. 2021, 77, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Dolladille, C.; Ederhy, S.; Sassier, M.; Cautela, J.; Thuny, F.; Cohen, A.A.; Fedrizzi, S.; Chrétien, B.; Da-Silva, A.; Plane, A.-F.; et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol. 2020, 6, 865. [Google Scholar] [CrossRef] [PubMed]
- Sala, I.; Pagan, E.; Pala, L.; Oriecuia, C.; Musca, M.; Specchia, C.; De Pas, T.; Cortes, J.; Giaccone, G.; Postow, M.; et al. Surrogate Endpoints for Overall Survival in Randomized Clinical Trials Testing Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Immunol. 2024, 15, 1340979. [Google Scholar] [CrossRef] [PubMed]
| Variable | Number | Frequency (%) | |
|---|---|---|---|
| Sex | Male | 313 | 72.0 |
| Female | 122 | 28.0 | |
| ECOG | ECOG 0 | 137 | 31.5 |
| ECOG 1 | 124 | 28.5 | |
| ECOG 2 | 167 | 38.4 | |
| ECOG 3 | 7 | 1.6 | |
| Smoking status * | Smoker | 234 | 53.9 |
| No smoker | 200 | 46.1 | |
| DM prior to ICIs | Yes | 85 | 19.5 |
| No | 350 | 80.5 | |
| HT prior to ICIs | Yes | 218 | 50.1 |
| No | 217 | 49.9 | |
| Hypercholesterolemia prior to ICIs | Yes | 96 | 22.1 |
| No | 339 | 77.9 | |
| Diagnosis | Lung cancer | 219 | 50.3 |
| Head and neck cancer | 137 | 31.5 | |
| Melanoma | 43 | 9.9 | |
| Renal cell carcinoma | 16 | 3.7 | |
| Urothelial carcinoma | 12 | 2.8 | |
| Hepatocellular carcinoma | 4 | 0.9 | |
| Breast cancer | 2 | 0.5 | |
| Cancer of Unknown Primary | 2 | 0.5 | |
| CHT prior to ICIs ** | Yes | 184 | 42.3 |
| No | 247 | 56.8 | |
| RT prior to ICIs | Yes | 236 | 54.3 |
| No | 199 | 45.7 | |
| ICIs | Atezolizumab | 20 | 4.6 |
| Avelumab | 7 | 1.6 | |
| Durvalumab | 12 | 2.8 | |
| Nivolumab | 140 | 32.2 | |
| Nivolumab + Ipilimumab | 22 | 5.1 | |
| Pembrolizumab | 234 | 53.8 |
| Cardiovascular events (n = 144, 33.1%) | Cardiomyopathy | 45 | 10.3 |
| Hypertension | 20 | 4.6 | |
| Vascular toxicity (thrombosis) | 55 | 12.6 | |
| Cardiac irAEs (n = 55, 12.6%) | Arrhythmias | 35 | 8 |
| Grade 2 | 19 | 4.4 | |
| Grade 3 | 5 | 1.1 | |
| Grade 1 | 10 | 2.3 | |
| Grade 4 | 1 | 0.2 | |
| Pericardial disease | 15 | 3.4 | |
| Grade 2 | 6 | 1.4 | |
| Grade 3 | 6 | 1.4 | |
| Grade 1 | 2 | 0.5 | |
| Grade 4 | 1 | 0.2 | |
| Myocarditis | 13 | 3 | |
| Grade 2 | 8 | 1.8 | |
| Grade 3 | 4 | 0.9 | |
| Grade 4 | 1 | 0.2 |
| OS | |||||
| Number | RMST | Median | 95% Confidence Interval | Log-Rank Test p-Value | |
| Global | N = 435 | 26.18 | 15.2 | 13.5–19.0 | - |
| Cardiac irAEs | No N = 380 | 26.51 | 15.0 | 13.3–19.3 | 0.56 |
| Yes N = 55 | 23.91 | 15.8 | 12.0–30.3 | ||
| Non-cardiac irAEs | No N = 339 | 24.39 | 13.9 | 12.4–16.5 | 0.008 |
| Yes N = 96 | 32.45 | 26.0 | 15.5–NA | ||
| Severe cardiac irAEs | No N = 417 | 26.3 | 15.1 | 13.4–19.0 | 0.76 |
| Yes N = 18 | 24.22 | 17.1 | 7.3–NA | ||
| Cardiac events other than irAEs | No N = 291 | 25.6 | 14.4 | 12.4–17.5 | 0.44 |
| Yes N = 144 | 27.42 | 20.1 | 14.4–27.4 | ||
| PFS | |||||
| Number | RMST | Median | 95% Confidence Interval | Log-Rank Test p-Value | |
| Global | N = 435 | 19.25 | 9.0 | 7.6–10.5 | - |
| Cardiac irAEs | No N = 380 | 19.31 | 9.1 | 7.6–10.4 | 0.97 |
| Yes N = 55 | 18.69 | 8.1 | 5.3–19.3 | ||
| Non-cardiac irAEs | No N = 339 | 17.83 | 8.7 | 7.3–10.3 | 0.029 |
| Yes N = 96 | 24.25 | 12.3 | 8.1–26.0 | ||
| Severe cardiac irAEs | No N = 417 | 19.34 | 9.0 | 7.6–10.5 | 0.87 |
| Yes N = 18 | 14.19 | 9.2 | 4.6–NA | ||
| Cardiac events other than irAEs | No N = 291 | 18.54 | 8.1 | 7.1–9.7 | 0.2 |
| Yes N = 144 | 27.42 | 20.1 | 14.4–27.4 | ||
| Variable | Hazard Ratio | p-Value | |
|---|---|---|---|
| OS | Non-cardiac irAEs (yes) | 0.66 (0.49 to 0.9) | 0.008 |
| Cardiac irAEs (yes) | 1.11 (0.78 to 1.57) | 0.56 | |
| PFS | Non-cardiac irAEs (yes) | 0.74 (0.56 to 0.97) | 0.029 |
| Cardiac irAEs (yes) | 1.003 (0.72 to 1.39) | 0.99 | |
| OS | ||||||
| Number | RMST | Median | 95% Confidence Interval | Log-Rank Test p-Value | ||
| Lung | Global | N = 219 | 25.92 | 15.1 | 13.1–19.8 | - |
| Cardiac irAEs | No N = 181 | 27.35 | 16.6 | 13.7–26.0 | 0.025 | |
| Yes N = 38 | 18.65 | 12.0 | 7.2–19.0 | |||
| Non-cardiac irAEs | No N = 160 | 25.72 | 14.4 | 12.1–23.5 | 0.73 | |
| Yes N = 59 | 26.43 | 16.6 | 14.0–34.3 | |||
| HN | Global | N = 137 | 21.30 | 11.4 | 9.3–15.5 | - |
| Cardiac irAEs | No N = 130 | There were less than 10 patients with cardiac irAEs | ||||
| Yes N = 7 | ||||||
| Non-cardiac irAEs | No N = 117 | 18.9 | 11.3 | 8.7–14.4 | 0.051 | |
| Yes N = 20 | 32.22 | 31.7 | 8.2–NA | |||
| PFS | ||||||
| Number | RMST | Median | 95% Confidence Interval | Log-Rank Test p-Value | ||
| Lung | Global | N = 219 | 18.92 | 9.3 | 7.4–11.1 | - |
| Cardiac irAEs | No N = 181 | 19.87 | 9.7 | 8.0–11.3 | 0.83 | |
| Yes N = 38 | 14.30 | 5.9 | 4.3–12.0 | |||
| Non-cardiac irAEs | No N = 160 | 18.43 | 9.3 | 7.5–11.2 | 0.76 | |
| Yes N = 59 | 20.06 | 9.0 | 5.8–21.9 | |||
| HN | Global | N = 137 | 16.07 | 6.6 | 5.0–8.7 | - |
| Cardiac irAEs | No N = 130 | There were less than 10 patients with cardiac irAEs | ||||
| Yes N = 7 | ||||||
| Non-cardiac irAEs | No N = 117 | 13.22 | 5.9 | 5.0–8.7 | 0.055 | |
| Yes N = 20 | 26.78 | 8.2 | 4.5–NA | |||
| Variable | Hazard Ratio | p-Value | ||
|---|---|---|---|---|
| OS | Lung | Non-cardiac irAEs (yes) | 0.94 (0.64 to 1.36) | 0.73 |
| Cardiac irAEs (yes) | 1.59 (1.06 to 2.4) | 0.026 | ||
| HN | Non-cardiac irAEs (yes) | 0.52 (0.27 to 1.01) | 0.55 | |
| PFS | Lung | Non-cardiac irAEs (yes) | 0.94 (0.67 to 1.34) | 0.75 |
| Cardiac irAEs (yes) | 1.41 (0.95 to 2.09) | 0.08 | ||
| HN | Non-cardiac irAEs (yes) | 0.55 (0.3 to 1.02) | 0.58 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pătru, I.-R.; Anghel, A.-V.; Galeschi, E.R.; Bătăuș, L.C.; Ionescu, A.-I.; Negru, A.G.; Barbu, M.A.; Iordache, M.; Antone-Iordache, I.-L. The Impact of Immune-Related Adverse Events on the Survival of Patients Treated with Immune Checkpoint Inhibitors: The Distinct Role of Cardiac Toxicities. J. Clin. Med. 2025, 14, 7794. https://doi.org/10.3390/jcm14217794
Pătru I-R, Anghel A-V, Galeschi ER, Bătăuș LC, Ionescu A-I, Negru AG, Barbu MA, Iordache M, Antone-Iordache I-L. The Impact of Immune-Related Adverse Events on the Survival of Patients Treated with Immune Checkpoint Inhibitors: The Distinct Role of Cardiac Toxicities. Journal of Clinical Medicine. 2025; 14(21):7794. https://doi.org/10.3390/jcm14217794
Chicago/Turabian StylePătru, Ileana-Raluca, Alexandra-Valentina Anghel, Eusebiu Robert Galeschi, Lorena Carolina Bătăuș, Andreea-Iuliana Ionescu, Alina Gabriela Negru, Maria Alexandra Barbu, Maria Iordache, and Ionuț-Lucian Antone-Iordache. 2025. "The Impact of Immune-Related Adverse Events on the Survival of Patients Treated with Immune Checkpoint Inhibitors: The Distinct Role of Cardiac Toxicities" Journal of Clinical Medicine 14, no. 21: 7794. https://doi.org/10.3390/jcm14217794
APA StylePătru, I.-R., Anghel, A.-V., Galeschi, E. R., Bătăuș, L. C., Ionescu, A.-I., Negru, A. G., Barbu, M. A., Iordache, M., & Antone-Iordache, I.-L. (2025). The Impact of Immune-Related Adverse Events on the Survival of Patients Treated with Immune Checkpoint Inhibitors: The Distinct Role of Cardiac Toxicities. Journal of Clinical Medicine, 14(21), 7794. https://doi.org/10.3390/jcm14217794







