1. Introduction
Acute Type A aortic dissection (ATAAD) is a life-threatening emergency that requires immediate surgical intervention [
1]. Surgical replacement of the ascending aorta with reinforcement of the distal anastomosis remains the standard treatment. However, achieving a secure and hemostatic distal anastomosis is technically challenging. Bleeding from needle holes or anastomotic mismatch can lead to serious perioperative and long-term complications, contributing to significant morbidity and mortality even years after the repair [
2,
3,
4,
5].
A major concern in ATAAD repair is the persistence of the false lumen (PFL), which may result from inadequate reapproximation of the aortic wall layers, incomplete suture apposition, or the development of a new distal entry tear—referred to as distal anastomotic new entry (DANE) [
2,
6]. Both DANE and PFL increase the risk of ongoing false lumen pressurization, aortic expansion, reintervention, and reduced long-term survival [
6].
Various reinforcement strategies have been proposed, including the use of Teflon felt or pericardial strips [
7,
8], BioGlue fixation [
9], adventitial inversion [
10], stent graft approaches [
11], and various modifications thereof. Among these, the double patch “
sandwich technique” is one of the most widely used. This method involves placing two strips of reinforcement material—typically Teflon felt or pericardium—on both the outer and inner surfaces of the aortic wall to minimize bleeding and reduce the risk of new entry tears during suturing, followed by implantation of a vascular prosthesis [
12]. However, more recent evidence suggests that a surgeon’s expertise and meticulous surgical technique may be the most critical determinants influencing rates of DANE and PFL, rather than the reinforcement material itself. Notably, the use of Teflon felt or biological glue—compared with no reinforcement material—has not consistently demonstrated superiority in preventing these complications [
13].
Despite this, the optimal choice of reinforcement material remains a subject of debate due to differences in mechanical properties, tissue integration, and handling characteristics. To address this gap, our study compares these two materials in a standardized human cadaver model of ATAAD repair, aiming to provide experimental evidence that may inform surgical decision-making and improve clinical outcomes.
2. Materials and Methods
Twenty fresh human cadavers with artificially created type A aortic dissections (
Figure 1) underwent surgical repair using the
sandwich technique with either felt or pericardial strips. Only cadavers with a post-mortem interval of less than 72 h and no prior history of thoracic aortic surgery were included. The study was approved by the Ethics Committee of the Medical University of Vienna (No. 1997/2024). All donors had provided informed consent during their lifetime for body donation for physician education, continuing medical training, and medical research. This consent was verified by an additional confirmation letter from the institutional body donation program, which was submitted to the journal.
Ten procedures used felt strips for the sandwich technique, while the remaining ten utilized pericardial strips. The time required to complete the sandwich technique and prosthetic graft anastomosis was recorded in minutes (min) and seconds (s). Leakage volume, simulating blood loss, was measured in milliliters (mL). The integrity of the sandwich technique and anastomosis was assessed by identifying complications such as persistent false lumen (PFL) and distal anastomotic new entry (DANE). Perfusion was maintained at a standardized pressure of approximately 160/90 mmHg. To evaluate potential factors influencing the outcomes of the simulated surgical procedure, demographic information and medical histories of the cadavers were recorded.
2.1. Cadaver Model Preparation and Setup
Surgical access was obtained through a median sternotomy. The pericardium was opened, and the ascending aorta along with its branch vessels, was carefully exposed and mobilized. A transverse aortotomy was performed approximately 2 cm proximal to the brachiocephalic trunk. An artificial dissection was created by separating the intimal layer from the medial layer of the aortic wall with an 11-blade scalpel and a dissector. The dissection was subsequently extended approximately 1.5 cm distally toward the aortic arch and widened in a semicircular fashion using a dissector.
2.2. Surgical Repair and Model Perfusion
Aortic repair was performed by reapproximating the dissected aortic wall layers using the
sandwich technique, followed by implantation of a vascular graft. For the
sandwich technique, two strips—either felt or pericardium—approximately 7 mm in width were prepared, with their lengths adjusted to match the specific diameter of the aorta (see schematic representation in
Figure 2 and
Figure 3a,b). The structural and mechanical characteristics of the two reinforcement materials are summarized in
Table 1. The primary strip was positioned externally to encircle the aortic wall, while the secondary strip was placed on the inner/luminal surface. The dissected aortic wall layers were then secured between the two strips using a horizontal mattress suture with 4-0 polypropylene, ensuring proper reapproximation of the tissue layers. Subsequently, an aortic replacement graft was anastomosed to the reinforced aortic stump in an end-to-end fashion with a continuous running suture (
Figure 3b). Finally, the aortic arch was resected just proximal to the origins of the left common carotid and subclavian arteries, corresponding to zone 1 according to the Ishimaru classification [
14].
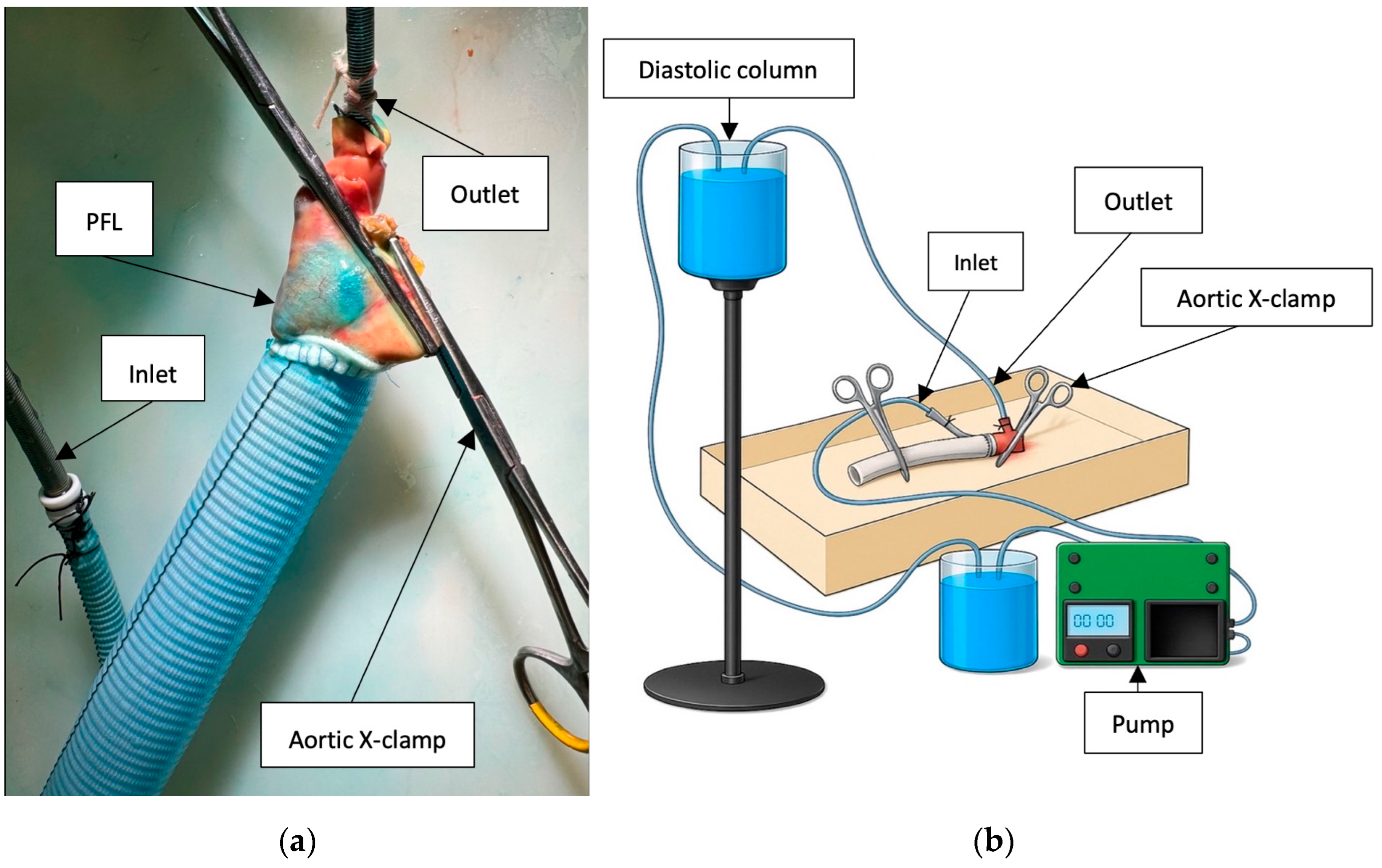
Following surgical repair, each specimen underwent both static and dynamic perfusion using a mechanical pump to evaluate the integrity of the aortic repair. The pump was programmed to deliver a pulsatile flow pattern consisting of a 2 s active flow phase followed by a 5 s pause. During the active phase, the flow rate was maintained at 100 mL/s, corresponding to an average overall flow rate of 1 L/min. Each perfusion cycle was conducted for a total duration of 20 min per simulation. A fluid column generating a diastolic pressure of 90 mmHg was used to replicate physiological condition, while systolic pressure was regulated and maintained at 160 mmHg using an external pressure monitoring system.
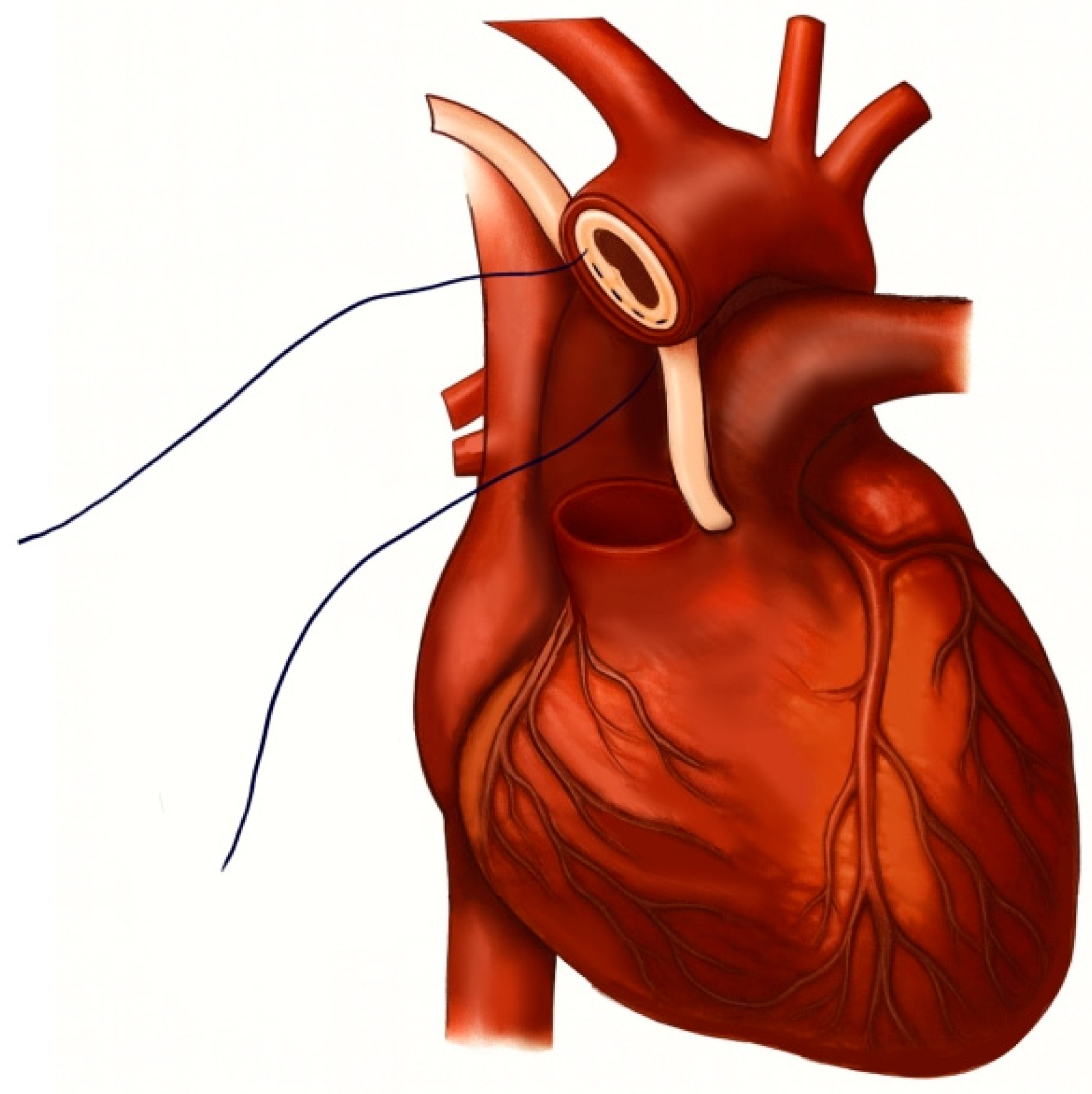
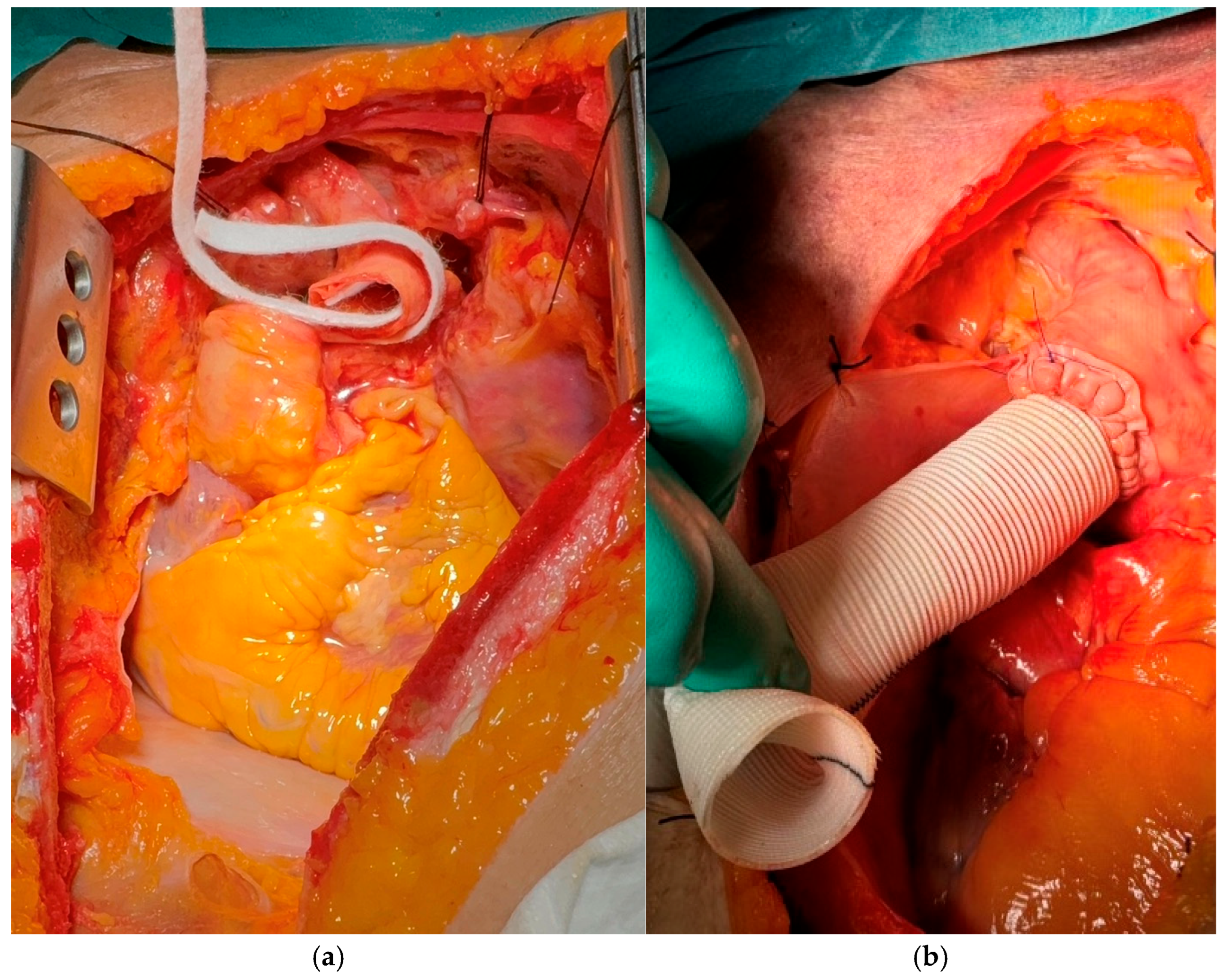
The inlet ECMO cannula was inserted into the side branch of the aortic prosthesis, and the outlet ECMO cannula was positioned within the brachiocephalic trunk. Both cannulas were securely ligated to maintain a closed circulatory circuit. Large vascular cross-clamps were applied to the distal end of the prosthesis and the native aortic arch (
Figure 4a,b).
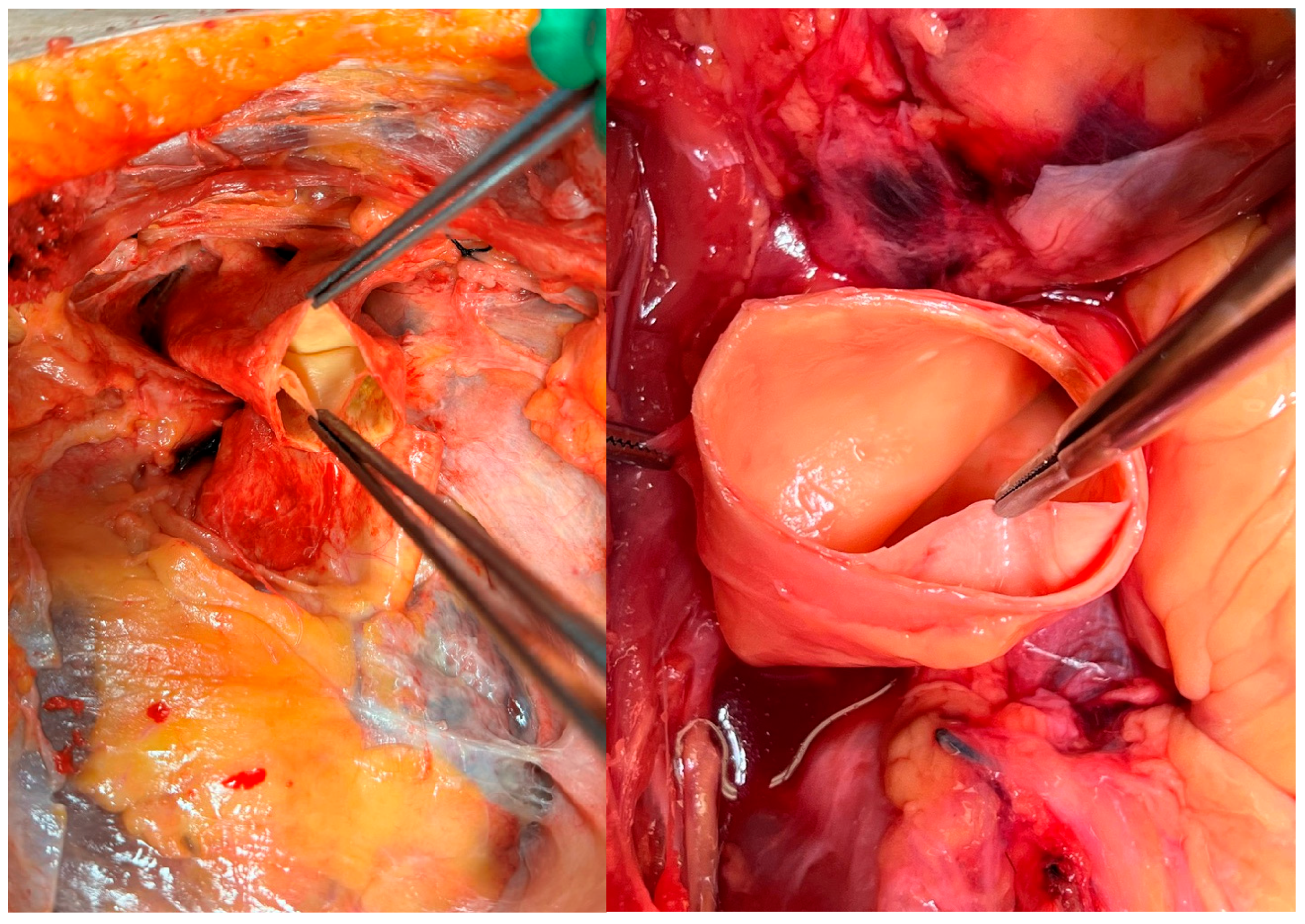
To simulate blood viscosity, a fluid mixture consisting of 40% glycerol and 60% water was used. The solution was stained with methylene blue at a concentration of 16 mL/L to enhance visualization of fluid leakage, PFL (
Figure 5a) and DANE [
15].
2.3. Evaluation of Integrity of Sandwich Technique and Vascular Graft Anastomosis
The explanted aorta was placed in an appropriate container to enable collection and quantification of fluid loss. Prior to the 20 min perfusion phase, leakage sites were identified, and additional sutures were applied as required. To assess iatrogenic dissection, all sutures were carefully removed following perfusion. The presence of new intimal tears and persistent false lumen was evaluated by detecting blue staining between the dissected layers (
Figure 5a).
2.4. Statistics
Continuous variables were expressed as medians with interquartile ranges (IQRs) and categorical variables as frequencies and percentages. Group comparisons for continuous variables were performed using the Mann–Whitney U test, and Fisher’s exact test was applied for categorical variables. The composite endpoint was defined as the occurrence of either distal anastomotic new entry (DANE) or persistent false lumen (PFL). Statistical significance was assessed at a two-sided α level of 0.05. All statistical analyses were performed using SPSS version 29 (IBM Corp., Armonk, NY, USA).
4. Discussion
This study is, to our knowledge, the first to directly compare felt and pericardium for anastomotic sealing in an experimental model. The use of pericardium as a sandwich material resulted in a significantly lower leakage volume compared to synthetic felt. Despite its superior sealing performance, no measurable differences were observed in the occurrence of DANE, PFL, or the composite endpoint.
Optimization of the proximal and distal anastomosis has been a major focus in aortic surgery for several decades. While the
sandwich technique with Teflon felt remains the standard [
5], alternative methods such as the adventitia inversion technique have been described in the surgical literature to further optimize hemostasis [
16]. Additionally, techniques using tissue glue have been explored for several decades; although they may improve local hemostasis, they have not consistently reduced reintervention rates [
17]. Furthermore, concerns regarding stroke have led some centers to avoid the routine use of these methods [
18].
A perfectly sealed distal anastomosis is particularly crucial, as inadequate closure can potentially lead to serious complications. The occurrence of DANE or PFL exposes patients to immediate risks such as malperfusion of vital organs, and long-term consequences including progressive aneurysmal disease, both of which increase the likelihood of reintervention. Another important concern during aortic repair is hemorrhage arising from suture needle tracts or from insufficient sealing at the aortic-prosthetic interface, potentially resulting in considerable intraoperative blood loss. Therefore, even minor improvements in sealing quality may translate into significantly better short- and long-term outcomes.
The significantly reduced leakage volume with pericardium may be explained by the inherent material properties. Reasons may be the lower porosity of pericardium as compared to felt, as well as the superior pliability of the biological material, which may allow for a more uniform distribution of suture tension and better adaptation to the aortic wall, thereby potentially enhancing local hemostasis and seal integrity. While these findings did not correspond to measurable differences in clinically relevant surrogate parameters such as DANE, PFL, or intimal tears, improved sealing could nonetheless reduce intraoperative bleeding and transfusion requirements. This potential clinical advantage, however, remains to be confirmed in further studies.
A possible explanation for the lack of clinical benefit may also lie in the multifactorial nature of anastomosis complications. Anatomical factors such as aortic wall quality or the presence of calcifications, as well as surgical factors including needle blunting after repeated penetration or technical difficulties with the anastomosis itself, could play a crucial role in the occurrence of adverse events, making the choice of buttressing material not the sole determinant [
13,
19,
20]. Additionally, as emphasized by Khonsari, it is equally “important […] to provide appropriate tension on the suture line” to prevent bleeding from loose suture lines or intimal tears from improper suture handling [
19].
The use of pericardial strips is likely associated with slightly greater procedural complexity, as the softer and more elastic nature of pericardium, compared with the stiffer felt, makes it more challenging to handle and suture. This is reflected by somewhat longer anastomotic times, although the difference was not statistically significant. Whether this discrepancy has clinical relevance remains unclear. Nevertheless, this finding highlights the importance of simulation and training—such as on cadaver models—in cardiac surgery, especially for low-volume centers [
15,
21]. For example, in the present study, a significant improvement was observed between the first four and the last four
sandwich procedures with pericardium (
p = 0.029).
Several limitations must be acknowledged, which may affect the generalizability of the results. The small sample size (n = 10 per group) limits statistical power, particularly for detecting differences in outcomes such as DANE and PFL. This was mainly due to the restricted availability of body donors. Moreover, the experimental setup cannot fully replicate in vivo conditions, as it lacks coagulation, tissue repair, prolonged vascular remodeling and realistic dissection mechanisms. The glycerol solution only mimics blood viscosity without the cellular and hemostatic components required for physiological healing. Finally, long-term effects such as re-operations or clinical outcomes cannot be assessed with this model.