Alzheimer Syndrome or Age-Related Dementia—History, Therapy and Prevention
Abstract
1. Introduction
2. Methods
3. Historical Facts
3.1. Historical Outline of AD Nosology
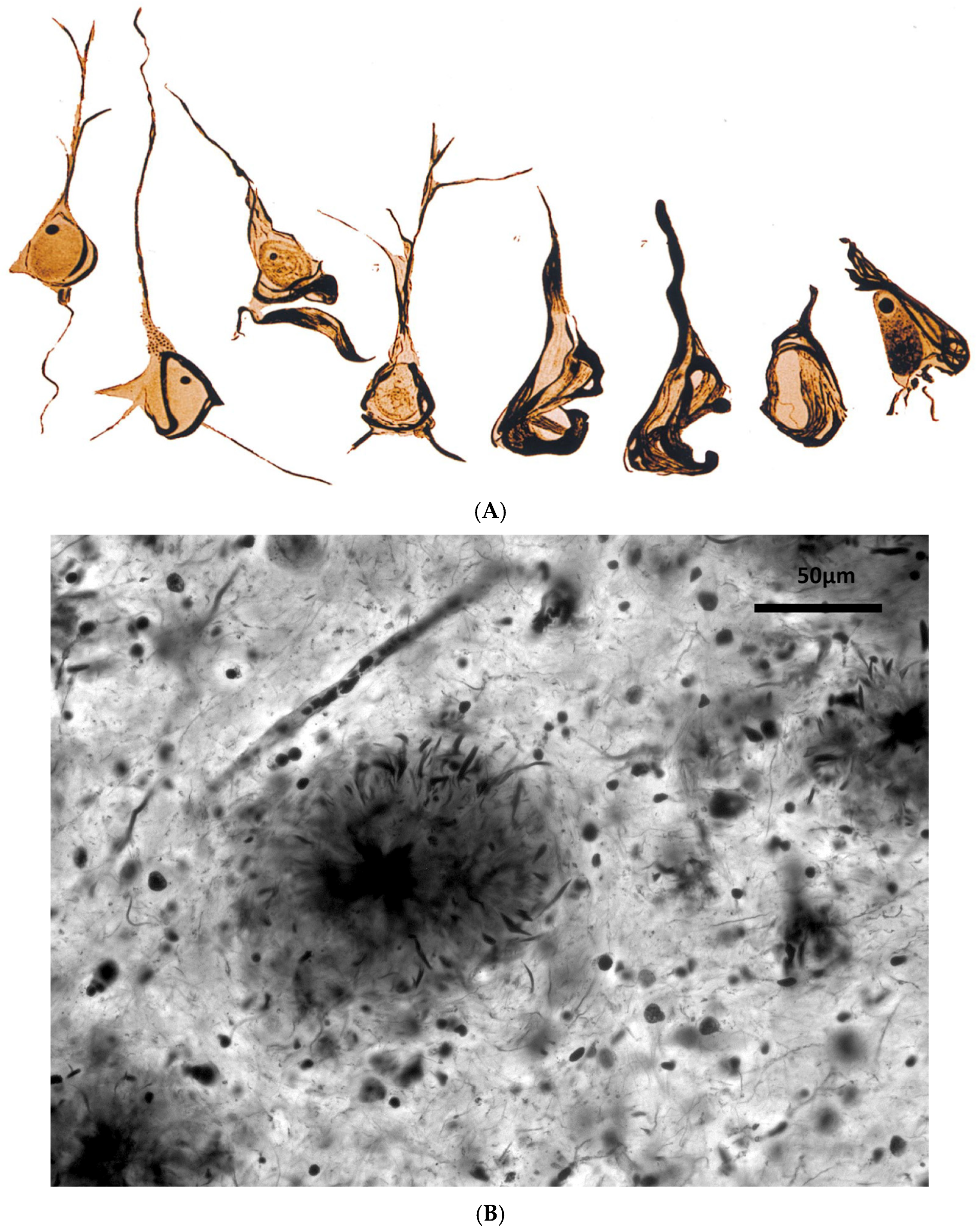
The Controversial Beginnings of AD
| Author/Year | Very Brief Comments |
|---|---|
| Redlich/1898 [48] | Initial description of senile plaque (SP), “miliary sclerosis” in dementia |
| Fischer/1907 [49] | Complete description of SPs in senile dementia (SeD) |
| Alzheimer/1907 [9] | Description of NFT in a case of presenile dementia (PSD) |
| Kraepelin/1910 [8] | Alzheimer’s disease (AD) creation (only four cases of his disciples) |
| Fuller/1907 [50] | DNF description in SeD and various neurological disorders |
| Alzheimer/1911 [10] | Express doubts that AD could be a different entity from SeD |
| Perusini/1911 [51] | AD is probably a new disease, in agreement with Kraepelin |
| Simchowicz/1911 [52] | Coined the term “senile plaque” and agrees with Kraepelin on AD |
| Bielschowsky/1911[53] | Propose a link between neuritic SPs and NFT |
| Fischer/1912 [41] | AD is not a separate entity from SeD |
| Fuller/1912 [54] | AD is an atypical form of SeD |
| Barrett/1913 [55] | AD may determine dementia and motor deficits |
| Lambert/1916 [56] | AD and SD are the same entity |
| Simchowicz/1924 [57] | Change his previous opinion. AD is a severe form of SeD |
| Malamud/1929 [42] | AD is a syndrome with various causal factors |
| Henderson/1930 [58] | AD cases must be less than 40 years old |
| Lowenberg/1931 [43] | AD is a group of multiple heterogeneous etiologies |
| Gellerstedt/1933 [40] | NFTs and SPs are nonspecific lesions associated with aging |
| Rothschild/1934 [59] | AD pathology is analogous to SeD. Psychogenic component |
| Rothschild/1936 [60] | AD is due to an exogenous component plus aging |
| Jervis/1936 [61] | AD is a clinicopathological entity |
| Rothschild/1937 [62] | There is no relationship between PS and cognitive decline |
| Hannah/1936 [63] | AD and SeD are clinically different entities |
| McMenemey/1940 [44] | Constitutional and toxic-infectious causes of AD. Introduces “the reserve power of the brain”. |
| Newton/1948 [46] | AD and SeD are the same entity related to aging |
| Goodman/1953 [64] | AD is due to a microglia insufficiency |
| Schenk/1954 [45] | AD is a syndrome |
| Roth/1955 [65] | Differentiates vascular SeD vs. degenerative SeD |
| Corsellis/1962 [66] | SPs and NFTs are more frequent in SeD cases |
| Kidd/1963 [67] | Description of the ultrastructure of NFT (paired helicoidally fibrils) |
| Larsson/1963 [68] | Genetic risk of SeD vs. controls |
| Terry/1964 [69] | Description of the ultrastructure of SPs, NFT and other markers |
| Blessed/1968 [70] | SPs correlate with psychological tests, but poorly with dementia |
| Margolis/1959 [71] | Description of histologic markers in PSD and SeD |
| McDonald/1969 [72] | Clinical diversity of SeD |
3.2. The Conceptual Shift (1970s): Senile Dementia Medicalization
The Internationalization of the New AD Nosology
3.3. The 21st Century and the Third Nosological Shift: AD as a Biological Entity
3.3.1. Foundations of This Historical Shift
3.3.2. The Amyloid Cascade Hypothesis (ACH) as the Origin of AD
- Passive immunotherapy (anti-βA monoclonal antibodies, AβA-MA). The history of this therapeutic development is relevant despite the large number of attempts and resources spent over more than two decades without positive results. The main trials of these drugs (bapineuzumab, solanezumab, and gantenerumab) failed to alter the progression of AD. A combined analysis of these drugs, using Bayesian methodology, which allows for the integration of previous results, demonstrated that six clinical trials of AβA-MA showed no therapeutic effect (Bayes factor of 11.27 against 0.09 in favor of cognitive benefit) [23]. Other authors expressed similar opinions [103,104,105].
3.3.3. New Discoveries About Alzheimer’s Disease
Neurotransmission Deficits in AD and Its Therapy
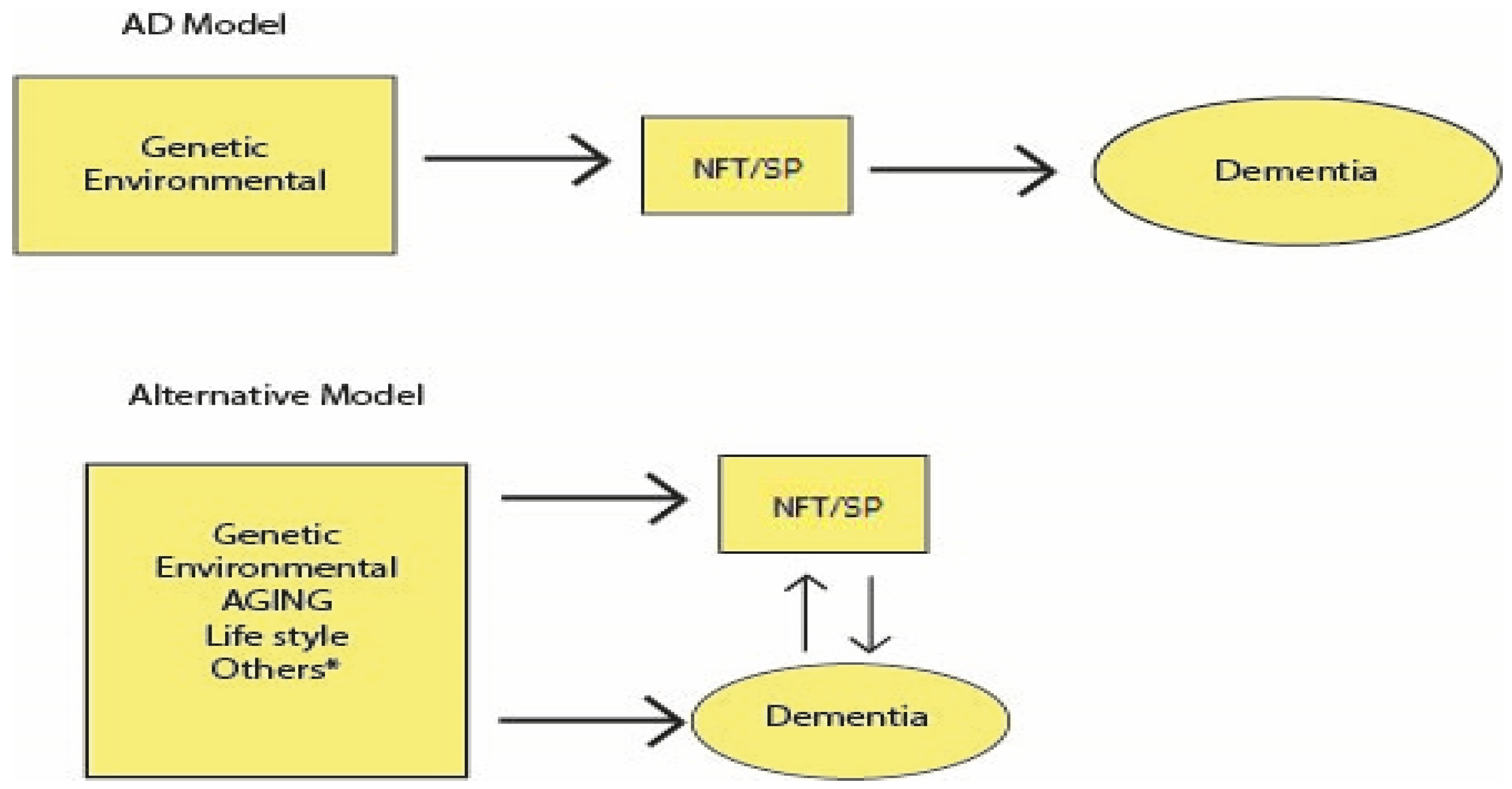
AD Develops Throughout Life: AD as a Biological Construct
ARD Pathology is More Complex than AD Pathology
Declining Dementia Risk in High-Income Countries
Absence of Curative Therapy for ARD and AD. Current and Future Possibilities
| (A). Pharmacological | |
|---|---|
| Year/Author | Drug Treatment |
| 1972/Giurgea [168] | Nootropics (cognitive enhancers) and dementia treatment |
| 1984/Hollister [169] | Hydergine (vasodilator, metabolic enhancer), used in ARD |
| 1986/Growdon [170] | Piracetam (nootropic): unproven efficacy in AD |
| 1986/Summers [171] | Tacrine (AChEI) was the first drug FDA-approved for AD |
| 1989/Thal [172] | Vinpocetine (vasodilator, enhancer): unproven efficacy in AD |
| 1995/Saletu [173] | Nicergoline (vasodilator, enhancer): unproven efficacy in AD |
| 1995/Fritze [174] | Nimodipine (calcium antagonist) has unproven efficacy in AD * |
| 1996/Rogers [175] | Donepezil (AChEI) for AD, the first AChEI widely prescribed |
| 1999/Rösler [176] | Rivastigmine (AChEI) showed efficacy in AD |
| 1999/Winblad. [177] | Memantine (NMDA antagonist) has a mild benefit in AD |
| 2000/Raskind [178] | Galantamine (AChEI) showed efficacy in AD |
| 2002/Orgogozo [100] | Elan AN-1792, an active anti-amyloid vaccine in humans, has been halted |
| 2005/Schneider [179] | Ginkgo biloba is not effective in treating AD |
| 2008/DeKosky [180] | Ginkgo biloba does not prevent AD |
| 2014/Salloway [181] | Bapineuzumab (AAbmA) phase III trials failed in AD |
| 2014/Doody [182] | Solaneuzumab (AAbmA) phase III trials failed in AD |
| 2022/Budd Haeberlein [183] | Doubtful efficacy of Aducanumab, but FDA approved |
| 2023/Congdon [184] | Tau targeting therapies in AD |
| 2023/van Dyck [106] | Small efficacy of Lecanemab in early AD |
| 2023/Sims [185] | Small efficacy of Donanemab in early AD |
| 2025/Terao [186] | Lithium therapy better than the three previous AβA-MA? |
| (B). Non-Pharmacological †† | |
| 2010/Olazarán [187] | Review of non-pharmacological therapies in AD * |
| 2012/Yang [188] | Several types of sensorial stimulation and their benefits reviewed |
| 2016/Petersson [189] | Diet (Mediterranean) delays the onset of ARD |
| 2018/Rosenberg [166] | Multidomain intervention reduces cognitive decline (FINGER) ** |
| 2022/Matziorinis [190] | Long music therapy for memory and ARD prevention |
| 2024/Brown [191] | Cognitive and biological benefits of yoga in AD |
| 2024/Desai [192] | Cognitive stimulation therapy in mild & moderate AD |
| 2025/Terao [193] | *** Transcranial magnetic stimulation (cognitive impairment/ARD) |
| 2025 Howard [194] | *** Deep brain stimulation is effective in AD (hippocampus) |
| (C). New Approaches ††† | |
| 2018 Raikwar [195] | Genetic and epigenetic gene therapies |
| 2019 Cummings [196] | Combination therapy in AD (mainly drugs) |
| 2019 Bednar [197] | Combination therapy in AD (drugs and devices) |
| 2024 Cao [198] | Stem cell therapy (several technologies) in AD |
4. The Current Situation: AD Nosology and Therapy
4.1. Nosology
4.2. Therapy and Prevention

5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boorse, C. Second rebuttal of health. J. Med. Philosophy 2014, 39, 683–724. [Google Scholar] [CrossRef]
- Nordenfelt, L. On the relevance and importance of the notion of disease. Theoretical Med. 1993, 14, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Kingma, E. Naturalism about health and disease: Adding nuance for progress. J. Med. Philosophy 2014, 39, 590–608. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.P. The concept of health: Beyond normativism and naturalism. J. Eval. Clin. Pract. 2010, 16, 323–329. [Google Scholar] [CrossRef]
- van der Linden, R.; Schermer, M. Health and disease as practical concepts: Exploring function in context-specific definitions. Med. Health Care Philos. 2022, 25, 131–140. [Google Scholar] [CrossRef]
- Xiao, X.; Song, H.; Sang, T.; Wu, Z.; Xie, Y.; Yang, Q. Analysis of Real-World Implementation of the Biopsychosocial Approach to Healthcare: Evidence From a Combination of Qualitative and Quantitative Methods. Front. Psychiatry 2021, 12, 725596. [Google Scholar] [CrossRef]
- Schleidgen, S.; Fernau, S.; Fleischer, H.; Schickhardt, C.; Oßa, A.K.; Winkler, E.C. Applying systems biology to biomedical research and health care: A précising definition of system- medicine. BMC Health Ser. Res. 2017, 17, 761. [Google Scholar] [CrossRef]
- Kraepelin, E. Psychiatrie: Ein Lehrbuch Fuer Studierende Und Aerzte; Verlag von Johann Ambrosius Barth: Lepzig, Germany, 1910; (Description in English in: “Schorer, C.E. Historical essay: Kraepelin’s description of Alzheimer’s disease. Int. J. Aging Hum. Dev. 1985, 21, 235–238. https://doi.org/10.2190/gnq1-gdux-eptl-0f2l”). [Google Scholar]
- Alzheimer, A. Ueber eine eigenartige Erkrankung der Hirnrinde. In Allgemeine Zeitschrift Für Psychiatrie und Psychisch-gerichtliche Medizin; Forgotten Books: London, UK, 1907; Volume 64, (English version in: Jarvik, L.; Greenson, H. About a peculiar disease of the cerebral cortex. By Alois Alzheimer, 1907. Alzheimer Dis. Assoc. Disord. 1987, 1, 3–8. PMID: 3331112). [Google Scholar]
- Alzheimer, A. Über eigenartige Krankheitsfälle des späteren Alters. Z. Ges. Neurol. Psychiatr. 1911, 4, 356–385, (English version: Alzheimer, A.; Förstl, H.; Levy, R. On certain peculiar diseases of old age. Hist. Psychiatry 1991, 2, 71–101. https://doi.org/10.1177/0957154X9100200505). [Google Scholar]
- Bermejo-Pareja, F. The changing nosology of Alzheimer disease over more than 100 years. A historical overview. Neurosc. History 2025, in press. [Google Scholar]
- Ballenger, J.F. Self, Senility, and Alzheimer’s Disease in Modern America: A History; The John Hopkins University Press: Baltimore, MD, USA, 2006. [Google Scholar]
- Bick, K.L. The early story of Alzheimer’s disease. In Alzheimer Disease; Terry, R.D., Katzman, R., Bick, K.L., Sisodia, S.S., Eds.; Lippincott W&W: Philadelphia, PA, USA, 1999; pp. 1–10. [Google Scholar]
- Katzman, R.; Terry, R.D.; y Bick, K.L. Recommendation of nosology, epidemiology, and pathophysiology commissions of the Workshop-Conference on Alzheimer’s dementia and related disorders. In Alzheimer’s Disease: Senile Dementia and Related Disorders; Katzman, R., Terry, R.D., Bick, K.L., Eds.; Raven Press: New York, NY, USA, 1978; pp. 579–585. [Google Scholar]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Pareja, F.; Del Ser, T. Controversial past, splendid present, unpredictable future: A brief review of Alzheimer disease history. J. Clin. Med. 2024, 13, 536. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Herrup, K. How not to Study a Disease: The Story of Alzheimer’s Disease. The MIT Press: Cambridge, UK, 2021. [Google Scholar]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Dubois, B.; Villain, N.; Frisoni, G.B.; Rabinovici, G.D.; Sabbagh, M.; Cappa, S.; Bejanin, A.; Bombois, S.; Epelbaum, S.; Teichmann, M.; et al. Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurol. 2021, 20, 484–496. [Google Scholar] [CrossRef]
- Nelson, P.T.; Brayne, C.; Flanagan, M.E.; Abner, E.L.; Agrawal, S.; Attems, J.; Castellani, R.J.; Corrada, M.M.; Cykowski, M.D.; Di, J.; et al. Frequency of LATE neuropathologic change across the spectrum of Alzheimer’s disease neuropathology: Combined data from 13 community-based or population-based autopsy cohorts. Acta Neuropathol. 2022, 144, 27–44. [Google Scholar] [CrossRef]
- Schneider, J.A.; Arvanitakis, Z.; Bang, W.; Bennett, D.A. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007, 69, 2197–2204. [Google Scholar] [CrossRef]
- Richard, E.; den Brok, M.G.H.E.; van Gool, W.A. Bayes analysis supports null hypothesis of anti-amyloid beta therapy in Alzheimer’s disease. Alzheimer’s Dement. 2021, 17, 1051–1055. [Google Scholar] [CrossRef]
- Behl, C. Alzheimer’s Disease Research: What Has Guided Research So Far and Why It Is High Time for a Paradigm Shift; Springer Nature: Cham, Switzerland, 2023. [Google Scholar]
- Wolters, F.J.; Chibnik, L.B.; Waziry, R.; Anderson, R.; Berr, C.; Beiser, A.; Bis, J.C.; Blacker, D.; Bos, D.; Brayne, C.; et al. Twenty-seven-year time trends in dementia incidence in Europe and the United States: The Alzheimer Cohorts Consortium. Neurology 2020, 95, e519–e531. [Google Scholar] [CrossRef]
- Zeiler, L. An analytic framework for conceptualisations of disease: Nine structuring questions and how some conceptualisations of Alzheimer’s disease can lead to ‘diseasisation’. Med. Health Care Philos. 2020, 23, 677–693. [Google Scholar] [CrossRef] [PubMed]
- George, D.R.; Whitehouse, P.J. American Dementia Brain Health in an Unhealthy Society; Johns Hopkins University Press: Baltimore, MD, USA, 2021. [Google Scholar]
- Herrup, K. Reimagining Alzheimer’s disease—An age-based hypothesis. J. Neurosci. 2010, 30, 16755–16762. [Google Scholar] [CrossRef] [PubMed]
- Lock, M. The Alzheimer Conundrum: Entanglements of Dementia and Aging; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Liu, R.M. Aging, cellular senescence, and Alzheimer’s disease. Int. J. Mol. Sci. 2022, 23, 1989. [Google Scholar] [CrossRef] [PubMed]
- Sain-Jean, O.; Favereau, E. Alzheimer, le Gran Leurre; Michalon Edit: Paris, France, 2018. [Google Scholar]
- Fiorini, N.; Canese, K.; Starchenko, G.; Kireev, E.; Kim, W.; Miller, V.; Osipov, M.; Kholodov, M.; Ismagilov, R.; Mohan, S.; et al. Best match: New relevance search for PubMed. PLoS Biol. 2018, 16, e2005343. [Google Scholar] [CrossRef]
- Amaducci, L. Alzheimer’s original patient. Science 1996, 274, 328a. [Google Scholar] [CrossRef]
- Keohane, K.; Grace, V. What is ‘Alzheimer’s Disease’? The ‘Auguste D’ Case. Re-opened. Cult. Med. Psychiatry 2019, 43, s11013–s11019. [Google Scholar] [CrossRef]
- Rupp, C.; Beyreuther, K.; Maurer, K.; Kins, S. A presenilin 1 mutation in the first case of Alzheimer’s disease: Revisited. Alzheimer’s Dement. 2014, 10, 869–872. [Google Scholar] [CrossRef]
- Hansen, L.A.; Masliah, E.; Galasko, D.; Terry, R.D. Plaque-only Alzheimer disease is usually the Lewy body variant, and vice versa. J. Neuropathol. Exp. Neurol. 1993, 52, 648–654. [Google Scholar] [CrossRef]
- Amaducci, L.A.; Rocca, W.A.; Schoenberg, B.S. Origin of the distinction on between Alzheimer’s disease and senile dementia. Neurology 1986, 35, 1497–1499. [Google Scholar] [CrossRef]
- Beach, T.G. The history of Alzheimer’s disease: Three debates. J. Hist. Med. Allied Sci. 1987, 42, 327–349. [Google Scholar] [CrossRef]
- Piller, C. Doctored: Fraud, Arrogance, and Tragedy in the Quest to Cure Alzheimer’s; Simond & Schuster: New York, NY, USA, 2025. [Google Scholar]
- Gellerstedt, N. Zur Kenntnis der Hirnveranderungen bei der normalen Altersinvolution. Upsala Lakar Forh. 1933, 38, 193–408. [Google Scholar] [CrossRef]
- Fischer, O. Ein weiterer Beitrag zur Klinik und Pathologie der presbyophrenen Demenz. Z. Ges Neurol. Psychiatry 1912, 12, 99–135. [Google Scholar] [CrossRef]
- Malamud, W.; Lowenberg, K. Alzheimer’s disease: A contribution to its etiology and classification. Arch. Neurol. Psychiatry 1929, 21, 805–827. [Google Scholar] [CrossRef]
- Lowenberg, K.; Rothschild, D. Alzheimer’s disease: Its occurrence on the basis of a variety of etiologic factors. Am. J. Psychiatry 1931, 11, 269–287. [Google Scholar] [CrossRef]
- McMenemey, W.H. Alzheimer’s disease: A report of six cases. J. Neurol. Psychiatry 1940, 3, 211–240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schenk, V.W.D. Syndroom van Alzheimer. Acta Neurol. Psychiatr. Belg. 1954, 54, 213–217. [Google Scholar] [PubMed][Green Version]
- Newton, R.D. The identity of Alzheimer’s disease and senile dementia and their relationship to senility. J. Ment. Sci. 1948, 94, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Perusini, G. Uber klinisch und histologisch eigenartige psychische Erkrankungen des späteren Lebensalters. Histologische und histopathologische. Arbeiten 1910, 3, 297–351. [Google Scholar]
- Redlich, E. Uber miliaere Sklerose der Hirnrinde bei seniler Atrophie. Jahr. Psychiatr. Neurol. 1898, 17, 208–216. [Google Scholar]
- Fischer, O. Miliare Nekrosen mit drusigenWucherungen der Neurofibrillen, eine regelmassige Veranderung der Hirnrinde bei seniler Demenz. Monatsschr. Psychiat. Neurol. 1907, 22, 361–372. [Google Scholar] [CrossRef]
- Fuller, S.C. A study of the neurofibrils in dementia paralytica, dementia senilis, chronic alcoholism cerebral lues and microcephalic idiocy. Am. J. Insan 1907, 63, 5468. [Google Scholar] [CrossRef]
- Perusini, G. Sul valore nosografico di alcuni reperti istopatologici caratteristiche per la senilita. Riv. Ital. Neirroputol Psichiutr Eiettroter. 1911, 4, 193–213. [Google Scholar]
- Simchowicz, T. Histologische Studien uber die Senile Demenz. Histol. Histoputhol Arb. Grosshirnr. 1911, 4, 267–444. [Google Scholar]
- Bielschowsky, M. Zur Kenntnis der Alzheimerschen Krankheit (präsenilen Demenz mit Herdsymptomen). J. Psychol. Neurol. 1911, 18, 1–20. [Google Scholar]
- Fuller, S.C. Alzheimer’s disease (senium praecox): The report of a case and review of published cases. J. Nerv. Ment. Dis. 1912, 39, 440–455. [Google Scholar] [CrossRef]
- Barrett, A.M. A case of Alzheimer’s disease with unusual neurological disturbances. J. Nerv. Ment. Dis. 1913, 40, 361–374. [Google Scholar] [CrossRef]
- Lambert, C.L. The clinical and anatomic features of Alzheimer’s disease. Psychiat. Bull. N. Y. State Hosp. 1916, 9, 169–170. [Google Scholar]
- Simchowicz, T. Sur la signification de plaques séniles et sur la formule sénil de l’écorce cérébrale. Rev. Neurol. 1924, 31, 220–227. [Google Scholar]
- Henderson, D.K.; MacLachlan, S.H. Alzheimer’s Disease. J. Ment. Sci. 1930, 76, 646. [Google Scholar] [CrossRef]
- Rothschild, D. Alzheimer’s Disease: A Clinicopathologic Study of Five Cases. Am. J. Psychiatry 1934, 91, 485–519. [Google Scholar] [CrossRef]
- Rothschild, D.; Kasanin, J. Clinic-pathologic study of Alzheimer’s disease: Relationship to senile condition. Arch. Neurol. Psychiatry 1936, 36, 293–321. [Google Scholar] [CrossRef]
- Jervis, G.; Soltz, S.E. Alzheimer’s disease—The so-called juvenile type. Am. J. Psychiatry 1936, 93, 39–56. [Google Scholar] [CrossRef]
- Rothschild, D.; Trainor, M.A. Pathologic changes in senile psychoses and their psychobiologic significance. Am. J. Psychiatry 1937, 93, 757–788. [Google Scholar]
- Hannah, J.A. A case of Alzheimer’s disease with neuropathological findings. Can. Med. Assoc. J. 1936, 35, 361–366. [Google Scholar] [PubMed]
- Goodman, L. Alzheimer’s disease; a clinico-pathologic analysis of twenty-three cases with a theory on pathogenesis. J. Nerv. Ment. Dis. 1953, 118, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Roth, M. The natural history of mental disorder in old age. J. Ment. Sci. 1955, 101, 281–301. [Google Scholar] [CrossRef] [PubMed]
- Corsellis, J.A.N. Mental Illness and the Aging Brain: The Distribution of Pathological Changes in a Mental Hospital Population; University Press: London, UK; Oxford, UK, 1962. [Google Scholar]
- Kidd, M. Paired helical filaments in electron microscopy of Alzheimer’s disease. Nature 1963, 197, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Larsson, T.; Sjogren, T.; Jacobson, G. Senile dementia. A clinical, sociomedical and genetic study. Acta Psychiat. Scand. 1963, 39 (Suppl. 167), 1–259. [Google Scholar]
- Terry, R.D.; Gonatas, N.K.; Weiss, M. Ultrastructural studies in Alzheimer’s presenile dementia. Am. J. Pathol. 1964, 44, 269–297. [Google Scholar] [PubMed]
- Blessed, G.; Tomlinson, B.E.; Roth, M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br. J. Psychiatry 1968, 114, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Margolis, G. Senile cerebral disease. A critical survey of traditional concepts based upon observations with newer techniques. Lab. Investig. 1959, 8, 335–370. [Google Scholar] [PubMed]
- McDonald, C. Clinical heterogeneity in senile dementia. Br. J. Psychiatry 1969, 115, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Fox, P. From senility to Alzheimer’s disease: The rise of the Alzheimer’s disease movement. Milbank Q. 1989, 67, 58–102. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.J.; Kelly, S.E.; Tobin, S.L. Defining dementia: Social and historical background of Alzheimer disease. Genet. Test. 1999, 3, 13–19. [Google Scholar] [CrossRef]
- Karlawish, J. The Problem of Alzheimer’s Disease. How Science Culture, and Politics Turned a Rare Disease into a Crisis and What We Can Do about It; St. Martin’s Publishing Group: New York, NY, USA, 2021. [Google Scholar]
- Katzman, R. Editorial: The prevalence and malignancy of Alzheimer disease. A major killer. Arch. Neurol. 1976, 33, 217–218. [Google Scholar] [CrossRef]
- Kuhn, T.S. The Structure of Scientific Revolutions; University of Chicago: Chicago, IL, USA, 1970. [Google Scholar]
- De la Torre, J.C.; Mussivand, T. Can disturbed brain microcirculation cause Alzheimer’s disease? Neurol. Res. 1993, 15, 146–153. [Google Scholar] [CrossRef]
- Robert, C.; Wilson, C.S.; Lipton, R.B.; Arreto, C.D. Evolution of the research literature and the scientific community of Alzheimer’s disease from 1983-2017, A 35-year survey. J. Alzheimer’s Dis. 2020, 75, 1105–1134. [Google Scholar] [CrossRef]
- Nicolas, G. Lessons from genetic studies in Alzheimer disease. Rev. Neurol. 2024, 180, 368–377. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Yang, L.G.; March, Z.M.; Stephenson, R.A.; Narayan, P.S. Apolipoprotein E in lipid metabolism and neurodegenerative disease. Trends Endocrinol. Metab. 2023, 34, 430–445. [Google Scholar] [CrossRef]
- Corbo, R.M.; Scacchi, R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’ allele? Ann. Hum. Genet. 1999, 63 Pt 4, 301–310. [Google Scholar] [CrossRef]
- Reiman, E.M.; Arboleda-Velasquez, J.F.; Quiroz, Y.T.; Huentelman, M.J.; Beach, T.G.; Caselli, R.J.; Chen, Y.; Su, Y.; Myers, A.J.; Hardy, J.; et al. Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat. Commun. 2020, 11, 667. [Google Scholar] [CrossRef]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef]
- Raichlen, D.A.; Alexander, G.E. Exercise, APOE genotype, and the evolution of the human lifespan. Trends Neurosci. 2014, 37, 247–255. [Google Scholar] [CrossRef]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J. Alzheimer’s Dis. 2001, 3, 75–80. [Google Scholar] [CrossRef]
- Soscia, S.J.; Kirby, J.E.; Washicosky, K.J.; Tucker, S.M.; Ingelsson, M.; Hyman, B.; Burton, M.A.; Goldstein, L.E.; Duong, S.; Tanzi, R.E.; et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS ONE 2010, 5, e9505. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.K.; Choi, S.H.; Washicosky, K.J.; Eimer, W.A.; Tucker, S.; Ghofrani, J.; Lefkowitz, A.; McColl, G.; Goldstein, L.E.; Tanzi, R.E.; et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci. Transl. Med. 2016, 8, 340ra72. [Google Scholar] [CrossRef]
- Eimer, W.A.; Vijaya Kumar, D.K.; Navalpur Shanmugam, N.K.; Rodriguez, A.S.; Mitchell, T.; Washicosky, K.J.; György, B.; Breakefield, X.O.; Tanzi, R.E.; Moir, R.D. Alzheimer’s Disease-Associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron 2018, 99, 56–63.e3. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, S.L.; Tucker, A.; Jacobson, S. The elusive role of herpesviruses in Alzheimer’s disease: Current evidence and future directions. Neuroimmune Pharm. Ther. 2023, 2, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, P.J.; George, D. The Myth of Alzheimer’ Disease; St Martin’s Griffin: New York, NY, USA, 2008. [Google Scholar]
- Wirak, D.O.; Bayney, R.; Ramabhadran, T.V.; Fracasso, R.P.; Hart, J.T.; Hauer, P.E.; Hsiau, P.; Pekar, S.K.; Scangos, G.A.; Trapp, B.D.; et al. Deposits of amyloid beta protein in the central nervous system of transgenic mice. Science 1991, 253, 323–325. [Google Scholar] [CrossRef]
- Lesné, S.; Koh, M.T.; Kotilinek, L.; Kayed, R.; Glabe, C.G.; Yang, A.; Gallagher, M.; Ashe, K.H. A specific amyloid-beta protein assembly in the brain impairs memory. Nature 2006, 440, 352–357, Retraction in: Nature 2024, 631, 240. https://doi.org/10.1038/s41586-024-07691-8. [Google Scholar] [CrossRef]
- Piller, C. Blots on a field? Science 2022, 377, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Appenzeller, T. Editor’s note. Science 2022, 377, 935. [Google Scholar] [CrossRef] [PubMed]
- Piller, C. Picture imperfect. Science 2024, 385, 1406–1412. [Google Scholar] [CrossRef]
- Piller, C. Firm misled investors on Alzheimer’s drug, SEC charges. Science 2024, 386, 15. [Google Scholar] [CrossRef]
- Orgogozo, J.M.; Gilman, S.; Dartigues, J.F.; Laurent, B.; Puel, M.; Kirby, L.C.; Jouanny, P.; Dubois, B.; Eisner, L.; Flitman, S.; et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 2003, 61, 46–54. [Google Scholar] [CrossRef]
- Nicoll, J.A.R.; Buckland, G.R.; Harrison, C.H.; Page, A.; Harris, S.; Love, S.; Neal, J.W.; Holmes, C.; Boche, D. Persistent neuropathological effects 14 years following amyloid-β immunization in Alzheimer’s disease. Brain 2019, 142, 2113–2126. [Google Scholar] [CrossRef]
- Bhadane, P.; Roul, K.; Belemkar, S.; Kumar, D. Immunotherapeutic approaches for Alzheimer’s disease: Exploring active and passive vaccine progress. Brain Res. 2024, 1840, 149018. [Google Scholar] [CrossRef]
- Herrup, K. The case for rejecting the amyloid cascade hypothesis. Nat. Neurosc. 2015, 18, 794–799. [Google Scholar] [CrossRef]
- Fedele, E. Anti-amyloid therapies for Alzheimer’s disease and the Amyloid Cascade Hypothesis. Int. J. Mol. Sci. 2023, 24, 14499. [Google Scholar] [CrossRef] [PubMed]
- Robakis, N.K.; Høilund-Carlsen, P.F.; Sensi, S.L.; Vissel, B. The amyloid cascade hypothesis: An updated critical review. Brain 2023, 146, 3969–3990. [Google Scholar] [CrossRef] [PubMed]
- Christopher, H.; van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Joseph, J.; Shukitt-Hale, B.; Denisova, N.A.; Martin, A.; Perry, G.; Smith, M.A. Copernicus revisited: Amyloid beta in Alzheimer’s disease. Neurobiol. Aging 2001, 22, 131–146. [Google Scholar] [CrossRef]
- Begley, S. The Maddening Saga of How Alzheimer’s ‘Cabal’ Thwarted Progress Toward a Cure for Decades. STAT [Internet]. 2019. Available online: https://www.statnews.com/2019/06/25/alzheimers-cabal-thwarted-progress-toward-cure/ (accessed on 28 February 2025).
- Whitehouse, P.J.; Price, D.L.; Clark, A.W.; Coyle, J.T.; DeLong, M.R. Alzheimer disease: Evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 1981, 10, 122–126. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging 1995, 16, 271–278. [Google Scholar] [CrossRef]
- Braak, H.; Thal, D.R.; Ghebremedhin, E.; Del Tredici, K. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011, 70, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K. Neuroanatomy and pathology of sporadic Alzheimer’s disease. In Advances in Anatomy, Embryology and Cell Biology; Spring Nature: Berlin/Heidelberg, Germany, 2015; Volume 215, pp. 1–162. [Google Scholar] [PubMed]
- Mudher, A.; Lovestone, S. Alzheimer’s disease-do tauists and baptists finally shake hands? Trends Neurosci. 2002, 25, 22–26. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Datta, D.; Preuss, T.M. Studies of aging nonhuman primates illuminate the etiology of early-stage Alzheimer’s-like neuropathology: An evolutionary perspective. Am. J. Primatol. 2021, 83, e23254. [Google Scholar] [CrossRef]
- Aisen, P.S.; Cummings, J.; Jack, C.R., Jr.; Morris, J.C.; Sperling, R.; Frölich, L.; Jones, R.W.; Dowsett, S.A.; Matthews, B.R.; Raskin, J.; et al. On the path to 2025: Understanding the Alzheimer’s disease continuum. Alzheimer’s Res. Ther. 2017, 9, 60. [Google Scholar] [CrossRef]
- Jellinger, K.A. Neuropathology of the Alzheimer’s continuum: An update. Free Neuropathol. 2020, 1, 1–32. [Google Scholar] [CrossRef]
- Satizabal, C.L.; Beiser, A.S.; Chouraki, V.; Chêne, G.; Dufouil, C.; Seshadri, S. Incidence of dementia over three decades in the Framingham Heart Study. N. Engl. J. Med. 2016, 374, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Amieva, H.; Mokri, H.; Le Goff, M.; Meillon, C.; Jacqmin-Gadda, H.; Foubert-Samier, A.; Orgogozo, J.M.; Stern, Y.; Dartigues, J.F. Compensatory mechanisms in higher-educated subjects with Alzheimer’s disease: A study of 20 years of cognitive decline. Brain 2014, 137 Pt 4, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Pareja, F. Alzheimer: Prevention from Childhood; Lamberth Academic Publishing: Vacoas, Maurithius, 2018. [Google Scholar]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Dekosky, S.T.; Barberger-Gateau, P.; Cummings, J.; Delacourte, A.; Galasko, D.; Gauthier, S.; Jicha, G.; et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS–ADRDA criteria. Lancet Neurol. 2007, 6, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B. Pour une nouvelle conception de la maladie d’Alzheimer. Rev. Med. Interne 2008, 29, 763–765. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Proceedings of the Meeting of the International Working Group (IWG) and the American Alzheimer’s Association. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s Dement. 2016, 12, 292–323. [Google Scholar] [CrossRef]
- Dubois, B.; Villain, N.; Schneider, L.; Fox, N.; Campbell, N.; Galasko, D.; Kivipelto, M.; Jessen, F.; Hanseeuw, B.; Boada, M.; et al. Alzheimer Disease as a clinical-biological construct—An International Working Group Recommendation. JAMA Neurol. 2024, 81, 1304–1311. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Therneau, T.M.; Wiste, H.J.; Weigand, S.D.; Knopman, D.S.; Lowe, V.J.; Mielke, M.M.; Vemuri, P.; Roberts, R.O.; Machulda, M.M.; et al. Transition rates between amyloid and neurodegeneration biomarker states and to dementia: A population-based, longitudinal cohort study. Lancet Neurol. 2016, 15, 56–64. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimer’s Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Lista, S.; Teipel, S.J.; Garaci, F.; Nisticò, R.; Blennow, K.; Zetterberg, H.; Bertram, L.; Duyckaerts, C.; Bakardjian, H.; et al. Perspective on future role of biological markers in clinical therapy trials of Alzheimer’s disease: A long-range point of view beyond 2020. Biochem. Pharmacol. 2014, 88, 426–449. [Google Scholar] [CrossRef] [PubMed]
- Schermer, M.H.N. Preclinical disease or risk factor? Alzheimer’s disease as a case study of changing conceptualizations of disease. J. Med. Philos. 2023, 48, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, R.; Arzberger, T.; Alafuzoff, I.; Al-Sarraj, A.; Budka, H.; Duyckaerts, C.; Falkai, P.; Ferrer, I.; Ironside, J.W.; Kovács, G.G.; et al. New lexicon and criteria for the diagnosis of Alzheimer’s disease. Lancet Neurol. 2011, 10, 298–299. [Google Scholar] [CrossRef]
- Oksengard, A.R.; Cavallin, L.; Axelsson, R.; Andersson, C.; Nägga, K.; Winblad, B.; Eriksdotter-Jönhagen, M.; Wahlund, L.O. Lack of accuracy for the proposed ‘Dubois Criteria’ in Alzheimer’s disease: A validation study from the Swedish Brain Power Initiative. Dement. Geriatr. Cogn. Disord. 2010, 30, 374–380. [Google Scholar] [CrossRef]
- Alafuzoff, I. Techniques in neuropathology. In Handbook of Clinical Neurology; Kovacs, G.G., Alafuzoff, I., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 145. [Google Scholar]
- Nichols, E.; Merrick, R.; Hay, S.I.; Himali, D.; Himali, J.J.; Hunter, S.; Keage, H.A.D.; Latimer, C.S.; Scott, M.R.; Steinmetz, J.D.; et al. The prevalence, correlation, and co-occurrence of neuropathology in old age: Harmonisation of 12 measures across six community-based autopsy studies of dementia. Lancet Healthy Longev. 2023, 4, e115–e125. [Google Scholar] [CrossRef]
- Kawas, C.H.; Legdeur, N.; Corrada, M.M. What have we learned from cognition in the oldest-old. Curr. Opin. Neurol. 2021, 34, 258–265. [Google Scholar] [CrossRef]
- Nelson, P.T.; Dickson, D.W.; Trojanowski, J.Q.; Jack, C.R.; Boyle, P.A.; Arfanakis, K.; Rademakers, R.; Alafuzoff, I.; Attems, J.; Brayne, C.; et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain 2019, 142, 1503–1527. [Google Scholar] [CrossRef]
- Savva, G.M.; Wharton, S.B.; Ince, P.G.; Forster, G.; Matthews, F.E.; Brayne, C.; Medical Research Council Cognitive Function and Ageing Study. Age, neuropathology, and dementia. N. Engl. J. Med. 2009, 360, 2302–2309. [Google Scholar] [CrossRef]
- King, A.; Bodi, I.; Troakes, C. The Neuropathological diagnosis of Alzheimer’s disease—The challenges of pathological mimics and concomitant pathology. Brain Sci. 2020, 10, 479. [Google Scholar] [CrossRef]
- Robinson, J.L.; Richardson, H.; Xie, S.X.; Suh, E.; Van Deerlin, V.M.; Alfaro, B.; Loh, N.; Porras-Paniagua, M.; Nirschl, J.J.; Wolk, D.; et al. The development and convergence of co-pathologies in Alzheimer’s disease. Brain 2021, 144, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Godrich, D.; Martin, E.R.; Schellenberg, G.; Pericak-Vance, M.A.; Cuccaro, M.; Scott, W.K.; Kukull, W.; Montine, T.; Beecham, G.W. Neuropathological lesions and their contribution to dementia and cognitive impairment in a heterogeneous clinical population. Alzheimer’s Dement. 2022, 18, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Boyle, P.A.; Wingo, A.P.; Yang, J.; Wang, T.; Buchman, A.S.; Wang, T.; Buchman, A.S.; Wingo, T.S.; Seyfried, N.T.; et al. Neuropathologic correlates of human cortical proteins in Alzheimer disease and related dementias. Neurology 2022, 98, e1031–e1039. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, A.H.; Markus, H.S.; Schneider, J.A. Cerebral small vessel disease, hypertension, and vascular contributions to cognitive impairment and dementia. Hypertension 2024, 81, 75–86. [Google Scholar] [CrossRef]
- Burgueño-García, I.; López-Martínez, M.J.; Uceda-Heras, A.; García-Carracedo, L.; Zea-Sevilla, M.A.; Rodrigo-Lara, H.; Rego-García, I.; Saiz-Aúz, L.; Ruiz-Valderrey, P.; López-González, F.J.; et al. Neuropathological heterogeneity of dementia due to combined pathology in aged patients: Clinicopathological findings in the Vallecas Alzheimer’s Reina Sofía Cohort. J. Clin. Med. 2024, 13, 6755. [Google Scholar] [CrossRef]
- Ren, Y.; Shahbaba, B.; Stark, C.E.L. Identifying dementia neuropathology using low-burden clinical data. Alzheimer’s Dement. 2025, 21, e70539. [Google Scholar] [CrossRef]
- Haan, M.N.; Wallace, R. Can dementia be prevented? Brain aging in a population-based context. Annu. Rev. Public. Health 2004, 25, 1–24. [Google Scholar] [CrossRef]
- Lobo, A.; Lopez-Anton, R.; Santabárbara, J.; de-la-Cámara, C.; Ventura, T.; Quintanilla, M.A.; Roy, J.F.; Campayo, A.J.; Lobo, E.; Palomo, T.; et al. Incidence and lifetime risk of dementia and Alzheimer’s disease in a Southern European population. Acta Psychiatr. Scand. 2011, 124, 372–383. [Google Scholar] [CrossRef]
- Rocca, W.A.; Petersen, R.C.; Knopman, D.S.; Hebert, L.E.; Evans, D.A.; Hall, K.S.; Gao, S.; Unverzagt, F.W.; Langa, K.M.; Larson, E.B.; et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimer’s Dement. 2011, 7, 80–93. [Google Scholar] [CrossRef]
- Schrijvers, E.M.; Verhaaren, B.F.; Koudstaal, P.J.; Hofman, A.; Ikram, M.A.; Breteler, M.M. Is dementia incidence declining?: Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology 2012, 78, 1456–1463. [Google Scholar] [CrossRef]
- Matthews, F.E.; Stephan, B.C.; Robinson, L.; Jagger, C.; Barnes, L.E.; Arthur, A.; Brayne, C.; Stephan, B.C.M.; Robinson, L. A two decades dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat. Commun. 2016, 7, 11398. [Google Scholar] [CrossRef] [PubMed]
- Grasset, L.; Brayne, C.; Joly, P.; Jacqmin-Gadda, H.; Peres, K.; Foubert-Samier, A.; Dartigues, J.F.; Helmer, C. Trends in dementia incidence: Evolution over a 10-year period in France. Alzheimer’s Dement. 2016, 12, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Abhari, S.; Guzman-Castillo, M.; Bandosz, P.; Shipley, M.J.; Muniz-Terrera, G.; Singh-Manoux, A.; Kivimäki, M.; Steptoe, A.; Capewell, S.; O’Flaherty, M.; et al. Temporal trend in dementia incidence since 2002 and projections for prevalence in England and Wales to 2040, modelling study. BMJ 2017, 358, j2856. [Google Scholar] [CrossRef] [PubMed]
- Derby, C.A.; Katz, M.J.; Lipton, R.B.; Hall, C.B. Trends in dementia incidence in a birth cohort analysis of the Einstein Aging Study. JAMA Neurol. 2017, 74, 1345–1351. [Google Scholar] [CrossRef]
- Noble, J.M.; Schupf, N.; Manly, J.J.; Andrews, H.; Tang, M.X.; Mayeux, R. Secular trends in the incidence of dementia in a multi-ethnic community. J. Alzheimer’s Dis. 2017, 60, 1065–1075. [Google Scholar] [CrossRef]
- Wu, Y.T.; Beiser, A.S.; Breteler, M.M.B.; Fratiglioni, L.; Helmer, C.; Hendrie, H.C.; Honda, H.; Ikram, M.A.; Langa, K.M.; Lobo, A.; et al. The changing prevalence and incidence of dementia over time—Current evidence. Nat. Rev. Neurol. 2017, 13, 327–339. [Google Scholar] [CrossRef]
- Gao, S.; Burney, H.N.; Callahan, C.M.; Purnell, C.E.; Hendrie, H.C. Incidence of dementia and Alzheimer disease over time: A meta-analysis. J. Am. Geriatr. Soc. 2019, 67, 1361–1369. [Google Scholar] [CrossRef]
- Seblova, D.; Quiroga, M.L.; Fors, S.; Johnell, K.; Lövdén, M.; de Leon, A.P.; Svensson, A.C.; Wicks, S.; Lager, A. Thirty-year trends in dementia: A nationwide population study of Swedish inpatient records. Clin. Epidemiol. 2018, 10, 1679–1693. [Google Scholar] [CrossRef]
- Sullivan, K.J.; Dodge, H.H.; Hughes, T.F.; Chang, C.H.; Zhu, X.; Liu, A.; Ganguli, M. Declining incident dementia rates across four population-based birth cohorts. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1439–1445. [Google Scholar] [CrossRef]
- Aranda, M.P.; Kremer, I.N.; Hinton, L.; Zissimopoulos, J.; Whitmer, R.A.; Hummel, C.H.; Trejo, L.; Fabius, C. Impact of dementia: Health disparities, population trends, care interventions, and economic costs. J. Am. Geriatr. Soc. 2021, 69, 1774–1783. [Google Scholar] [CrossRef]
- Contador, I.; Buch-Vicente, B.; Del Ser, T.; Llamas-Velasco, S.; Villarejo-Galende, A.; Benito-León, J.; Bermejo-Pareja, F. Charting Alzheimer’s disease and dementia: Epidemiological insights, risk factors and prevention pathways. J. Clin. Med. 2024, 13, 4100. [Google Scholar] [CrossRef]
- Vitali, F.; Branigan GLBrinton, R.G. Preventing Alzheimer’s disease within reach by 2025, Targeted-risk-AD-prevention (TRAP) strategy. Transl. Res. Clin. Interv. 2021, 7, e12190. [Google Scholar] [CrossRef]
- Farina, M.P.; Zhang, Y.S.; Kim, J.K.; Hayward, M.D.; Crimmins, E.M. Trends in dementia prevalence, incidence, and mortality in the United States (2000–2016). J. Aging Health 2022, 34, 100–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.D.; Deng, Y.T.; You, J.; He, X.Y.; Wu, X.R.; Wu, B.S.; Yang, L.; Zhang, Y.R.; Kuo, K.; et al. Identifying modifiable factors and their joint effect on dementia risk in the UK Biobank. Nat. Hum. Behav. 2023, 7, 1185–1195. [Google Scholar] [CrossRef]
- Lee, M.; Whitsel, E.; Avery, C.; Hughes, T.M.; Griswold, M.E.; Sedaghat, S.; Gottesman, R.F.; Mosley, T.H.; Heiss, G.; Lutsey, P.L. Variation in population attributable fraction of dementia associated with potentially modifiable risk factors by race and ethnicity in the US. JAMA Netw. Open. 2022, 5, e2219672. [Google Scholar] [CrossRef] [PubMed]
- Welberry, H.J.; Tisdell, C.C.; Huque, M.H.; Jorm, L.R. Have We Been Underestimating Modifiable Dementia Risk? An alternative approach for calculating the combined population attributable fraction for modifiable dementia risk factors. Am. J. Epidemiol. 2023, 192, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Stephan, B.C.M.; Cochrane, L.; Kafadar, A.H.; Cochrane, L.; Kafadar, A.H.; Brain, J.; Burton, E.; Myers, B.; Brayne, C.; Naheed, A.; et al. Population attributable fractions of modifiable risk factors for dementia: A systematic review and meta-analysis. Lancet Healthy Longev. 2024, 5, e406–e421. [Google Scholar] [CrossRef] [PubMed]
- Friedland, R.P.; Nandi, S. A modest proposal for a longitudinal study of dementia prevention (with apologies to Jonathan Swift, 1729). J. Alzheimer’s Dis. 2013, 33, 313–315. [Google Scholar] [CrossRef]
- Rosenberg, A.; Ngandu, T.; Rusanen, M.; Antikainen, R.; Bäckman, L.; Havulinna, S.; Hänninen, T.; Laatikainen, T.; Lehtisalo, J.; Levälahti, E.; et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: The FINGER trial. Alzheimer‘s Dement. 2018, 14, 263–270. [Google Scholar] [CrossRef]
- Reuben, D.B.; Kremen, S.; Maust, D.T. Dementia prevention and treatment: A narrative review. JAMA Intern. Med. 2024, 184, 563–572. [Google Scholar] [CrossRef]
- Giurgea, C. Vers une pharmacologie de l’activité intégrative du cerveau. Tentative du concept nootrope en psychopharmacologie. Actual Pharmacol. 1972, 25, 115–156. [Google Scholar] [PubMed]
- Hollister, L.E.; Yesavage, J. Ergoloid mesylates for senile dementias: Unanswered questions. Ann. Intern. Med. 1984, 100, 894–898. [Google Scholar] [CrossRef]
- Growdon, J.H.; Corkin, S.; Huff, F.J.; Rosen, T.J. Piracetam combined with lecithin in the treatment of Alzheimer’s disease. Neurobiol. Aging 1986, 7, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Summers, W.K.; Majovski, L.V.; Marsh, G.M.; Tachiki, K.; Kling, A. Oral tetrahydroaminoacridine in long-term treatment of senile dementia, Alzheimer type. N. Engl. J. Med. 1986, 315, 1241–1245. [Google Scholar] [CrossRef]
- Thal, L.J.; Salmon, D.P.; Lasker, B.; Bower, D.; Klauber, M.R. The safety and lack of efficacy of vinpocetine in Alzheimer’s disease. J. Am. Geriatr. Soc. 1989, 37, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Saletu, B.; Paulus, E.; Linzmayer, L.; Anderer, P.; Semlitsch, H.V.; Grünberger, J.; Wicke, L.; Neuhold, A.; Podreka, I. Nicergoline in senile dementia of Alzheimer type and multi-infarct dementia: A double-blind, placebo-controlled, clinical and EEG/ERP mapping study. Psychopharmacology 1995, 117, 385–395. [Google Scholar] [CrossRef]
- Fritze, J.; Walden, J. Clinical findings with nimodipine in dementia: Test of the calcium hypothesis. J. Neural Transm. Suppl. 1995, 46, 439–453. [Google Scholar] [PubMed]
- Rogers, S.L.; Friedhoff, L.T. The efficacy and safety of donepezil in patients with Alzheimer’s disease: Results of a US Multicentre, Randomized, Double-Blind, Placebo-Controlled Trial. The Donepezil Study Group. Dementia 1996, 7, 293–303. [Google Scholar] [CrossRef]
- Rösler, M.; Anand, R.; Cicin-Sain, A.; Gauthier, S.; Agid, Y.; Dal-Bianco, P.; Stähelin, H.B.; Hartman, R.; Gharabawi, M. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: International randomised controlled trial. BMJ 1999, 318, 633–638. [Google Scholar] [CrossRef]
- Winblad, B.; Poritis, N. Memantine in severe dementia: Results of the 9M-Best Study (Benefit and efficacy in severely demented patients during treatment with memantine). Int. J. Geriatr. Psychiatry 1999, 14, 135–146. [Google Scholar] [CrossRef]
- Raskind, M.A.; Peskind, E.R.; Wessel, T.; Yuan, W. Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology 2000, 54, 2261–2268. [Google Scholar] [CrossRef]
- Schneider, L.S.; DeKosky, S.T.; Farlow, M.R.; Tariot, P.N.; Hoerr, R.; Kieser, M. A randomized, double-blind, placebo-controlled trial of two doses of Ginkgo biloba extract in dementia of the Alzheimer’s type. Curr. Alzheimer Res. 2005, 2, 541–551. [Google Scholar] [CrossRef] [PubMed]
- DeKosky, S.T.; Williamson, J.D.; Fitzpatrick, A.L.; Kronmal, R.A.; Ives, D.G.; Saxton, J.A.; Lopez, O.L.; Burke, G.; Carlson, M.C.; Fried, L.P.; et al. Ginkgo Evaluation of Memory (GEM) Study Investigators. Ginkgo biloba for prevention of dementia: A randomized controlled trial. JAMA 2008, 300, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- Salloway, S.; Sperling, R.; Fox, N.C.; Blennow, K.; Klunk, W.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Porsteinsson, A.P.; Ferris, S.; et al. Bapineuzumab 301 and 302 Clinical Trial Investigators. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014, 370, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Doody, R.S.; Farlow, M.; Aisen, P.S.; Alzheimer’s Disease Cooperative Study Data Analysis and Publication Committee. Phase 3 trials of solanezumab and bapineuzumab for Alzheimer’s disease. N. Engl. J. Med. 2014, 370, 1460. [Google Scholar] [CrossRef]
- Budd Haeberlein, S.; Aisen, P.S.; Barkhof, F. Two randomized phase 3 studies of Aducanumab in early Alzheimer’s disease. J. Prev. Alzheimer’s Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Nery, E.S.; et al. Donanemab in early symptomatic Alzheimer disease: The TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef]
- Congdon, E.E.; Ji, C.; Tetlow, A.M.; Jiang, Y.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease: Current status and future directions. Nat. Rev. Neurol. 2023, 19, 715–736. [Google Scholar] [CrossRef]
- Terao, I.; Kodama, W. Comparative efficacy, tolerability and acceptability of donanemab, lecanemab, aducanumab and lithium on cognitive function in mild cognitive impairment and Alzheimer’s disease: A systematic review and network meta-analysis. Ageing Res. Rev. 2024, 94, 102203. [Google Scholar] [CrossRef]
- Olazarán, J.; Reisberg, B.; Clare, L.; Cruz, I.; Peña-Casanova, J.; Del Ser, T.; Woods, B.; Beck, C.; Auer, S.; Lai, C.; et al. Nonpharmacological therapies in Alzheimer’s disease: A systematic review of efficacy. Dement. Geriatr. Cogn. Disord. 2010, 30, 161–178. [Google Scholar] [CrossRef]
- Yang, H.; Luo, Y.; Hu, Q.; Tian, X.; Wen, H. Benefits in Alzheimer’s disease of sensory and multisensory stimulation. J. Alzheimer’s Dis. 2021, 82, 463–484. [Google Scholar] [CrossRef]
- Petersson, S.D.; Philippou, E. Mediterranean diet, cognitive function, and dementia: A systematic review of the evidence. Adv. Nutr. 2016, 7, 889–904. [Google Scholar] [CrossRef]
- Matziorinis, A.M.; Koelsch, S. The promise of music therapy for Alzheimer’s disease: A review. Ann. N. Y Acad. Sci. 2022, 1516, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Bayley, P.J. The therapeutic potential of yoga for Alzheimer’s Disease: A Critical Review. J. Alzheimer’s Dis. 2024, 101, S521–S535. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Leung, W.G.; Fearn, C.; John, A.; Stott, J.; Spector, A. Effectiveness of Cognitive Stimulation Therapy (CST) for mild to moderate dementia: A systematic literature review and meta-analysis of randomised control trials using the original CST protocol. Ageing Res. Rev. 2024, 97, 102312. [Google Scholar] [CrossRef] [PubMed]
- Terao, I.; Kodama, W. Comparative efficacy, tolerability, and acceptability of aducanumab, lecanemab, and donanemab with repetitive transcranial magnetic stimulation on cognitive function in mild cognitive impairment and Alzheimer’s disease: A systematic review and network meta-analysis. J. Psychopharmacol. 2025, 19, 2698811251340901. [Google Scholar] [CrossRef]
- Howard, C.W.; Reich, M.; Luo, L.; Pacheco-Barrios, N.; Alterman, R.; Rios, A.S.; Guo, M.; Luo, Z.; Friedrich, H.; Pines, A.; et al. Cognitive outcomes of deep brain stimulation depend on age and hippocampal connectivity in Parkinson’s and Alzheimer’s disease. Alzheimer’s Dement. 2025, 21, e70498. [Google Scholar] [CrossRef]
- Raikwar, S.P.; Kikkeri, N.S.; Sakuru, R.; Saeed, D.; Zahoor, H.; Premkumar, K.; Mentor, S.; Thangavel, R.; Dubova, I.; Ahmed, M.E.; et al. Next generation precision medicine: CRISPR-mediated genome editing for the treatment of neurodegenerative disorders. J. Neuroimmune Pharmacol. 2019, 14, 608–641. [Google Scholar] [CrossRef]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment combinations for Alzheimer’s disease: Current and future pharmacotherapy options. J. Alzheimer’s Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef]
- Bednar, M.M. Combination therapy for Alzheimer’s disease and related dementias. Prog. Mol. Biol. Transl. Sci. 2019, 168, 289–296. [Google Scholar] [CrossRef]
- Cao, Z.; Kong, F.; Ding, J.; Chen, C.; He, F.; Deng, W. Promoting Alzheimer’s disease research and therapy with stem cell technology. Stem Cell Res. Ther. 2024, 15, 136. [Google Scholar] [CrossRef]
- Salloway, S.; Pain, A.; Lee, E.; Papka, M.; Ferguson, M.B.; Wang, H.; Hu, H.; Lu, M.; Oru, E.; Ardayfio, P.A.; et al. TRAILBLAZER-ALZ 4: A phase 3 trial comparing donanemab with aducanumab on amyloid plaque clearance in early, symptomatic Alzheimer’s disease. Alzheimer’s Dement. 2025, 21, e70293. [Google Scholar] [CrossRef]
- Rubin, R. Treating Alzheimer disease with antiamyloid therapies-The real-world experience grows. JAMA 2025, 334, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Høilund-Carlsen, P.F.; Alavi, A.; Barrio, J.R.; Castellani, R.J.; Costa, T.; Herrup, K.; Kepp, K.P.; Neve, R.L.; Perry, G.; Revheim, M.E.; et al. Donanemab, another anti-Alzheimer’s drug with risk and uncertain benefit. Ageing Res. Rev. 2024, 99, 102348. [Google Scholar] [CrossRef] [PubMed]
- Pereira da Silva, A.M.; Falcão, L.; Virgilio, F.; Rodrigues Menezes, I.; Leite, M.; Farias, E.; Nascimento, M.; Lee Han, M.; Mota Telles, J.P.; de Souza Franco, E.; et al. Efficacy and APOE ε4-stratified risk of donanemab in Alzheimer’s disease: A systematic review and meta-analysis of randomized clinical trials. J. Alzheimer’s Dis. 2025, 107, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Kepp, K.P.; Høilund-Carlsen, P.F.; Cristea, I.A.; Cumming, R.G.; Daly, T.; Emilsson, L.; Flacco, M.E.; Hemkens, L.G.; Janiaud, P.; Johnsen, K.B.; et al. Communicating scientific evidence: Drugs for Alzheimer’s disease as a case study. Curr. Med. Res. Opin. 2025, 41, 347–354. [Google Scholar] [CrossRef]
- Bobbins, A.; Davies, M.; Lynn, E.; Roy, D.; Yeomans, A.; Shakir, S.A.W. Safety and effectiveness of the anti-amyloid monoclonal antibody (mAb) drug lecanemab for early Alzheimer’s disease: The pharmacovigilance perspective. Br. J. Clin. Pharmacol. 2025, 91, 1352–1360. [Google Scholar] [CrossRef]
- Burke, J.F.; Kerber, K.A.; Langa, K.M.; Albin, R.L.; Kotagal, V. Lecanemab: Looking before we leap. Neurology 2023, 101, 661–665. [Google Scholar] [CrossRef]
- Claus, J.J.; Vom Hofe, I.; van Ijlzinga Veenstra, A.; Licher, S.; Seelaar, H.; de Jong, F.J.; Neitzel, J.; Vernooij, M.W.; Ikram, M.A.; Wolters, F.J. Generalizability of trial criteria on amyloid-lowering therapy against Alzheimer’s disease to individuals with mild cognitive impairment or early Alzheimer’s disease in the general population. Eur. J. Epidemiol. 2025, 40, 327–337. [Google Scholar] [CrossRef]
- Urso, D.; Introna, A.; Gnoni, V.; Giugno, A.; Vilella, D.; Zecca, C.; De Blasi, R.; Giannoni-Luza, S.; Logroscino, G. Donanemab eligibility in early Alzheimer’s disease: A real-world study. J. Alzheimer’s Dis. 2025, 105, 745–750. [Google Scholar] [CrossRef]
- Aron, L.; Ngian, Z.K.; Qiu, C.; Choi, J.; Liang, M.; Drake, D.M.; Hamplova, S.E.; Lacey, E.K.; Roche, P.; Yuan, M.; et al. Lithium deficiency and the onset of Alzheimer’s disease. Nature 2025, 645, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.K.; Fuh, J.L. A 2025 update on treatment strategies for the Alzheimer’s disease spectrum. J. Chin. Med. Assoc. 2025, 88, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Garret, M.D. A Critique of the 2018 National Institute on Aging’s. Research Framework: Toward a biological definition of Alzheimer’s disease. Curr. Neurobiol. 2018, 9, 49–58. Available online: https://www.researchgate.net/publication/326995050 (accessed on 20 March 2025).
- Carandini, T.; Arighi, A.; Sacchi, L.; Fumagalli, G.G.; Pietroboni, A.M.; Ghezzi, L.; Colombi, A.; Scarioni, M.; Fenoglio, C.; De Riz, M.A.; et al. Testing the 2018 NIA-AA research framework in a retrospective large cohort of patients with cognitive impairment: From biological biomarkers to clinical syndromes. Alzheimer’s Res. Ther. 2019, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Ackley, S.F.; Zimmerman, S.C.; Brenowitz, W.D.; Tchetgen Tchetgen, E.J.; Gold, A.L.; Manly, J.J.; Mayeda, E.R.; Filshtein, T.J.; Power, M.C.; Elahi, F.M.; et al. Effect of reductions in amyloid levels on cognitive change in randomized trials: Instrumental variable meta-analysis. BMJ 2021, 372, n156. [Google Scholar] [CrossRef]
- Souchet, B.; Michaïl, A.; Billoir, B.; Braudeau, J. Biological diagnosis of Alzheimer’s Disease based on amyloid status: An. illustration of confirmation bias in medical research? Int. J. Mol. Sci. 2023, 24, 17544. [Google Scholar] [CrossRef]
- Hazan, J.; Liu, K.Y.; Costello, H.; Isaacs, J.D.; Thambisetty, M.; Howard, R. Challenges in a biological definition of Alzheimer disease. Neurology 2024, 103, e209884. [Google Scholar] [CrossRef]
- Drachman, D.A. Rethinking Alzheimer’s disease: The role of age-related changes. Curr. Neurol. Neurosci. Rep. 2007, 7, 265–268. [Google Scholar] [CrossRef]
- Pierce, A.L.; Kawas, C.H. Dementia in the oldest old: Beyond Alzheimer disease. PLoS Med. 2017, 14, e1002263. [Google Scholar] [CrossRef]
- Schermer, M.H.M.; Richard, E. On the reconceptualization of Alzheimer’s disease. Bioethics 2019, 33, 138–145. [Google Scholar] [CrossRef]
- Moseholm, K.F.; Tybjerg, K.; Jensen, M.K.; Westendorp, R.G.J. Too narrow and too broad: Differentiating late-onset dementia from its historical entanglement with Alzheimer’s disease. Aging Brain 2021, 1, 100010. [Google Scholar] [CrossRef] [PubMed]
- van der Schaar, J.; Visser, L.N.C.; Bouwman, F.H.; Ket, J.C.F.; Scheltens, P.; Bredenoord, A.L.; van der Flier, W.M. Considerations regarding a diagnosis of Alzheimer’s disease before dementia: A systematic review. Alzheimer’s Res. Ther. 2022, 14, 31. [Google Scholar] [CrossRef]
- Petersen, R.C.; Mormino, E.; Schneider, J.A. Alzheimer Disease-What’s in a Name? JAMA Neurol. 2024, 1, 1245–1246. [Google Scholar] [CrossRef]
- Latta, C.H.; Brothers, H.M.; Wilcock, D.M. Neuroinflammation in Alzheimer’s disease; A source of heterogeneity and target for personalized therapy. Neuroscience 2015, 30, 103–111. [Google Scholar] [CrossRef]
- Qiu, Y.; Jacobs, D.M.; Messer, K.; Salmon, D.P.; Feldman, H.H. Cognitive heterogeneity in probable Alzheimer disease: Clinical and neuropathologic features. Neurology 2019, 93, e778–e790. [Google Scholar] [CrossRef]
- Mehta, R.I.; Schneider, J.A. What is ‘Alzheimer’s disease’? The neuropathological heterogeneity of clinically defined Alzheimer’s dementia. Curr. Opin. Neurol. 2021, 34, 237–245. [Google Scholar] [CrossRef]
- Duara, R.; Barker, W. Heterogeneity in Alzheimer’s disease diagnosis and progression rates: Implications for therapeutic trials. Neurotherapeutics 2022, 19, 8–25. [Google Scholar] [CrossRef]
- Jellinger, K.A. Recent update on the heterogeneity of the Alzheimer’s disease spectrum. J. Neural Transm. 2022, 129, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I. Hypothesis review: Alzheimer’s overture guidelines. Brain Pathol. 2023, 33, e13122. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, B. Looking for trouble? Diagnostics expanding disease and producing patients. J. Eval. Clin. Pract. 2018, 24, 978–982. [Google Scholar] [CrossRef]
- Doust, J.A.; Bell, K.J.L.; Glasziou, P.P. Potential consequences of changing disease classifications. JAMA 2020, 323, 921–922. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.; Brayne, C. What do we mean by Alzheimer’s disease? BMJ 2010, 341, c4670. [Google Scholar] [CrossRef] [PubMed]
- Khachaturian, Z.S.; Khachaturian, A.S. Politics of science: Progress toward prevention of the dementia–Alzheimer’s syndrome. Mol. Asp. Med. 2015, 43–44, 3–15. [Google Scholar] [CrossRef]
- Khachaturian, Z.S. Prospects for effective treatment of the Dementia-Alzheimer syndrome: A renewed Odyssey in search of the magic elixir. J. Prev. Alzheimer’s Dis. 2017, 4, 215–217. [Google Scholar] [CrossRef]
- Pearce, J.M.S. Disease, diagnosis or syndrome? Pract. Neurol. 2011, 11, 91–97. [Google Scholar] [CrossRef]
- Abbate, C. Research on Alzheimer’s syndromes is critical to improve diagnosis, patient management and non-pharmacological treatments, but is under-pursued. Front. Aging Neurosci. 2022, 14, 1039899. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Bhat, M.A.; Dhaneshwar, S. Neurodegenerative diseases: New hopes and perspectives. Curr. Mol. Med. 2024, 24, 1004–1032. [Google Scholar] [CrossRef]
- Bradley, J.; Pottier, C.; da Fonseca, E.L.; Kurup, J.T.; Western, D.; Wang, C.; Neupane, A.; Ray, N.R.; Jean-Francois, M.; Ali, M.; et al. Novel early-onset Alzheimer-associated genes influence risk through dysregulation of glutamate, immune activation, and intracellular signaling pathways. Alzheimer‘s Dement. 2025, 21, e70377. [Google Scholar] [CrossRef]
- Smirnov, D.S.; Galasko, D.; Hiniker, A.; Edland, S.D.; Salmon, D.P. Age-at-onset and APOE-related heterogeneity in pathologically confirmed sporadic Alzheimer disease. Alzheimer’s Dement. 2021, 96, e2272–e2283. [Google Scholar] [CrossRef]
- Gao, J.; Bi, X.; Jiang, W.; Wang, Y. Integration of multi-omics quantitative trait loci evidence reveals novel susceptibility genes for Alzheimer’s disease. Sci. Rep. 2025, 15, 30158. [Google Scholar] [CrossRef] [PubMed]
- Rajabli, F.; Benchek, P.; Tosto, G.; Kushch, N.; Sha, J.; Bazemore, K.; Zhu, C.; Lee, W.P.; Haut, J.; Hamilton-Nelson, K.L.; et al. Multi-ancestry genome-wide meta-analysis of 56,241 individuals identifies known and novel cross-population and ancestry-specific associations as novel risk loci for Alzheimer’s disease. Genome Biol. 2025, 26, 210. [Google Scholar] [CrossRef] [PubMed]
- Nikolac Perkovic, M.; Videtic Paska, A.; Konjevod, M.; Kouter, K.; Svob Strac, D.; Nedic Erjavec, G.; Pivac, N. Epigenetics of Alzheimer’s disease. Biomolecules 2021, 11, 195. [Google Scholar] [CrossRef]
- Hunter, S. Friedland SP, Brayne C. Time for a change in the research paradigm for Alzheimer’s disease: The value of a chaotic matrix modeling approach. CNS Neurosc. Ther. 2010, 16, 254–262. [Google Scholar] [CrossRef]
- Zheng, C.; Xu, R. The Alzheimer’s comorbidity phenome: Mining from a large patient database and phenome-driven genetics prediction. JAMIA Open 2019, 2, 131–138. [Google Scholar] [CrossRef]
- Morgan, S.L.; Naderi, P.; Koler, K.; Pita-Juarez, Y.; Prokopenko, D.; Vlachos, I.S.; Tanzi, R.E.; Bertram, L.; Hide, W.A. Most pathways can be related to the pathogenesis of Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 846902. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Lau, V.; Ramer, L.; Tremblay, M.È. An aging, pathology burden, and glial senescence build-up hypothesis for late onset Alzheimer’s disease. Nat. Commun. 2023, 14, 1670. [Google Scholar] [CrossRef]
- Saurat, N.; Minotti, A.P.; Rahman, M.T.; Sikder, T.; Zhang, C.; Cornacchia, D.; Jungverdorben, J.; Ciceri, G.; Betel, D.; Studer, L. Genome-wide CRISPR screen identifies neddylation as a regulator of neuronal aging and AD neurodegeneration. Cell Stem Cell 2024, 31, 1162–1174.e8. [Google Scholar] [CrossRef]
- Ge, Y. Vascular contributions to healthy aging and dementia. Aging Dis. 2024, 15, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Riboli, E. Can noncommunicable diseases be prevented? Lessons from studies of populations and individuals. Science 2012, 337, 1482. [Google Scholar] [CrossRef]
- Piccini, C.; Bracco, L.; Amaducci, L. Treatable and reversible dementias: An update. J. Neurol. Sci. 1998, 153, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Pareja, F.; Llamas-Velasco, S.; Villarejo-Galende, A. Alzheimer’s disease prevention: A way forward. Rev. Clin. Esp. 2016, 216, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Chaufan, C.; Hollister, B.; Nazareno, J.; Fox, P. Medical ideology as a double-edged sword: The politics of cure and care in the making of Alzheimer’s disease. Soc. Sci. Med. 2012, 74, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M. The Diseased Brain and the Failing Mind. Dementia in Science, Medicine and Literature of the Long Twentieth Century; Bloomsbury Academic: London, UK, 2020. [Google Scholar]
- Larson, E.B. Prevention of late-life dementia: No magic bullet. Ann. Intern. Med. 2018, 168, 77–79. [Google Scholar] [CrossRef]
- Hachinski, V. Dementia: New vistas and opportunities. Neurol. Sci. 2019, 40, 763–767. [Google Scholar] [CrossRef]
- Peters, R.; Booth, A.; Rockwood, K.; Peters, J.; D’Este, C.; Anstey, K.J. Combining modifiable risk factors and risk of dementia: A systematic review and meta-analysis. BMJ Open 2019, 9, e022846. [Google Scholar] [CrossRef]
- Yu, J.T.; Xu, W.; Tan, C.C.; Andrieu, S.; Suckling, J.; Evangelou, E.; Pan, A.; Zhang, C.; Jia, J.; Feng, L.; et al. Evidence-based prevention of Alzheimer’s disease: Systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1201–1209. [Google Scholar] [CrossRef]
- Weiss, J.; Puterman, E.; Prather, A.A.; Ware, E.B.; Rehkopf, D.H. A data-driven prospective study of dementia among older adults in the United States. PLoS ONE 2020, 15, e0239994. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef]
- Wilkinson, R.; Marmot, M. The Solid Facts; World Health Organization: Copenhagen, Denmark, 2003. [Google Scholar]
- Beeri, M.S. Prevention of dementia presents a potentially critical platform for improvement of long-term public health. Dialogues Clin. Neurosci. 2019, 21, 93–99. [Google Scholar] [CrossRef] [PubMed]
- WHO Guidelines. Risk Reduction of Cognitive Decline and Dementia; World Health Organization: Copenhagen, Denmark, 2019. [Google Scholar]
- Daly, T. Ethics, evidence, and the environment in dementia risk reduction. Lancet Healthy Longev. 2022, 3, e131. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Govia, I.; Peters, R.; Richard, E.; Stephan, B.C.M.; Wilson, N.A.; Wallace, L.; Anstey, K.J.; Brayne, C. What would a population-level approach to dementia risk reduction look like, and how would it work? Alzheimer’s Dement. 2023. [Google Scholar] [CrossRef] [PubMed]
- Bodryzlova, J.; Mehrabi, F.; Bosson, A.; Maïano, C.; André, C.; Bélanger, E.; Moullec, G. The potential of social policies in preventing dementia: An ecological study using systematic review and meta-analysis. J. Aging Soc. Policy 2024, 36, 1004–1025. [Google Scholar] [CrossRef]
- Bermejo-Pareja, F.; Gómez de la Cámara, A.; Del Ser, T.; Contador, I.; Llamas-Velasco, S.; López-Arrieta, J.M.; Martín-Arriscado, C.; Hernández-Gallego, J.; Vega, S.; Benito-León, J. The health status: The ignored risk factor in dementia incidence. NEDICES cohort. Aging Clin. Exp. Res. 2022, 34, 1275–1283. [Google Scholar] [CrossRef]
- Viña, J.; Jorge Sanz-Ros, J. Alzheimer’s disease: Only prevention makes sense. Eur. J. Clin. Investig. 2018, 48, e13005. [Google Scholar] [CrossRef]
- Walsh, S.; Merrick, R.; Milne, R.; Nurock, S.; Richard, E.; Brayne, C. Considering challenges for the new Alzheimer’s drugs: Clinical, population, and health system perspectives. Alzheimer’s Dement. 2024, 20, 6639–6646. [Google Scholar] [CrossRef]
- Stoiljkovic, M.; Horvath, T.L.; Hajó, M. Therapy for Alzheimer’s disease: Missing targets and functional markers? Ageing Res. Rev. 2022, 68, 101318. [Google Scholar] [CrossRef]
- Jin, S.; Lu, W.; Zhang, J.; Zhang, L.; Tao, F.; Zhang, Y.; Hu, X.; Liu, Q. The mechanisms, hallmarks, and therapies for brain aging and age-related dementia. Sci. Bull. 2024, 69, 3756–3776. [Google Scholar] [CrossRef]
- Cummings, J.L.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Leisgang-Osse, A.M.; Cheng, F. Alzheimer’s disease drug development pipeline: 2025. Alzheimer’s Dement. 2025, 11, e70098. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Aho, E.; Brayne, C.; Ciccarelli, O.; Dubois, B.; Fox, N.C.; Frederiksen, K.S.; Gabay, C.; Garibotto, V.; Hofmarcher, T.; et al. Alzheimer’s disease outlook: Controversies and future directions. Lancet 2025, 406, 1424–1442. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, G.B.; Ritchie, C.; Carrera, E.; Nilsson, P.; Ousset, P.J.; Molinuevo, J.L.; Dubois, B.; Scheltens, P.; Minoshima, S. Re-aligning scientific and lay narratives of Alzheimer’s disease. Lancet Neurol. 2019, 18, 918–919. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Shue, F.; Bu, G.; Kanekiyo, T. Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer’s disease. Mol. Neurodegener. 2023, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M. Vascular dementia: From pathophysiology to therapeutic frontiers. J. Clin. Med. 2025, 14, 6611. [Google Scholar] [CrossRef]
- Pinheiro, A.; Ekenze, O.; Aparicio, H.J.; Beiser, A.S.; Decarli, C.S.; Demissie, S.; Seshadri, S.; Romero, J.R. Multimarker Cerebral Small Vessel Disease Score and Risk of Incident Dementia in the Framingham Heart Study. Neurology 2025, 105, e214113. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef]
- Melo dos Santos, L.S.; Trombetta-Lima, M.; Eggen, B.J.L.; Demaria, M. Cellular senescence in brain aging and neurodegeneration. Ageing Res. Rev. 2024, 93, 102141. [Google Scholar] [CrossRef]
- Puri, S.; Shaheen, M.; Grover, B. Nutrition and cognitive health: A life course approach. Front. Public. Health 2023, 11, 1023907. [Google Scholar] [CrossRef]
- Twarowski, B.; Herbet, M. Inflammatory Processes in Alzheimer’s Disease-pathomechanism, Diagnosis and treatment: A Review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L.C. Alzheimer’s disease: From immunotherapy to immunoprevention. Cell 2023, 186, 4260–4270. [Google Scholar] [CrossRef]
- Feizpour, A.; Doecke, J.D.; Doré, V.; Krishnadas, N.; Huang, K.; Bourgeat, P.; Laws, S.M.; Fowler, C.; Robertson, J.; Mackintosh, L.; et al. Detection and staging of Alzheimer’s disease by plasma pTau217 on a high throughput immunoassay platform. EBioMedicine 2024, 109, 105405. [Google Scholar] [CrossRef]
- Cai, H.; Pang, Y.; Fu, X.; Ren, Z.; Jia, L. Plasma biomarkers predict Alzheimer’s disease before clinical onset in Chinese cohorts. Nat. Commun. 2023, 14, 6747. [Google Scholar] [CrossRef]
- Salvadó, G.; Janelidze, S.; Bali, D.; Dolado, A.O.; Therriault, J.; Brum, W.S.; Pichet Binette, A.; Stomrud, E.; Mattsson-Carlgren, N.; Palmqvist, S.; et al. Plasma Phosphorylated Tau 217 to Identify Preclinical Alzheimer Disease. JAMA Neurol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, S.; Warmenhoven, N.; Anastasi, F.; Pilotto, A.; Janelidze, S.; Tideman, P.; Stomrud, E.; Mattsson-Carlgren, N.; Smith, R.; Ossenkoppele, R.; et al. Plasma phospho-tau217 for Alzheimer’s disease diagnosis in primary and secondary care using a fully automated platform. Nat. Med. 2025, 31, 2036–2043. [Google Scholar] [CrossRef] [PubMed]
- Monane, M.; Maraganore, D.M.; Carlile, R.M.; Johnson, K.G.; Merrill, D.A.; Gitelman, D.R.; Sharlin, K.S.; VandeVrede, L.A.; George, K.K.; Wang, J.; et al. Clinical utility of an Alzheimer’s Disease blood test among cognitively impaired patients: Results from the Quality Improvement PrecivityAD2 (QUIP II) Clinician Survey Study. Diagnostics 2025, 15, 167. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Wang, Q.; Yang, M.; Lin, G.; Yao, K.; Wu, Z.; Xu, D.; Zhou, H.; Chen, B.; Shi, H.; et al. Plasma p-tau217 and p-tau217/Aβ1-42 are effective biomarkers for identifying CSF- and PET imaging-diagnosed Alzheimer’s disease: Insights for research and clinical practice. Alzheimer’s Dement. 2025, 21, e14536. [Google Scholar] [CrossRef]
- Antonioni, A.; Raho, E.M.; Di Lorenzo, F.; Manzoli, L.; Flacco, M.E.; Koch, G. Blood phosphorylated Tau217 distinguishes amyloid-positive from amyloid-negative subjects in the Alzheimer’s disease continuum. A systematic review and meta-analysis. J. Neurol. 2025, 272, 252. [Google Scholar] [CrossRef]
- Moon, H.; Chen, X.; Alzheimer’s Disease Neuroimaging Initiative. Plasma p-tau217 predicting brain-wide tau accumulation in preclinical, AD. J. Prev. Alzheimer’s Dis. 2025, 12, 100252. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; Buckley, R.F.; van der Flier, W.M.; Han, Y.; Molinuevo, J.L.; Rabin, L.; Rentz, D.M.; Rodriguez-Gomez, O.; Saykin, A.J.; et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020, 19, 271–278. [Google Scholar] [CrossRef]
- Giacomucci, G.; Crucitti, C.; Ingannato, A.; Moschini, V.; Bagnoli, S.; Pozzi, F.E.; Marcantelli, E.; Padiglioni, S.; Morinelli, C.; Mazzeo, S.; et al. The two cut-offs approach for plasma p-tau217 in detecting Alzheimer’s disease in subjective cognitive decline and mild cognitive impairment. Alzheimer’s Dement 2025, 17, e70116. [Google Scholar] [CrossRef]
- Jonaitis, E.M.; Janelidze, S.; Cody, K.A.; Langhough, R.; Du, L.; Chin, N.A.; Mattsson-Carlgren, N.; Hogan, K.J.; Christian, B.T.; Betthauser, T.J.; et al. Plasma phosphorylated tau 217 in preclinical Alzheimer’s disease. Brain Commun. 2023, 5, fcad057. [Google Scholar] [CrossRef]
- Parra, M.A.; Butler, S.; McGeown, W.J.; Brown Nicholls, L.A.; Robertson, D.J. Globalising strategies to meet global challenges: The case of ageing and dementia. J. Glob. Health 2019, 9, 020310. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bermejo-Pareja, F.; del Ser, T. Alzheimer Syndrome or Age-Related Dementia—History, Therapy and Prevention. J. Clin. Med. 2025, 14, 7752. https://doi.org/10.3390/jcm14217752
Bermejo-Pareja F, del Ser T. Alzheimer Syndrome or Age-Related Dementia—History, Therapy and Prevention. Journal of Clinical Medicine. 2025; 14(21):7752. https://doi.org/10.3390/jcm14217752
Chicago/Turabian StyleBermejo-Pareja, Félix, and Teodoro del Ser. 2025. "Alzheimer Syndrome or Age-Related Dementia—History, Therapy and Prevention" Journal of Clinical Medicine 14, no. 21: 7752. https://doi.org/10.3390/jcm14217752
APA StyleBermejo-Pareja, F., & del Ser, T. (2025). Alzheimer Syndrome or Age-Related Dementia—History, Therapy and Prevention. Journal of Clinical Medicine, 14(21), 7752. https://doi.org/10.3390/jcm14217752





