Serum Biomarkers in Acute Ischemic Stroke: Clinical Applications and Emerging Insights
Abstract
1. Introduction
2. Materials and Methods
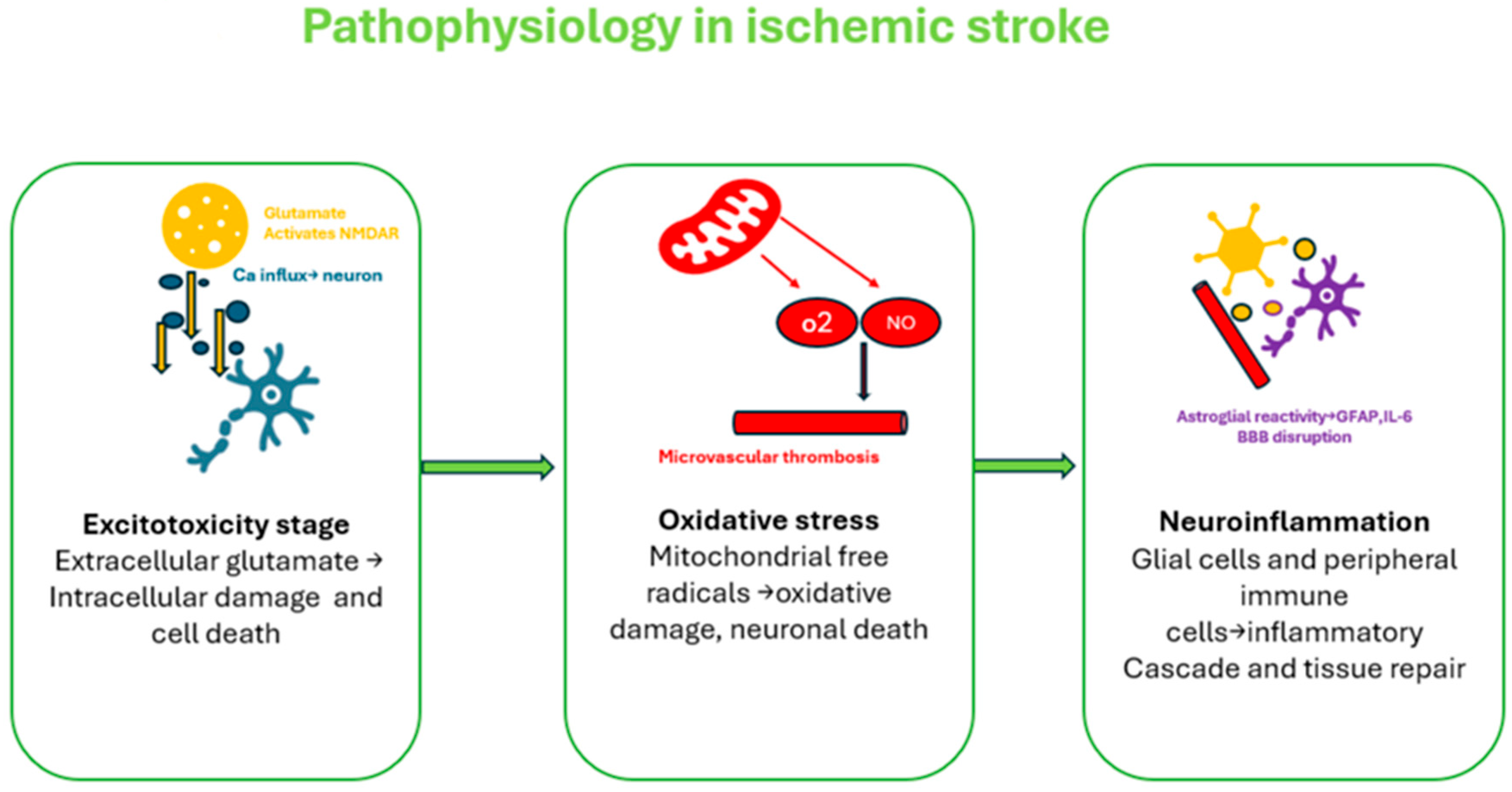
3. Acute Ischemic Stroke Pathogenesis Pathway
- Hyperacute Phase (0–6 h): Energy failure leads to neuronal swelling and excitotoxicity. Early reperfusion can save tissue but may worsen brain edema due to blood–brain barrier (BBB) disruption.
- Acute Phase (6 h to 3–4 days): Inflammation dominates, with reactive oxygen species and cellular debris activating immune cells, worsening BBB damage and maintaining the inflammatory cycle.
- Subacute Phase (Day 7 onward): Inflammation shifts toward repair, with anti-inflammatory responses, BBB stabilization, and angiogenesis promoting recovery.
- Chronic Phase (After 6 weeks): The BBB nearly normalizes, though low-grade inflammation persists. Recovery continues through neuroplasticity and tissue remodeling [11].
4. AIS Serum Biomarkers: Toward a Pathophysiology-Guided Framework for Clinical Translation
4.1. Oxidative Stress Biomarkers
4.2. Inflammatory Biomarkers
4.3. Thrombus Formation Biomarkers
4.4. Cardiac Function Biomarkers
4.5. Neuronal and Axonal Injury Markers
5. Discussion
6. Future Perspective: Microglia as a Promising Therapeutic Target
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.O.; Pandian, J.; Lindsay, P.; Grupper, M.F.; Rautalin, I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int. J. Stroke 2025, 20, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Rossi, R.; Jabrah, D.; Doyle, K. Detection, Diagnosis and Treatment of Acute Ischemic Stroke: Current and Future Perspectives. Front. Med. Technol. 2022, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Chalela, J.A.; Alsop, D.C.; Gonzalez-Atavales, J.B.; Maldjian, J.A.; Kasner, S.E.; Detre, J.A. Magnetic Resonance Perfusion Imaging in Acute Ischemic Stroke Using Continuous Arterial Spin Labeling. Stroke 2000, 31, 680–687. [Google Scholar] [CrossRef]
- Dias, B.A.; Bezerra, K.B.; Bezerra, A.S.d.A.; Santana, V.G.; Borges, R.R.; Reinaux, J.C.d.F.; Souza, D.L.; Maluf, F.B. Importance of computed tomography angiography in acute/hyperacute ischemic stroke. Radiol. Bras. 2021, 54, 360–366. [Google Scholar] [CrossRef]
- Arch, A.E.; Weisman, D.C.; Coca, S.; Nystrom, K.V.; Wira, C.R.; Schindler, J.L. Missed Ischemic Stroke Diagnosis in the Emergency Department by Emergency Medicine and Neurology Services. Stroke 2016, 47, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, J.; Chen, Y.; Tong, Y.; Li, L.; Xu, Y.; Wu, S. Advances in the detection of biomarkers for ischemic stroke. Front. Neurol. 2025, 16, 1488726. [Google Scholar] [CrossRef]
- Miao, Y.; Liao, J.K. Potential serum biomarkers in the pathophysiological processes of stroke. Expert Rev. Neurother. 2014, 14, 173–185. [Google Scholar] [CrossRef]
- Babić, A.; Bonifačić, D.; Komen, V.; Kovačić, S.; Mamić, M.; Vuletić, V. Blood Biomarkers in Ischemic Stroke Diagnostics and Treatment—Future Perspectives. Medicina 2025, 61, 514. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Andone, S.; Bajko, Z.; Motataianu, A.; Mosora, O.; Balasa, R. The Role of Biomarkers in Atherothrombotic Stroke—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 9032. [Google Scholar] [CrossRef]
- Salaudeen, M.A.; Bello, N.; Danraka, R.N.; Ammani, M.L. Understanding the Pathophysiology of Ischemic Stroke: The Basis of Current Therapies and Opportunity for New Ones. Biomolecules 2024, 14, 305. [Google Scholar] [CrossRef]
- Maida, C.D.; Norrito, R.L.; Rizzica, S.; Mazzola, M.; Scarantino, E.R.; Tuttolomondo, A. Molecular Pathogenesis of Ischemic and Hemorrhagic Strokes: Background and Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 6297. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Z.; Liao, Y.; Sun, S.; Dai, Y.; Tang, Y. Ischemic stroke: From pathological mechanisms to neuroprotective strategies. Front. Neurol. 2022, 13, 1013083. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Kumar, S.; Acharya, S.; Shukla, S.; Hulkoti, V.; Patel, M.; Gupte, Y.; Verma, P. Serum uric acid as a biomarker in predicting outcome in patients of acute ischemic stroke: A cross-sectional study at limited resources rural setup. Int. J. Nutr. Pharmacol. Neurol. Dis. 2023, 13, 68–73. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, Z.; Fu, F.; Zhan, Z. Serum uric acid and prognosis in acute ischemic stroke: A dose–response meta-analysis of cohort studies. Front. Aging Neurosci. 2023, 15, 1223015. [Google Scholar] [CrossRef]
- Teixeira, B.C.; Lopes, A.L.; Macedo, R.C.O.; Correa, C.S.; Ramis, T.R.; Ribeiro, J.L.; Reischak-Oliveira, A. Inflammatory markers, endothelial function and cardiovascular risk. J. Vasc. Bras. 2014, 13, 108–115. [Google Scholar] [CrossRef][Green Version]
- Liu, W.; Sahin, C.; Güner Sak, N.; Giraud, A.; Messina, P.; Bozsak, F.; Darcourt, J.; Sacchetti, F.; Januel, A.C.; Bellanger, G.; et al. C-reactive protein expression in acute ischemic stroke blood clots: Implications for etiology. Eur. Stroke J. 2025, 10, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Steliga, A.; Kowiański, P.; Czuba, E.; Waśkow, M.; Moryś, J.; Lietzau, G. Neurovascular Unit as a Source of Ischemic Stroke Biomarkers—Limitations of Experimental Studies and Perspectives for Clinical Application. Transl. Stroke Res. 2020, 11, 553–579. [Google Scholar] [CrossRef]
- Ng, G.J.; Quek, A.M.; Cheung, C.; Arumugam, T.V.; Seet, R.C. Stroke biomarkers in clinical practice: A critical appraisal. Neurochem. Int. 2017, 107, 11–22. [Google Scholar] [CrossRef]
- Rosenberg, J.; Do, D.; Cucchiara, B.; Messé, S.R. D-dimer and Body CT to Identify Occult Malignancy in Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 105366. [Google Scholar] [CrossRef]
- Schutte, T.; Thijs, A.; Smulders, Y.M. Never ignore extremely elevated D-dimer levels: They are specific for serious illness. Neth. J. Med. 2016, 74, 443–448. [Google Scholar] [PubMed]
- Surma, S.; Banach, M. Fibrinogen and Atherosclerotic Cardiovascular Diseases—Review of the Literature and Clinical Studies. Int. J. Mol. Sci. 2021, 23, 193. [Google Scholar] [CrossRef] [PubMed]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Xiang, X.; Pan, Y.; Zhang, Q.; Li, H.; Meng, X.; Wang, Y.-J. Baseline or 90-day fibrinogen levels and long-term outcomes after ischemic stroke or TIA: Results from the China national stroke registry Ⅲ. Atherosclerosis 2021, 337, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, M.; Singh, P. Is Plasma Fibrinogen Useful in Evaluating Ischemic Stroke Patients? Stroke 2009, 40, 1549–1552. [Google Scholar] [CrossRef]
- He, L.; Wang, J.; Dong, W. The clinical prognostic significance of hs-cTnT elevation in patients with acute ischemic stroke. BMC Neurol. 2018, 18, 118. [Google Scholar] [CrossRef]
- Perry, L.A.; Lucarelli, T.; Penny-Dimri, J.C.; McInnes, M.D.; Mondello, S.; Bustamante, A.; Montaner, J.; Foerch, C.; Kwan, P.; Davis, S.; et al. Glial fibrillary acidic protein for the early diagnosis of intracerebral hemorrhage: Systematic review and meta-analysis of diagnostic test accuracy. Int. J. Stroke 2019, 14, 390–399. [Google Scholar] [CrossRef]
- Herrmann, M.; Vos, P.; Wunderlich, M.T.; de Bruijn, C.H.M.M.; Lamers, K.J.B. Release of Glial Tissue–Specific Proteins After Acute Stroke. Stroke 2000, 31, 2670–2677. [Google Scholar] [CrossRef]
- Kalra, L.; Zylyftari, S.; Blums, K.; Barthelmes, S.; Baum, H.; Meckel, S.; Heilgeist, A.; Luger, S.; Foerch, C. Rapid Diagnosis of Intracerebral Hemorrhage in Patients with Acute Stroke by Measuring Prehospital GFAP Levels on a Point-of-Care Device (DETECT). Neurology 2025, 105, e213823. [Google Scholar] [CrossRef]
- Holmegaard, L.; Jensen, C.; Pedersen, A.; Blomstrand, C.; Blennow, K.; Zetterberg, H.; Jood, K.; Jern, C. Circulating levels of neurofilament light chain as a biomarker of infarct and white matter hyperintensity volumes after ischemic stroke. Sci. Rep. 2024, 14, 16180. [Google Scholar] [CrossRef] [PubMed]
- Kaspa, C.; Govindu, S. Serum uric acid as a prognostic indicator in acute ischemic stroke. Int. J. Res. Med. Sci. 2020, 8, 1435. [Google Scholar] [CrossRef]
- Prasad, C.; Dwivedi, N.; Gupta, P.; Shukla, S.; Shukla, R.; Yadav, R.; Verma, S. Serum uric acid level in patients of acute stroke. Int. J. Adv. Med. 2016, 3, 393–397. [Google Scholar] [CrossRef][Green Version]
- Tong, X.; Lyu, C.; Guo, M.; Gu, J.; Zhao, Y. Serum uric acid as a predictor of mortality in patients with stroke: Results from National Health and Nutrition Examination Survey 2007–2016. Front. Neurol. 2024, 15, 1383300. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Cai, H.; Zhang, Z.; Wang, J.; Xiao, L.; Zhang, P.; Xu, Y.; Tu, W.; Zhu, W.; Liu, X.; et al. Serum uric acid and prognosis of ischemic stroke: Cohort study, meta-analysis and Mendelian randomization study. Eur. Stroke J. 2024, 9, 235–243. [Google Scholar] [CrossRef]
- Soeki, T.; Sata, M. Inflammatory Biomarkers and Atherosclerosis. Int. Heart J. 2016, 57, 134–139. [Google Scholar] [CrossRef]
- Wakugawa, Y.; Kiyohara, Y.; Tanizaki, Y.; Kubo, M.; Ninomiya, T.; Hata, J.; Doi, Y.; Okubo, K.; Oishi, Y.; Shikata, K.; et al. C-Reactive Protein and Risk of First-Ever Ischemic and Hemorrhagic Stroke in a General Japanese Population. Stroke 2006, 37, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, M.; Zhuo, W.; Zhang, Y.; Xu, A. The Role of Hs-CRP, D-Dimer and Fibrinogen in Differentiating Etiological Subtypes of Ischemic Stroke. PLoS ONE 2015, 10, e0118301. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Z.; Li, J.; Xu, Y. Serum CRP concentrations and severity of ischemic stroke subtypes. Can. J. Neurol. Sci. 2012, 39, 69–73. [Google Scholar] [CrossRef]
- Terruzzi, A.; Valente, L.; Mariani, R.; Moschini, L.; Camerlingo, M. C-reactive protein and aetiological subtypes of cerebral infarction. Neurol. Sci. 2008, 29, 245–249. [Google Scholar] [CrossRef]
- Ladenvall, C.; Jood, K.; Blomstrand, C.; Nilsson, S.; Jern, C.; Ladenvall, P. Serum C-reactive protein concentration and genotype in relation to ischemic stroke subtype. Stroke 2006, 37, 2018–2023. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-D.; Wang, D.-Z.; Zhang, Q.; Wang, J.-H.; Li, B.-H.; Zhang, X.; Zhang, J.; Zhou, S.; Jia, L.-J.; Wang, L.-R.; et al. Predictive role of pre-thrombolytic hs-CRP on the safety and efficacy of intravenous thrombolysis in acute ischemic stroke. BMC Neurol. 2023, 23, 244. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, M.; Derbisz, J.; Drabik, L.; Slowik, A. C-Reactive Protein and White Blood Cell Count in Non-Infective Acute Ischemic Stroke Patients Treated with Intravenous Thrombolysis. J. Clin. Med. 2021, 10, 1610. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, G.B.; Liu, Y.B.; Pan, H.B.; Yan, L.; Zhao, L.B.; Zhao, Y.; Ji, Q. Predictive effects of S100β and CRP levels on hemorrhagic transformation in patients with AIS after intravenous thrombolysis: A concise review based on our center experience. Medicine 2023, 102, e35149. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, L.; Lang, Y.; Wu, D.; Chen, J.; Zhao, W.; Li, C.; Ji, X. Association between high-sensitivity C-reactive protein levels and clinical outcomes in acute ischemic stroke patients treated with endovascular therapy. Ann. Transl. Med. 2020, 8, 1379. [Google Scholar] [CrossRef]
- Zeng, Q.; Zeng, Y.; Slevin, M.; Guo, B.; Shen, Z.; Deng, B.; Zhang, W. C-Reactive Protein Levels and Clinical Prognosis in LAA-Type Stroke Patients: A Prospective Cohort Study. BioMed Res. Int. 2021, 2021, 6671043. [Google Scholar] [CrossRef]
- Finck, T.; Sperl, P.; Hernandez-Petzsche, M.; Boeckh-Behrens, T.; Maegerlein, C.; Wunderlich, S.; Zimmer, C.; Kirschke, J.; Berndt, M. Inflammation in stroke: Initial CRP levels can predict poor outcomes in endovascularly treated stroke patients. Front. Neurol. 2023, 14, 1167549. [Google Scholar] [CrossRef]
- Luwen, H.; Lei, X.; Qing-Rong, O.; Linlin, L.; Ming, Y. Association between hs-CRP/HDL-C ratio and three-month unfavorable outcomes in patients with acute ischemic stroke: A second analysis based on a prospective cohort study. BMC Neurol. 2024, 24, 418. [Google Scholar] [CrossRef]
- Geng, H.; Wang, X.; Fu, R.; Jing, M.; Huang, L.; Zhang, Q.; Wang, X.-X.; Wang, P.-X. The Relationship between C-Reactive Protein Level and Discharge Outcome in Patients with Acute Ischemic Stroke. Int. J. Environ. Res. Public Health 2016, 13, 636. [Google Scholar] [CrossRef]
- Na, S.; Kim, T.; Koo, J.; Hong, Y.J.; Kim, S. Vessel wall enhancement and high-sensitivity CRP as prognostic markers in intracranial atherosclerotic stroke: A prospective cohort study. Eur. Stroke J. 2025, 10, 862–870. [Google Scholar] [CrossRef]
- Bian, J.; Guo, S.; Huang, T.; Li, X.; Zhao, S.; Chu, Z.; Li, Z. CRP as a potential predictor of outcome in acute ischemic stroke. Biomed. Rep. 2023, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, R.; Ago, T.; Hata, J.; Wakisaka, Y.; Kuroda, J.; Kuwashiro, T.; Kitazono, T.; Kamouchi, M. Plasma C-Reactive Protein and Clinical Outcomes after Acute Ischemic Stroke: A Prospective Observational Study. PLoS ONE 2016, 11, e0156790. [Google Scholar] [CrossRef]
- Pu, Y.; Li, S.; Wang, L.; Fang, B.; Bai, X. Association Between High-Sensitivity C-Reactive Protein and Prognosis of Patients with Acute Cerebral Infarction. Neuropsychiatr. Dis. Treat. 2022, 18, 1771–1778. [Google Scholar] [CrossRef]
- Chen, L.; Wang, M.; Yang, C.; Wang, Y.; Hou, B. The role of high-sensitivity C-reactive protein serum levels in the prognosis for patients with stroke: A meta-analysis. Front. Neurol. 2023, 14, 1199814. [Google Scholar] [CrossRef]
- Mengozzi, M.; Kirkham, F.A.; Girdwood, E.E.R.; Bunting, E.; Drazich, E.; Timeyin, J.; Ghezzi, P.; Rajkumar, C. C-Reactive Protein Predicts Further Ischemic Events in Patients with Transient Ischemic Attack or Lacunar Stroke. Front. Immunol. 2020, 11, 1403. [Google Scholar] [CrossRef]
- Wang, G.; Jing, J.; Li, J.; Pan, Y.; Yan, H.; Meng, X.; Zhao, X.; Liu, L.; Li, H.; Wang, D.Z.; et al. Association of elevated hs-CRP and multiple infarctions with outcomes of minor stroke or TIA: Subgroup analysis of CHANCE randomised clinical trial. Stroke Vasc. Neurol. 2021, 6, 80–86. [Google Scholar] [CrossRef]
- Miwa, K.; Tanaka, M.; Okazaki, S.; Furukado, S.; Sakaguchi, M.; Mochizuki, H.; Kitagawa, K. Association between interleukin-6 levels and first-ever cerebrovascular events in patients with vascular risk fac tors. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 400–405. [Google Scholar] [CrossRef]
- Martinic-Popovic, I.; Simundic, A.M.; Dukic, L.; Lovrencic-Huzjan, A.; Popovic, A.; Seric, V.; Basic-Kes, V.; Demarin, V. The association of inflammatory markers with cerebral vasoreactivity and carotid atherosclerosis in transient ischaemic attack. Clin. Biochem. 2014, 47, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, R.M.; Elsaid, A.F. Infarction size, interleukin-6, and their interaction are predictors of short-term stroke outcome in young Egyptian adults. J Stroke Cerebrovasc. Dis. 2016, 25, 2475–2481. [Google Scholar] [CrossRef]
- Bsat, S.; Halaoui, A.; Kobeissy, F.; Moussalem, C.; El Houshiemy, M.N.; Kawtharani, S.; Omeis, I. Acute ischemic stroke biomarkers: A new era with diagnostic promise? Acute Med. Surg. 2021, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Aref, H.M.A.; Fahmy, N.A.; Khalil, S.H.; Ahmed, M.F.; ElSadek, A.; Abdulghani, M.O. Role of interleukin-6 in ischemic stroke outcome. Egypt J. Neurol. Psychiatry Neurosurg. 2020, 56, 12. [Google Scholar] [CrossRef]
- Kowalski, R.G.; Ledreux, A.; Violette, J.E.; Paustian, W.; Sillau, S.; Thompson, J.A.; Neumann, R.T.; Ornelas, D.; Monte, A.A.; Dylla, L.; et al. Circulating Interleukin-6 Levels and Timing of Acute Ischemic Stroke Onset. Ann. Clin. Transl. Neurol. 2025, 12, 1926–1931. [Google Scholar] [CrossRef] [PubMed]
- Stanton, K.; Philippou, H.; Ariëns, R.A. Ischaemic Stroke, Thromboembolism and Clot Structure. Neuroscience 2024, 550, 3–10. [Google Scholar] [CrossRef]
- Tieck, M.P.; Single, C.; Poli, S.; Kowarik, M.C.; Ziemann, U.; Mengel, A.; Feil, K. Screening tools for malignancy in patients with cryptogenic stroke: Systematic review. Eur. Stroke J. 2025, 10, 665–674. [Google Scholar] [CrossRef]
- Seystahl, K.; Gramatzki, D.; Wanner, M.; Weber, S.J.; Hug, A.; Luft, A.R.; Rohrmann, S.; Wegener, S.; Weller, M. A risk model for prediction of diagnosis of cancer after ischemic stroke. Sci. Rep. 2023, 13, 111. [Google Scholar] [CrossRef]
- Kim, T.; Song, I.; Chung, S.; Kim, J.; Koo, J.; Lee, K. Serum D-dimer Levels Are Proportionally Associated with Left Atrial Enlargement in Patients with an Acute Ischemic Stroke due to Non-valvular Atrial Fibrillation. Intern. Med. 2016, 55, 1447–1452. [Google Scholar] [CrossRef]
- Yoshimuta, T.; Yokoyama, H.; Okajima, T.; Tanaka, H.; Toyoda, K.; Nagatsuka, K.; Higashi, M.; Hayashi, K.; Kawashiri, M.-A.; Yasuda, S.; et al. Impact of Elevated D-Dimer on Diagnosis of Acute Aortic Dissection with Isolated Neurological Symptoms in Ischemic Stroke. Circ. J. 2015, 79, 1841–1845. [Google Scholar] [CrossRef]
- Xu, N.; Fu, Y.; Wang, S.; Li, S.; Cai, D. High level of D-dimer predicts ischemic stroke in patients with infective endocarditis. Clin. Lab. Anal. 2020, 34, e23206. [Google Scholar] [CrossRef] [PubMed]
- Folsom, A.R.; Gottesman, R.F.; Appiah, D.; Shahar, E.; Mosley, T.H. Plasma d -Dimer and Incident Ischemic Stroke and Coronary Heart Disease. Stroke 2016, 47, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Ageno, W.; Finazzi, S.; Steidl, L.; Biotti, M.G.; Mera, V.; MelziD’Eril, G.; Venco, A. Plasma measurement of D-dimer levels for the early diagnosis of ischemic stroke subtypes. Arch. Intern. Med. 2002, 162, 2589–2593. [Google Scholar] [CrossRef]
- Koch, H.J.; Horn, M.; Bogdahn, U.; Ickenstein, G.W. The relationship between plasma D-dimer concentrations and acute ischemic stroke subtypes. J. Stroke Cerebrovasc. Dis. 2005, 14, 75–79. [Google Scholar] [CrossRef]
- Montaner, J.; Perea Gainza, M.; Delgado, P.; Ribó, M.; Chacón, P.; Rosell, A.; Quintana, M.; Palacios, M.E.; Molina, C.A.; Alvares-Sabín, J. Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers. Stroke 2008, 39, 2280–2287. [Google Scholar] [CrossRef]
- Isenegger, J.; Meier, N.; Lämmle, B.; Alberio, L.; Fischer, U.; Nedeltchev, K.; Gralla, J.; Kohler, H.-P.; Mattle, H.P.; Arnold, M. D-dimers predict stroke subtype when assessed early. Cerebrovasc. Dis. 2010, 29, 82–86. [Google Scholar] [CrossRef]
- Abbas, N.I.; Sayed, O.; Samir, S.; Abeed, N. D-dimer Level is Correlated with Prognosis, Infarct Size, and NIHSS in Acute Ischemic Stroke Patients. Indian J. Crit. Care Med. 2021, 25, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Pachón, A.; López-Cancio, E.; Bustamante, A.; Pérez de la Ossa, N.; Millán, M.; Hernández-Pérez, M.; Garcia-Berrocoso, T.; Cardona, P.; Rubiera, M.; Serena, J.; et al. D-Dimer as Predictor of Large Vessel Occlusion in Acute Ischemic Stroke. Stroke 2021, 52, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Hira, K.; Ueno, Y.; Watanabe, M.; Shimura, H.; Kurita, N.; Miyamoto, N.; Haginiwa, H.; Yamashiro, K.; Hattori, N.; Urabe, T. Impact of D-dimer for pathologic differentiation on transesophageal echocardiography in embolic stroke of undetermined source: A single-center experience. BMC Neurol. 2022, 22, 338. [Google Scholar] [CrossRef] [PubMed]
- Hacialioglu, R.; Kielkopf, M.; Branca, M.; Clenin, L.; Boronylo, A.; Silimon, N.; Göldlin, M.B.; Scutelnic, A.; Kaesmacher, J.; Mujanovic, A.; et al. Factors impacting D-dimer levels in patients with acute ischemic cerebrovascular events. J. Stroke Cerebrovasc. Dis. 2024, 33, 107834. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Yan, D.; Luo, C.; Jiang, S.; Wang, Z.; Li, X. Correlation Between Serum D-Dimer, NLR, and CRP/ALB in Patients with Acute Ischemic Stroke. Int. J. Gen. Med. 2025, 18, 2749–2756. [Google Scholar] [CrossRef]
- Zhang, T.; Fu, S.; Cao, X.; Xia, Y.; Hu, M.; Feng, Q.; Cong, Y.; Zhu, Y.; Tang, X.; Wu, M. Correlation of Peripheral Blood Inflammatory Indicators to Prognosis After Intravenous Thrombolysis in Acute Ischemic Stroke: A Retrospective Study. Int. J. Gen. Med. 2024, 17, 985–996. [Google Scholar] [CrossRef]
- Jiang, S.; Guo, P.; Cai, L.; Qian, C.; Yu, J.; Xu, L.; Li, X.; Chen, X.; Bing, F.; Yuan, Y.; et al. Emergency Admission Plasma D-Dimer and Prothrombin Activity: Novel Predictors for Clinical Outcomes After Thrombectomy in Acute Ischemic Stroke with Large Artery Occlusion. CNS Neurosci. Ther. 2025, 31, e70267. [Google Scholar] [CrossRef]
- Zhao, M.; Dai, Z.; Liu, R.; Liu, X.; Xu, G. Post-procedural plasma D-dimer level may predict futile recanalization in stroke patients with endovascular treatment. J. Stroke Cerebrovasc. Dis. 2025, 34, 108248. [Google Scholar] [CrossRef]
- Lu, M.; Xue, J.; Wang, Y.; Chen, D.; Cao, Y.; Zhong, C.; Zhang, X. The Joint Effect of Renal Function Status and Coagulation Biomarkers on In-Hospital Outcomes in Acute Ischemic Stroke Patients with Intravenous Thrombolysis. Immun. Inflamm. Amp Dis. 2024, 12, e70099. [Google Scholar] [CrossRef]
- Li, G.; Wang, C.; Wang, S.; Hao, Y.; Xiong, Y.; Zhao, X. Clinical Significance and Dynamic Change of Coagulation Parameters in Ischemic Stroke Patients Treated with Intravenous Thrombolysis. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221121287. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Tian, B.; Li, G.; Cui, Q.; Wang, C.; Zhang, Q.; Peng, B.; Gao, Y.; Zhan, Y.-Q.; Hu, D.; et al. Elevated plasma D-dimer levels are associated with short-term poor outcome in patients with acute ischemic stroke: A prospective, observational study. BMC Neurol. 2019, 19, 175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, L.; Tao, J.; Song, Y.; Fan, Y.; Gou, M.; Xu, J. Prognostic role of early D-dimer level in patients with acute ischemic stroke. PLoS ONE 2019, 14, e0211458. [Google Scholar] [CrossRef]
- Faura, J.; Bustamante, A.; Reverté, S.; García-Berrocoso, T.; Millán, M.; Castellanos, M.; Lara-Rodríguez, B.; Zaragoza, J.; Ventura, O.; Hernández-Pérez, M.; et al. Blood Biomarker Panels for the Early Prediction of Stroke-Associated Complications. J. Am. Hear. Assoc. 2021, 10, e018946. [Google Scholar] [CrossRef]
- M’barek, L.; Jin, A.; Pan, Y.; Lin, J.; Jiang, Y.; Meng, X.; Wang, Y. Stroke Prognosis: The Impact of Combined Thrombotic, Lipid, and Inflammatory Markers. J. Atheroscler. Thromb. 2025, 32, 458–473. [Google Scholar] [CrossRef]
- Zheng, Z. D-Dimer Levels and NIHSS as Prognostic Predictors in Elderly Patients with Cerebral Infarction. Clin. Interv. Aging 2025, 20, 505–511. [Google Scholar] [CrossRef]
- Choi, K.; Kim, J.; Kim, J.; Kang, K.; Lee, C.; Kim, J.; Choi, S.-M.; Park, M.-S.; Cho, K.-H. d-dimer Level as a Predictor of Recurrent Stroke in Patients with Embolic Stroke of Undetermined Source. Stroke 2021, 52, 2292–2301. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Chen, D.; Cheng, H.; Tan, F.; Yan, J.; Deng, H.; Fang, W.; Wang, S.; Zhu, J. N-terminal pro-B-type natriuretic peptide and D-dimer combined with left atrial diameter to predict the risk of ischemic stroke in nonvalvular atrial fibrillation. Clin. Cardiol. 2023, 46, 41–48. [Google Scholar] [CrossRef]
- Fujinami, J.; Nagakane, Y.; Fujikawa, K.; Murata, S.; Maezono, K.; Ohara, T.; Mizuno, T. D-Dimer Trends Predict Recurrent Stroke in Patients with Cancer-Related Hypercoagulability. Cerebrovasc. Dis. Extra 2023, 14, 9–15. [Google Scholar] [CrossRef]
- Prasad, M.K.; Marandi, S.; Mishra, B.; Guria, R.T.; Kumar, A.; Birua, H.; Ray, H.N.; Dungdung, A.; Kumar, D.; Maitra, S. Association of Fibrinogen with Ischemic Stroke: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e34335. [Google Scholar] [CrossRef]
- Peycheva, M.; Deneva, T.; Zahariev, Z. The role of fibrinogen in acute ischaemic stroke. Neurol. Neurochir. Pol. 2021, 55, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Abebe, E.C.; Dejenie, T.A.; Anley, D.T.; Mengstie, M.A.; Gebeyehu, N.A.; Adella, G.A.; Kassie, G.A.; Tesfa, N.A.; Gesese, M.M.; Feleke, S.F.; et al. Diagnostic performance of plasma D-dimer, fibrinogen, and D-dimer to fibrinogen ratio as potential biomarkers to predict hypertension-associated acute ischemic stroke. Heliyon 2024, 10, e27192. [Google Scholar] [CrossRef]
- Chekol Abebe, E.; Mengstie, M.A.; Seid, M.A.; Gebeyehu, N.A.; Adella, G.A.; Kassie, G.A.; Gesese, M.M.; Tegegne, K.D.; Anley, D.T.; Feleke, S.F.; et al. Comparison of circulating lipid profiles, D-dimer and fibrinogen levels between hypertensive patients with and without stroke. Metab. Open 2023, 19, 100252. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xing, C.; Li, Y.; Zhu, X. Elevated plasma fibrinogen indicates short-term poor outcome in patients with acute ischemic stroke after intravenous thrombolysis. J. Stroke Cerebrovasc. Dis. 2020, 29, 104991. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Xian, W.; Liang, J.; Yang, H.; Weng, B. Early changes in fibrinogen after administration of alteplase are associated with the short-term efficacy of thrombolysis. Medicine 2018, 97, e0241. [Google Scholar] [CrossRef]
- Chen, X.; Xu, X.; Li, Y.; Liu, F.; Zhang, B.; Zuo, L. Association between fibrinogen-to-albumin ratio and functional prognosis of 3 months in patients with acute ischemic stroke after intravenous thrombolysis. Brain Behav. 2024, 14, e3364. [Google Scholar] [CrossRef]
- Machlus, K.R.; Cardenas, J.C.; Church, F.C.; Wolberg, A.S. Causal Relationship between Hyperfibrinogenemia, Thrombosis, and Resistance to Thrombolysis in Mice. Blood 2011, 117, 4953–4963. [Google Scholar] [CrossRef]
- DiNapoli, M.; Papa, F.; Bocola, V. Prognostic Influence of Increased C-Reactive Protein and Fibrinogen Levels in Ischemic Stroke. Stroke 2001, 32, 133–138. [Google Scholar] [CrossRef]
- Theodorou, A.; Psychogios, K.; Kargiotis, O.; Safouris, A.; Chondrogianni, M.; Bakola, E.; Melanis, K.; Fanouraki, S.; Frantzeskaki, F.; Polyzogopoulou, E.; et al. Fibrinogen time course in acute ischemic stroke patients treated with intravenous thrombolysis with alteplase or tenecteplase. Eur. Stroke J. 2025, 32, 133–138. [Google Scholar] [CrossRef]
- Neacă, I.; Negroiu, C.E.; Tudorașcu, I.; Dănoiu, R.; Moise, C.G.; Toader, D.M.; Dănoiu, S. Risk Factors and Outcomes of Hemorrhagic Transformation in Acute Ischemic Stroke Following Thrombolysis: Analysis of a Single-Center Experience and Review of the Literature. Medicina 2025, 61, 722. [Google Scholar] [CrossRef]
- Huang, P.; Yi, X.Y. Predictive role of admission serum glucose, baseline NIHSS score, and fibrinogen on hemorrhagic transformation after intravenous thrombolysis with alteplase in acute ischemic stroke. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 9710–9720. [Google Scholar]
- Liu, X.; Jia, H.; Wang, G. Predictive value of the fibrinogen-to-albumin ratio for hemorrhagic transformation following intravenous thrombolysis in ischemic stroke: A retrospective propensity score-matched analysis. Front. Neurol. 2025, 16, 1465508. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Yuan, C.; Liu, Y.; Zeng, Y.; Cheng, H.; Cheng, Q.; Chen, Y.; Huang, G.; He, W.; He, J. High fibrinogen-to-albumin ratio is associated with hemorrhagic transformation in acute ischemic stroke patients. Brain Behav. 2021, 11, e01855. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Chen, J.; Huang, G.; Chen, Z.; Zhang, H.; Zhang, Y.; Duan, Q.; Wu, B.; He, J. The differences of fibrinogen levels in various types of hemorrhagic transformations. Front. Neurol. 2024, 15, 1364875. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Han, Y.; Hu, H.; Guo, Y.; Deng, Z.; Liu, D. Nonlinear association of fibrinogen levels with functional prognosis in patients with acute ischemic stroke: A prospective cohort study. BMC Neurol. 2024, 24, 163. [Google Scholar] [CrossRef]
- Yılmaz, B.Ö.; Şencan, R. The effect of fibrinogen levels on three-month neurological recovery in acute ischemic stroke patients. Sci. Rep. 2025, 15, 12644. [Google Scholar] [CrossRef]
- Zhai, M.; Cao, S.; Lu, J.; Xu, H.; Xia, M.; Li, Z. The Relationship Between the Fibrinogen to Albumin Ratio and Early Outcomes in Patients with Acute Pontine Infarction. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296211067260. [Google Scholar] [CrossRef]
- Wei, C.; Zhai, W.; Zhao, P.; Sun, L. Plasma fibrinogen as a potential biomarker of cognitive impairment after acute ischemic stroke. Sci. Rep. 2024, 14, 32120. [Google Scholar] [CrossRef]
- McCabe, J.J.; Walsh, C.; Gorey, S.; Harris, K.; Hervella, P.; Iglesias-Rey, R.; Jern, C.; Li, L.; Miyamoto, N.; Montaner, J.; et al. Plasma fibrinogen and risk of vascular recurrence after ischaemic stroke: An individual participant and summary-level data meta-analysis of 11 prospective studies. Eur. Stroke J. 2024, 9, 704–713. [Google Scholar] [CrossRef]
- Chang, X.; Xia, S.; Liu, Y.; Mao, X.; Wu, X.; Chu, M.; Niu, H.; Sun, L.; He, Y.; Liu, Y.; et al. Cardiac biomarkers are associated with increased risks of adverse clinical outcomes after ischemic stroke. J. Neurol. 2024, 271, 6313–6324. [Google Scholar] [CrossRef]
- Huang, Y.; Shao, Y.; Wang, Y.; Shi, T. Elevated troponin I levels on admission predict long-term mortality in patients with acute cerebral infarction following thrombolysis. Neurol. Sci. 2022, 43, 5431–5439. [Google Scholar] [CrossRef]
- Johansen, M.C.; von Rennenberg, R.; Nolte, C.H.; Jensen, M.; Bustamante, A.; Katan, M. Role of Cardiac Biomarkers in Stroke and Cognitive Impairment. Stroke 2024, 55, 2376–2384. [Google Scholar] [CrossRef]
- Chang, A.; Ricci, B.; Grory, B.M.; Cutting, S.; Burton, T.; Dakay, K.; Jayaraman, M.; Merkler, A.; Reznik, M.; Lerario, M.P.; et al. Cardiac Biomarkers Predict Large Vessel Occlusion in Patients with Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 1726–1731. [Google Scholar] [CrossRef]
- Liesirova, K.; Abela, E.; Pilgrim, T.; Bickel, L.; Meinel, T.; Meisterernst, J.; Rajeev, V.; Sarikaya, H.; Heldner, M.R.; Dobrocky, T.; et al. Baseline Troponin T level in stroke and its association with stress cardiomyopathy. PLoS ONE 2018, 13, e0209764. [Google Scholar] [CrossRef]
- Abdi, S.; Oveis-Gharan, S.; Sinaei, F.; Ghorbani, A. Elevated troponin T after acute ischemic stroke: Association with severity and location of infarction. Iran J. Neurol. 2015, 14, 35–40. [Google Scholar] [PubMed]
- Král, M.; Šaňák, D.; Veverka, T.; Hutyra, M.; Vindiš, D.; Kunčarová, A.; Bártková, A.; Dorňák, T.; Švábová, M.; Kubíčková, V.; et al. Troponin T in Acute Ischemic Stroke. Am. J. Cardiol. 2013, 112, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Liu, T.; Luo, J.; Xu, B.; Zheng, L.; Zhao, W.; Guan, Q.; Ren, L.; Dong, C.; Xiao, Y.; et al. Elevation of high-sensitivity cardiac troponin T at admission is associated with increased 3-month mortality in acute ischemic stroke patients treated with thrombolysis. Clin. Cardiol. 2019, 42, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Bai, X.; Wang, X.; Zhang, L.; Wang, F.; Huang, L.; Deng, J.; Geng, Z. Impact of high-sensitivity troponin elevation and dynamic changes on 90-day mortality in patients with acute ischemic stroke after mechanical thrombectomy: Results from an observational cohort. J. NeuroIntervent. Surg. 2023, 15, 1142–1147. [Google Scholar] [CrossRef]
- Fan, Y.; Jiang, M.; Gong, D.; Man, C.; Chen, Y. Cardiac troponin for predicting all-cause mortality in patients with acute ischemic stroke: A meta-analysis. Biosci. Rep. 2018, 38, BSR20171178. [Google Scholar] [CrossRef]
- Kim, B.S.; Park, J.; Chang, H.; Kim, S.H.; Kwon, C.H.; Chung, S.; Kim, H.Y.; Kim, H.J. Association of High-Sensitivity Troponin I with Cardiac and Cerebrovascular Events in Patient after Ischemic Stroke. Cerebrovasc. Dis. 2023, 52, 153–159. [Google Scholar] [CrossRef]
- Nam, K.; Kim, C.K.; Yu, S.; Chung, J.; Bang, O.Y.; Kim, G.; Jung, J.M.; Song, T.J.; Kim, Y.J.; Kim, B.J.; et al. Elevated troponin levels are associated with early neurological worsening in ischemic stroke with atrial fibrillation. Sci. Rep. 2020, 10, 12626. [Google Scholar] [CrossRef] [PubMed]
- Bolognese, M.; Weichsel, L.; Österreich, M.; Müller, M.; Karwacki, G.M.; Lakatos, L. Association of high-sensitivity cardiac troponin T with territorial middle cerebral artery brain infarctions and dynamic cerebral autoregulation. J. Cent. Nerv. Syst. Dis. 2024, 16, 11795735241302725. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Lee, J.; Yun, M.; Han, J.; Kim, S.; Kim, Y.; Lee, S.H.; Park, M.G.; Park, K.P.; Kang, D.W.; et al. Explanatory Power and Prognostic Implications of Factors Associated with Troponin Elevation in Acute Ischemic Stroke. J. Stroke 2023, 25, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Lee, J.; Yun, M.; Han, J.; Kim, S.; Lee, S.; Park, M.-G.; Park, K.-P.; Kang, D.-W.; Kim, J.S.; et al. Corrected QTc interval combined with troponin value and mortality in acute ischemic stroke. Front. Cardiovasc. Med. 2023, 10, 1253871. [Google Scholar] [CrossRef]
- Pitliya, A.; AlEdani, E.M.; Bhangu, J.K.; Javed, K.; Manshahia, P.K.; Nahar, S.; Kanda, S.; Chatha, U.; Odoma, V.; Mohammed, L. The Impact of Elevated Troponin Levels on Clinical Outcomes in Patients with Acute Ischemic Stroke: A Systematic Review. Ann. Indian Acad. Neurol. 2023, 26, 641–654. [Google Scholar] [CrossRef]
- Ahn, S.; Lee, J.; Kim, Y.; Kim, B.J.; Kim, Y.; Kang, D.; Kim, J.S.; Kwon, S.U. Prognostic Significance of Troponin Elevation for Long-Term Mortality after Ischemic Stroke. J. Stroke 2017, 19, 312–322. [Google Scholar] [CrossRef]
- Rosso, M.; Ramaswamy, S.; Mulatu, Y.; Little, J.N.; Kvantaliani, N.; Brahmaroutu, A.; Marczak, I.; Lewey, J.; Deo, R.; Messé, S.R.; et al. Rising Cardiac Troponin: A Prognostic Biomarker for Mortality After Acute Ischemic Stroke. J. Am. Hear. Assoc. 2024, 13, e032922. [Google Scholar] [CrossRef]
- Alhazzani, A.; Kumar, A.; Algahtany, M.; Rawat, D. Role of troponin as a biomarker for predicting outcome after ischemic stroke. Brain Circ. 2021, 7, 77–84. [Google Scholar] [CrossRef]
- Cui, Y.X.; Ren, H.; Lee, C.Y.; Li, S.F.; Song, J.X.; Gao, X.G.; Chen, H. Characteristics of elevated cardiac troponin I in patients with acute ischemic stroke. J. Geriatr. Cardiol. 2017, 14, 401–406. [Google Scholar]
- Esteak, T.; Hasan, M.; Atiqur Rahman, M.; Islam, D.M.K.; Ray, S.K.; Hosain, A.; Alam, S.; Zannat, T.; Hasan, A.H.; Khan, S.U. Elevated Troponin I as a Marker for Unfavorable Outcomes in Acute Ischemic Stroke. Cureus 2023, 15, e49568. [Google Scholar] [CrossRef]
- Willeit, K.; Boehme, C.; Toell, T.; Tschiderer, L.; Seekircher, L.; Mayer-Suess, L.; Komarek, S.; Lang, W.; Griesmacher, A.; Knoflach, M.; et al. High-Sensitivity Cardiac Troponin T and Cardiovascular Risk After Ischemic Stroke or Transient Ischemic Attack. JACC Adv. 2024, 3, 101022. [Google Scholar] [PubMed]
- Scheitz, J.F.; Lim, J.; Broersen, L.H.A.; Ganeshan, R.; Huo, S.; Sperber, P.S.; Piper, S.K.; Heuschmann, P.U.; Audebert, H.J.; Nolte, C.H.; et al. High-Sensitivity Cardiac Troponin T and Recurrent Vascular Events After First Ischemic Stroke. J. Am. Hear. Assoc. 2021, 10, e018326. [Google Scholar] [CrossRef] [PubMed]
- Camen, S.; Palosaari, T.; Reinikainen, J.; Sprünker, N.A.; Niiranen, T.; Gianfagna, F.; Vishram-Nielsen, J.K.K.; Costanzo, S.; Söderberg, S.; Palmieri, L.; et al. Cardiac Troponin I and Incident Stroke in European Cohorts. Stroke 2020, 51, 2770–2777. [Google Scholar] [CrossRef]
- Llombart, V.; Antolin-Fontes, A.; Bustamante, A.; Giralt, D.; Rost, N.S.; Furie, K.; Shibazaki, K.; Biteker, M.; Castillo, J.; Rodríguez-Yáñez, M.; et al. B-Type Natriuretic Peptides Help in Cardioembolic Stroke Diagnosis. Stroke 2015, 46, 1187–1195. [Google Scholar] [CrossRef]
- Palà, E.; Pagola, J.; Juega, J.; Francisco-Pascual, J.; Bustamante, A.; Penalba, A.; Comas, I.; Rodriguez, M.; De Lera Alfonso, M.; Arenillas, J.F.; et al. B-type natriuretic peptide over N-terminal pro-brain natriuretic peptide to predict incident atrial fibrillation after cryptogenic stroke. Euro J. Neurol. 2021, 28, 540–547. [Google Scholar] [CrossRef]
- Shiroto, H.; Hagii, J. Biomarkers for the detection of covert atrial fibrillation after ischemic stroke: NT-proBNP or BNP? J. Stroke Cerebrovasc. Dis. 2025, 34, 108239. [Google Scholar] [CrossRef]
- Patel, J.; Bhaskar, S.M.M. Diagnostic Utility of N-Terminal Pro-B-Type Natriuretic Peptide in Identifying Atrial Fibrillation Post-Cryptogenic Stroke: A Systematic Review and Meta-Analysis. Pathophysiology 2024, 31, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liao, J.; Jiang, Y.; Xu, Y.; Liu, M.; Zhang, X.; Dong, N.; Yu, L.; Chen, Q.; Fang, Q. Elevated NT-proBNP levels are associated with CTP ischemic volume and 90-day functional outcomes in acute ischemic stroke: A retrospective cohort study. BMC Cardiovasc. Disord. 2022, 22, 431. [Google Scholar] [CrossRef]
- Bustamante, A.; López-Cancio, E.; Pich, S.; Penalba, A.; Giralt, D.; García-Berrocoso, T.; Ferrer-Costa, C.; Gasull, T.; Hernández-Pérez, M.; Millan, M.; et al. Blood Biomarkers for the Early Diagnosis of Stroke. Stroke 2017, 48, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, D.; Liu, Q.; Chen, H.; Chai, E. Nomogram prediction model for the risk of intracranial hemorrhagic transformation after intravenous thrombolysis in patients with acute ischemic stroke. Front. Neurol. 2024, 15, 1361035. [Google Scholar] [CrossRef] [PubMed]
- Naveen, V.; Vengamma, B.; Mohan, A.; Vanajakshamma, V. N-terminal pro-brain natriuretic peptide levels and short term prognosis in acute ischemic stroke. Ann. Indian Acad. Neurol. 2015, 18, 435. [Google Scholar] [CrossRef]
- Rodríguez-Castro, E.; Hervella, P.; López-Dequidt, I.; Arias-Rivas, S.; Santamaría-Cadavid, M.; López-Loureiro, I.; da Silva-Candal, A.; Pérez-Mato, M.; Sobrino, T.; Campos, F.; et al. NT-pro-BNP: A novel predictor of stroke risk after transient ischemic attack. Int. J. Cardiol. 2020, 298, 93–97. [Google Scholar] [CrossRef]
- Barba, L.; Vollmuth, C.; Halbgebauer, S.; Ungethüm, K.; Hametner, C.; Essig, F.; Kollikowski, A.M.; Pham, M.; Schuhmann, M.K.; Heuschmann, P.U.; et al. Prognostic serum biomarkers of synaptic, neuronal and glial injury in patients with acute ischemic stroke of the anterior circulation. Euro. J. Neurol. 2025, 32, e16581. [Google Scholar] [CrossRef]
- Colasanti, T.; Delunardo, F.; Margutti, P.; Vacirca, D.; Piro, E.; Siracusano, A.; Ortona, E. Autoantibodies involved in neuropsychiatric manifestations associated with Systemic Lupus Erythematosus. J. Neuroimmunol. 2009, 212, 3–9. [Google Scholar] [CrossRef]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- Paul, J.F.; Ducroux, C.; Correia, P.; Daigneault, A.; Larochelle, C.; Stapf, C.; Gioia, L.C. Serum glial fibrillary acidic protein in acute stroke: Feasibility to determine stroke-type, timeline and tissue-impact. Front. Neurol. 2024, 6, 15. [Google Scholar] [CrossRef]
- Forró, T.; Manu, D.R.; Băjenaru, O.; Bălașa, R. GFAP as Astrocyte-Derived Extracellular Vesicle Cargo in Acute Ischemic Stroke Patients—A Pilot Study. Int. J. Mol. Sci. 2024, 25, 5726. [Google Scholar] [CrossRef] [PubMed]
- Foerch, C.; Niessner, M.; Back, T.; Bauerle, M.; De Marchis, G.M.; Ferbert, A.; Grehl, H.; Hamann, G.F.; Jacobs, A.; Kastrup, A.; et al. Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin. Chem. 2012, 58, 237–245. [Google Scholar] [CrossRef]
- Katsanos, A.H.; Makris, K.; Stefani, D.; Koniari, K.; Gialouri, E.; Lelekis, M.; Chondrogianni, M.; Zompola, C.; Dardiotis, E.; Rizos, I.; et al. Plasma Glial Fibrillary Acidic Protein in the Differential Diagnosis of Intracerebral Hemorrhage. Stroke 2017, 48, 2586–2588. [Google Scholar] [CrossRef] [PubMed]
- Luger, S.; Witsch, J.; Dietz, A.; Hamann, G.F.; Minnerup, J.; Schneider, H.; Sitzer, M.; Wartenberg, K.E.; Niessner, M.; Foerch, C.; et al. Glial Fibrillary Acidic Protein Serum Levels Distinguish between Intracerebral Hemorrhage and Cerebral Ischemia in the Early Phase of Stroke. Clin. Chem. 2017, 63, 377–385. [Google Scholar] [CrossRef]
- Luger, S.; Jæger, H.S.; Dixon, J.; Bohmann, F.O.; Schaefer, J.; Richieri, S.P.; Larsen, K.; Hov, M.R.; Bache, K.G.; Foerch, C. Diagnostic Accuracy of Glial Fibrillary Acidic Protein and Ubiquitin Carboxy-Terminal Hydrolase-L1 Serum Concentrations for Differentiating Acute Intracerebral Hemorrhage from Ischemic Stroke. Neurocrit. Care 2020, 33, 39–48. [Google Scholar] [CrossRef]
- Ren, C.; Kobeissy, F.; Alawieh, A.; Li, N.; Li, N.; Zibara, K.; Zoltewicz, S.; Guingab-Cagmat, J.; Larner, S.F.; Ding, Y.; et al. Assessment of Serum UCH-L1 and GFAP in Acute Stroke Patients. Sci. Rep. 2016, 6, 24588. [Google Scholar] [CrossRef]
- Almubayyidh, M.; Jenkins, D.A.; Gaude, E.; Parry-Jones, A.R. Integrating clinical predictors and glial fibrillary acidic protein in prediction models for the prehospital identification of intracerebral haemorrhage in suspected stroke. BMJ Neurol. Open. 2025, 7, e001160. [Google Scholar] [CrossRef]
- Kraljević, I.; Sablić, S.; Marinović Guić, M.; Budimir Mršić, D.; Štula, I.; Dolić, K.; Benzon, B.; Košta, V.; Čaljkušić, K.; Marčić, M.; et al. The Importance of Increased Serum GFAP and UCH-L1 Levels in Distinguishing Large Vessel from Small Vessel Occlusion in Acute Ischemic Stroke. Biomedicines 2024, 12, 608. [Google Scholar] [CrossRef]
- Durrani, Y.; Gerstl, J.V.E.; Murphy, D.; Harris, A.; Saali, I.; Gropen, T.; Shekhar, S.; Kappel, A.D.; Patel, N.J.; Du, R.; et al. Prospective Validation of Glial Fibrillary Acidic Protein, d -Dimer, and Clinical Scales for Acute Large-Vessel Occlusion Ischemic Stroke Detection. Stroke Vasc. Interv. Neurol. 2024, 4, e001304. [Google Scholar] [CrossRef]
- Gaude, E.; Murphy, D.; Gerstl, J.V.; Kappel, A.D.; Dmytriw, A.A.; Nawabi, N.L.; Izzy, S.; Alcedo Guardia, R.E.; Vicenty-Padilla, J.; Gropen, T.; et al. Detection of GFAP and D-Dimer in a Point-of-Care Test for Large Vessel Occlusion Ischemic Stroke. Stroke Vasc. Interv. Neurol. 2025, 5, e001559. [Google Scholar] [CrossRef]
- Ferrari, F.; Rossi, D.; Ricciardi, A.; Morasso, C.; Brambilla, L.; Albasini, S.; Vanna, R.; Fassio, R.; Begenisic, T.; Loi, M.; et al. Quantification and prospective evaluation of serum NfL and GFAP as blood-derived biomarkers of outcome in acute ischemic stroke patients. J. Cereb. Blood Flow Metab. 2023, 43, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Geng, J. Glial fibrillary acidic protein as a prognostic marker of acute ischemic stroke. Hum. Exp. Toxicol. 2018, 37, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Puspitasari, V.; Gunawan, P.Y.; Wiradarma, H.D.; Hartoyo, V. Glial Fibrillary Acidic Protein Serum Level as a Predictor of Clinical Outcome in Ischemic Stroke. Open Access Maced. J. Med. Sci. 2019, 7, 1471–1474. [Google Scholar] [CrossRef]
- Amalia, L. Glial Fibrillary Acidic Protein (GFAP): Neuroinflammation Biomarker in Acute Ischemic Stroke. J. Inflamm. Res. 2021, 14, 7501–7506. [Google Scholar] [CrossRef]
- Nielsen, H.H.; Soares, C.B.; Høgedal, S.S.; Madsen, J.S.; Hansen, R.B.; Christensen, A.A.; Madsen, C.; Clausen, B.H.; Frich, L.H.; Degn, M.; et al. Acute Neurofilament Light Chain Plasma Levels Correlate with Stroke Severity and Clinical Outcome in Ischemic Stroke Patients. Front. Neurol. 2020, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Tony, A.A.; Kholef, E.F.M.; Elgendy, D.B.; Shoyb, A. Neurofilament Light Chain Correlates with Stroke Severity and Clinical Outcome in Acute Cerebrovascular Stroke Patients. Cell Mol. Neurobiol. 2025, 45, 1–7. [Google Scholar] [CrossRef]
- Gendron, T.F.; Badi, M.K.; Heckman, M.G.; Jansen-West, K.R.; Vilanilam, G.K.; Johnson, P.W.; Burch, A.R.; Walton, R.L.; Ross, O.A.; Brott, T.G.; et al. Plasma neurofilament light predicts mortality in patients with stroke. Sci. Transl. Med. 2020, 12, eaay1913. [Google Scholar] [CrossRef]
- Ahn, J.W.; Hwang, J.; Lee, M.; Kim, J.H.; Cho, H.; Lee, H.; Eun, M.Y. Serum neurofilament light chain levels are correlated with the infarct volume in patients with acute ischemic stroke. Medicine 2022, 101, e30849. [Google Scholar] [CrossRef] [PubMed]
- Onatsu, J.; Vanninen, R.; Jäkälä, P.; Mustonen, P.; Pulkki, K.; Korhonen, M.; Hedman, M.; Zetterberg, H.; Blennow, K.; Höglund, K.; et al. Serum Neurofilament Light Chain Concentration Correlates with Infarct Volume but Not Prognosis in Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 2242–2249. [Google Scholar] [CrossRef]
- De Marchis, G.M.; Katan, M.; Barro, C.; Fladt, J.; Traenka, C.; Seiffge, D.J.; Hert, L.; Gensicke, H.; Disanto, G.; Sutter, R.; et al. Serum neurofilament light chain in patients with acute cerebrovascular events. Euro. J. Neurol. 2018, 25, 562–568. [Google Scholar] [CrossRef]
- Gundersen, J.K.; Gonzalez-Ortiz, F.; Karikari, T.; Kirsebom, B.; Mertes, K.; Zetterberg, H.; Kvartsberg, H.; Morten Rønning, O.; Gísladóttir, B.; Blennow, K.; et al. Neuronal plasma biomarkers in acute ischemic stroke. J. Cereb. Blood Flow Metab. 2025, 45, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, D.; Liang, Y.; Zhang, Z.; Zhuang, L.; Wang, Z. Plasma neurofilament light chain: A biomarker predicting severity in patients with acute ischemic stroke. Medicine 2022, 101, e29692. [Google Scholar] [CrossRef]
- Rattanawong, W.; Ongphichetmetha, T.; Hemachudha, T.; Thanapornsangsuth, P. Neurofilament light is associated with clinical outcome and hemorrhagic transformation in moderate to severe ischemic stroke. J. Cent Nerv. Syst. Dis. 2023, 15, 11795735221147212. [Google Scholar] [CrossRef]
- Pedersen, A.; Stanne, T.M.; Nilsson, S.; Klasson, S.; Rosengren, L.; Holmegaard, L.; Jood, K.; Blennow, K.; Zetterberg, H.; Jern, C. Circulating neurofilament light in ischemic stroke: Temporal profile and outcome prediction. J. Neurol. 2019, 266, 2796–2806. [Google Scholar] [CrossRef]
- Xu, C.; Yi, T.; Qing, T.; Jiang, Y.; Yi, X.; Xu, J.; Ma, J. Serum neurofilament light chain: A predictive marker for outcomes following mild-to-moderate ischemic stroke. Front. Neurol. 2024, 15, 1398826. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-Z.; Guo, F.-Q.; Guo, L.; Yang, S.; Yu, N.-W.; Wang, J.-H. Serum Neurofilament Light Predicts 6-Month Mental Health Outcomes in a Cohort of Patients with Acute Ischemic Stroke. Front. Psychiatry 2022, 12, 764656. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Huang, J.; Guo, F.-Q.; Wang, F.; Yang, S.; Yu, N.-W.; Zheng, B. Circulating Neurofilament Light Predicts Cognitive Decline in Patients with Post-stroke Subjective Cognitive Impairment. Front. Aging Neurosci. 2021, 13, 665981. [Google Scholar] [CrossRef]
- Qiao, H.; Wang, S.; Tao, M.; Fan, H.; Zhao, T.; Du, Y.; Dong, M. Serum neurofilament heavy chain predicts post-stroke cognitive impairment. Sci. Rep. 2025, 15, 13556. [Google Scholar] [CrossRef]
- Aulin, J.; Sjölin, K.; Lindbäck, J.; Benz, A.P.; Eikelboom, J.W.; Kultima, K.; Oldgren, J.; Wallentin, L.; Burman, J. Neuroglial Biomarkers for Risk Assessment of Ischemic Stroke and Other Cardiovascular Events in Patients with Atrial Fibrillation Not Receiving Oral Anticoagulation. J. Am. Hear. Assoc. 2025, 14, e038860. [Google Scholar] [CrossRef]
- Aulin, J.; Sjölin, K.; Lindbäck, J.; Benz, A.P.; Eikelboom, J.W.; Hijazi, Z.; Kultima, K.; Oldgren, J.; Wallentin, L.; Burman, J.; et al. Neurofilament Light Chain and Risk of Stroke in Patients with Atrial Fibrillation. Circulation 2024, 150, 1090–1100. [Google Scholar] [CrossRef]
- Dhana, A.; DeCarli, C.; Aggarwal, N.T.; Dhana, K.; Desai, P.; Evans, D.A.; Rajan, K.B. Serum neurofilament light chain, brain infarcts, and the risk of stroke: A prospective population-based cohort study. Eur. J. Epidemiol. 2023, 38, 427–434. [Google Scholar] [CrossRef]
- Dagonnier, M.; Donnan, G.A.; Davis, S.M.; Dewey, H.M.; Howells, D.W. Acute Stroke Biomarkers: Are We There Yet? Front. Neurol. 2021, 12, 619721. [Google Scholar] [CrossRef] [PubMed]
- Shui, X.; Chen, J.; Fu, Z.; Zhu, H.; Tao, H.; Li, Z. Microglia in Ischemic Stroke: Pathogenesis Insights and Therapeutic Challenges. J. Inflamm. Res. 2024, 17, 3335–3352. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Zhang, S.; Li, J.; Zheng, Y.; Fan, X. The natural (poly)phenols as modulators of microglia polarization via TLR4/NF-κB pathway exert anti-inflammatory activity in ischemic stroke. Eur. J. Pharmacol. 2022, 914, 174660. [Google Scholar] [CrossRef]
- Hyakkoku, K.; Hamanaka, J.; Tsuruma, K.; Shimazawa, M.; Tanaka, H.; Uematsu, S.; Akira, S.; Inagaki, N.; Nagai, H.; Hara, H. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience 2010, 171, 258–267. [Google Scholar] [CrossRef]
- Luo, L.; Liu, M.; Fan, Y.; Zhang, J.; Liu, L.; Li, Y.; Zhang, Q.; Xie, H.; Jiang, C.; Wu, J.; et al. Intermittent theta-burst stimulation improves motor function by inhibiting neuronal pyroptosis and regulating microglial polarization via TLR4/NFκB/NLRP3 signaling pathway in cerebral ischemic mice. J. Neuroinflamm. 2022, 19, 141. [Google Scholar] [CrossRef]
- Liao, S.; Wu, J.; Liu, R.; Wang, S.; Luo, J.; Yang, Y.; Qin, Y.; Li, T.; Zheng, X.; Song, J.; et al. A novel compound DBZ ameliorates neuroinflammation in LPS-stimulated microglia and ischemic stroke rats: Role of Akt(Ser473)/GSK3β(Ser9)-mediated Nrf2 activation. Redox Biol. 2020, 36, 101644. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; He, R.; Wang, P.; Shi, Y.; Zhao, L.; Liang, J. Exosomes from LPS-stimulated macrophages induce neuroprotection and functional improvement after ischemic stroke by modulating microglial polarization. Biomater. Sci. 2019, 7, 2037–2049. [Google Scholar] [CrossRef]
- Wang, D.; Liu, F.; Zhu, L.; Lin, P.; Han, F.; Wang, X.; Tan, X.; Lin, L.; Xiong, Y. FGF21 alleviates neuroinflammation following ischemic stroke by modulating the temporal and spatial dynamics of microglia/macrophages. J. Neuroinflamm. 2020, 17, 257. [Google Scholar] [CrossRef]
- Al Mamun, A.; Chauhan, A.; Yu, H.; Xu, Y.; Sharmeen, R.; Liu, F. Interferon regulatory factor 4/5 signaling impacts on microglial activation after ischemic stroke in mice. Euro J. Neurosci. 2018, 47, 140–149. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, Z.; Man, J.; Cui, K.; Fu, X.; Yu, L.; Gao, Y.; Liao, L.; Xiao, Q.; Guo, R.; et al. Wnt-3a alleviates neuroinflammation after ischemic stroke by modulating the responses of microglia/macrophages and astrocytes. Int. Immunopharmacol. 2019, 75, 105760. [Google Scholar] [CrossRef] [PubMed]
- Brifault, C.; Gras, M.; Liot, D.; May, V.; Vaudry, D.; Wurtz, O. Delayed pituitary adenylate cyclase-activating polypeptide delivery after brain stroke improves functional recovery by inducing m2 microglia/macrophage polarization. Stroke 2015, 46, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, J.; Huang, Y.; Wei, P.; Miao, W.; Yang, Y.; Gao, Y. Interleukin 13 promotes long-term recovery after ischemic stroke by inhibiting the activation of STAT3. J. Neuroinflammation 2022, 19, 112. [Google Scholar] [CrossRef]
- Zhong, Y.; Gu, L.; Ye, Y.; Zhu, H.; Pu, B.; Wang, J.; Li, Y.; Qiu, S.; Xiong, X.; Jian, Z. JAK2/STAT3 axis intermediates microglia/macrophage polarization during cerebral ischemia/reperfusion injury. Neuroscience 2022, 496, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Fan, W.H.; Liu, Q.; Shang, K.; Murugan, M.; Wu, L.J.; Wang, W.; Tian, D.S. Fingolimod protects against ischemic white matter damage by modulating microglia toward M2 polarization via STAT3 pathway. Stroke 2017, 48, 3336–3346. [Google Scholar] [CrossRef] [PubMed]
- Gaire, B.P. Microglia as the Critical Regulators of Neuroprotection and Functional Recovery in Cerebral Ischemia. Cell Mol. Neurobiol. 2022, 42, 2505–2525. [Google Scholar] [CrossRef] [PubMed]
- Gorostiola-Oyarzabal, N.; Joya, A.; Freijo, M.M.; Martín, A.; Mancini, S. Surrogate Molecular Biomarkers for Poststroke Cognitive Impairment: A Narrative Review. Stroke 2025. [Google Scholar] [CrossRef]
- Jin, C.; Bhaskar, S. Unifying Vascular Injury and Neurodegeneration: A Mechanistic Continuum in Cerebral Small Vessel Disease and Dementia. Eur. J. Neurosci. 2025, 62, e70246. [Google Scholar] [CrossRef]
| Pathophysiologic Category | Biomarker (Serum) | Clinical Relevance in AIS |
|---|---|---|
| Oxidative stress | SUA | Mildly elevated: neuroprotective role [15] Highly elevated: ↑ stroke recurrence [16] |
| Inflammation | CRP | ↑ Vascular inflammation ↑ atherothrombotic process [17,18] |
| IL-6 | Rapid ↑: mediates neuroinflammation [19,20] | |
| Thrombus formation | D-dimer | ↑ in AIS (within hours) [20] ↓ specificity: associated with thromboembolic activity [21,22] |
| Fibrinogen | ↑ thrombotic activity [23] ↑ atherosclerosis [23,24] ↑ in AIS within hours [25] ↓ specificity [26] | |
| Cardiac function | Troponin | ↑ risk of death/major disability [27] |
| NT-proBNP | ↑ myocardial injury [20] ↑ wall stress [20] | |
| Neuronal and axonal injury | GFAP | Marker of gliosis and astrocytic integrity [19]: ↑ in H.S (peak 2–6 h) [19,28], delayed elevation in AIS [29], early elevation in ICH [30]. |
| NFL | Early ↑ in AIS [31] |
| Biomarker | Relation to Etiology/Subtype | Relation to Diagnostic Characteristics | Relation to Differential Diagnosis | Relation to Response to Therapy | Relation to H.T. | Relation to Recurrent Risk | Relation to Outcome/Prognosis |
|---|---|---|---|---|---|---|---|
| SUA | No evidence | No evidence | No evidence | No evidence | No evidence | No evidence | High levels: ↑ NIHSS ↑ mRS [15] |
| CRP | High levels in CE > LAA > SAA [18,38,39,40,41] | No evidence | No evidence | High levels: poor post-IVT [42], post-MT outcome [45,46,47] | High levels: ↑ risk of H.T. [44] | High levels: ↑ risk of TIA/minor stroke [55] | High levels: mortality deterioration [49] |
| IL-6 | High levels: TIA [58], lacunar stroke [57] | High levels: infarct size ↑ NIHSS [59] | Early elevation in AIS: ~24 h ± 6 from onset; rate 28% every 2 h [62] | No evidence | No evidence | No evidence | High levels: ↑ mortality ↑ mRS [61] |
| D-dimer | Elevated in CE > ESUS, LAA > SAA [38,69,70,71,72,73,74,75] correlation with LVO [75] frequently elevated in cancer [64,65], LAE [66], NVAF [66], AAD [67], RLS [76], IE [65]. | No evidence | TIA [77] | High levels: poor post-IVT, post-MT outcome [78,79,80] | No evidence | ↑ embolic risk [89,90] | High levels: poor clinical outcome [84,85] |
| Fibrinogen | High levels: LAA, ESUS [93] | No evidence | No evidence | ↑ FAR post-IVT alteplase: poor outcome [96,97,98] | Lower levels after IVT: ↑H.T. [100] | ↑ stroke recurrence [111] | High levels: cognitive decline [110] |
| Troponin | High levels: LVO [114,115] | Relation to ↑ NIHSS [116,117] | No evidence | Elevation post-IVT: ↑ mortality [119] | No evidence | TIA, ischemic stroke [133]. | High levels: cardiovascular events, unfavorable discharge [122,123]. |
| NT-proBNP | High levels: CE [20,136], PAF [138], AF [137,139] | High levels: large infarct volume [140]. | No evidence | No evidence | Elevation post-IVT: ↑ risk of H.T. [142] | recurrent stroke with NVAF [144] | High levels: ↑ mRS [140], infarct mass effect [143] |
| GFAP | High levels in LVO > SVO [156] | No evidence | Early elevation in ICH [19,28] delayed elevation in AIS [29] | No evidence | No evidence | No evidence | High levels: ↑ brain damage [60,154] |
| NFL | High levels in CE, LAA [60] | Positive correlation with infarct volume [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167], ↑ NIHSS [164]; strong association with time onset [31]. | Elevated in AIS > TIA [49,169,170], mimics [169] | No evidence | No evidence | subclinical events, related with AIS in AF [179]. | High levels: ↑ mRS [170,171,172], BI [170] post-stroke cognitive impairment [175,176]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsogka, A.; Ellul, J.; Chroni, E.; Safouris, A.; Psychogios, K.; Veltsista, D.; Kargiotis, O. Serum Biomarkers in Acute Ischemic Stroke: Clinical Applications and Emerging Insights. J. Clin. Med. 2025, 14, 7748. https://doi.org/10.3390/jcm14217748
Tsogka A, Ellul J, Chroni E, Safouris A, Psychogios K, Veltsista D, Kargiotis O. Serum Biomarkers in Acute Ischemic Stroke: Clinical Applications and Emerging Insights. Journal of Clinical Medicine. 2025; 14(21):7748. https://doi.org/10.3390/jcm14217748
Chicago/Turabian StyleTsogka, Anthi, John Ellul, Elisabeth Chroni, Apostolos Safouris, Klearchos Psychogios, Dimitra Veltsista, and Odysseas Kargiotis. 2025. "Serum Biomarkers in Acute Ischemic Stroke: Clinical Applications and Emerging Insights" Journal of Clinical Medicine 14, no. 21: 7748. https://doi.org/10.3390/jcm14217748
APA StyleTsogka, A., Ellul, J., Chroni, E., Safouris, A., Psychogios, K., Veltsista, D., & Kargiotis, O. (2025). Serum Biomarkers in Acute Ischemic Stroke: Clinical Applications and Emerging Insights. Journal of Clinical Medicine, 14(21), 7748. https://doi.org/10.3390/jcm14217748





