Abstract
Transcatheter aortic valve replacement (TAVR) has evolved over the last two decades into a cornerstone therapy for patients with severe symptomatic aortic stenosis. This therapy was initially reserved for those at high or prohibitive surgical risk but is now firmly established across all surgical risk categories. Its non-inferiority to surgical aortic valve replacement has been demonstrated even in low-risk populations, supporting the rapid worldwide expansion of its use. Nevertheless, despite procedural refinements and the advent of newer-generation prostheses, conduction disturbances leading to permanent pacemaker implantation (PPI) remain one of the most frequent and clinically relevant complications. Reported incidence ranges between 8% and 20% depending on prosthesis type, implantation technique, and baseline patient characteristics. Multiple clinical, anatomical, and procedural factors have been identified as strong predictors of post-TAVR conduction disturbances. Taken together, the integration of anatomical and clinical risk assessment, precise procedural planning, careful device selection, structured monitoring, and emerging therapeutic strategies constitutes a comprehensive, evidence-based approach to reduce the burden of conduction disturbances following TAVR. Such a multimodal framework has the potential not only to lower the incidence of permanent pacemaker implantation but also to improve safety, optimize healthcare resource utilization, and support the broader adoption of TAVR in increasingly younger and lower-risk patient populations.
1. Introduction
Over the last decade, transcatheter aortic valve replacement (TAVR) has solidified its role as a first-line treatment for patients with severe symptomatic aortic stenosis who are at moderate-to-high surgical risk, and more recently, its non-inferiority has been demonstrated in low-risk populations [1]. Since the first successful TAVR procedure in 2002, adoption has grown steadily, with over 1.5 million procedures performed worldwide in 2023 and annual volumes increasing across all risk categories [1].
Despite procedural refinements and newer-generation devices, conduction disturbances remain one of the most common and challenging complications following TAVR. Early data reported permanent pacemaker implantation (PPI) rates as high as 25–30% with first-generation valves, while current-generation devices have reduced this to 8–15%, depending on valve type and center experience [2]. These conduction disturbances—largely due to mechanical trauma from the prosthesis frame impinging on the conduction system—can result in a high-grade atrioventricular block, necessitating PPI.
Several predictive factors have been identified, including the length and morphology of the membranous septum [3], valve type, and implantation depth. Self-expanding valves are associated with higher PPI rates than balloon-expandable ones, largely due to their sustained radial force [4]. Balloon-expandable valves, by contrast, provide greater deployment precision and exert less delayed pressure on conduction tissue due to their lack of post-deployment expansion, making them a preferred choice in patients with pre-existing conduction disease.
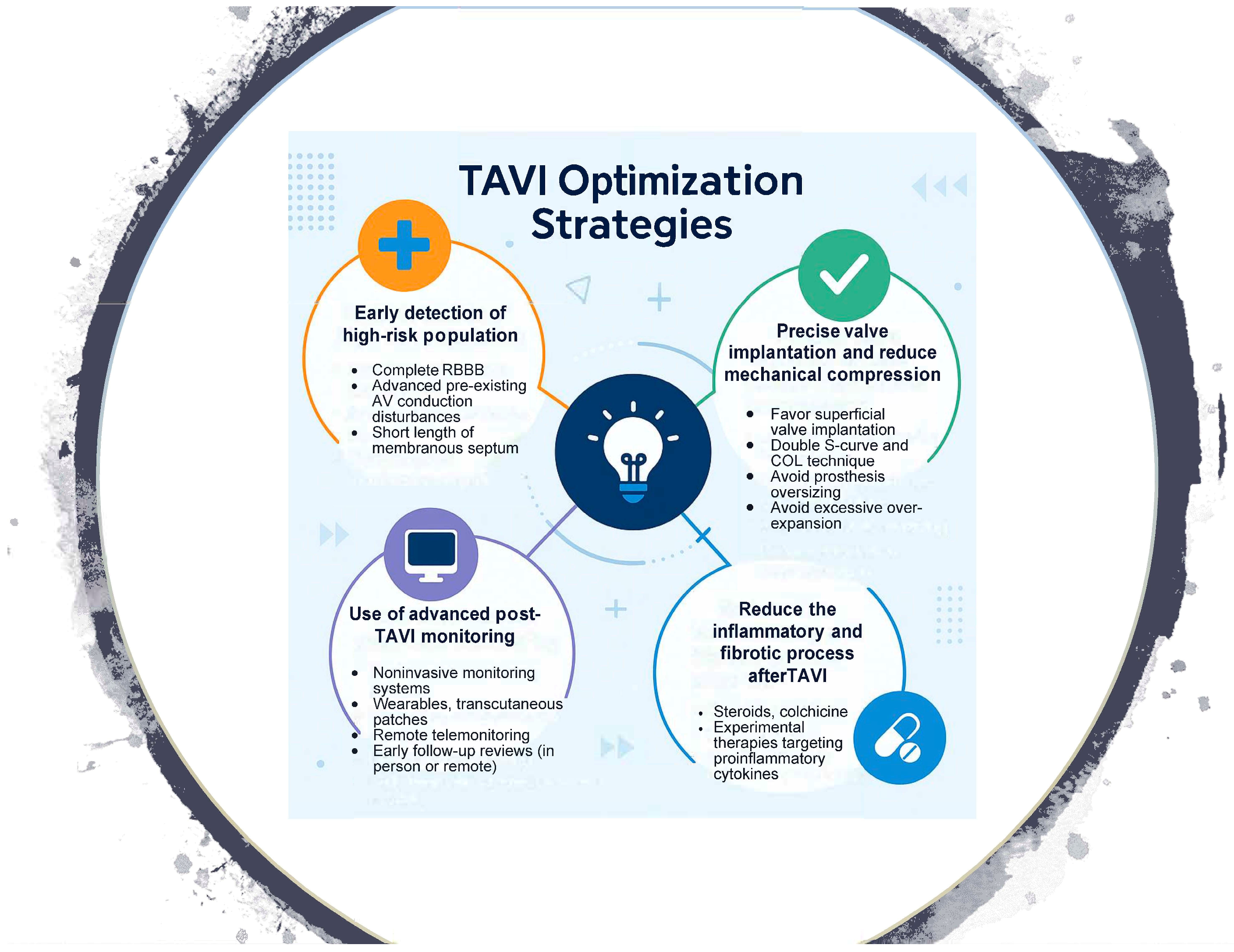
To mitigate these complications, an integrated strategy has emerged, ranging from the adaptation of valve implantation techniques to pharmacological interventions aimed at reducing post-TAVR edema and fibrosis, and structured monitoring systems that allow for early detection and timely treatment of conduction disturbances (Appendix A, Figure A1).
2. Procedural Techniques
- The double S-curve and the cusp-overlap technique: Recently introduced for self-expanding TAVR platforms, this method establishes the optimal fluoroscopic projection to guide valve implantation. Its objective is to achieve a shallower implantation at the aortic annulus, thereby reducing the risk of conduction disturbances by avoiding protrusion beyond the membranous septum, where the conduction system is more superficial. This strategy allows for more accurate control of implant depth and minimizes trauma to the conduction system, which may, in turn, decrease the incidence of permanent pacemaker implantation [5,6,7].
- The membranous septum: With current non-invasive imaging techniques, particularly computed tomography, the membranous septum can be accurately measured. Knowledge of its length provides valuable information for procedural planning, as it allows operators to perform higher valve implantations as a strategy to reduce the risk of high-grade atrioventricular block and, consequently, the need for permanent pacemaker implantation [8,9,10].
3. Patient and Valve Selection
Despite significant procedural and anatomical advances. Two key clinical factors remain strong predictors of the need for permanent pacemaker implantation (PPI) after TAVR. First, patients at intermediate or low surgical risk are increasingly undergoing TAVR, and observational data suggest that they may have a higher incidence of new PPI [11]. Second, the presence of underlying conduction abnormalities continues to be an independent risk factor for PPI [12].
- Baseline Conduction Disorders and Valve Type Selection: The presence of conduction abnormalities such as right bundle branch block (RBBB), left anterior fascicular block (LAFB), or bradyarrhythmias significantly increases the likelihood of permanent pacemaker implantation (PPI) after TAVR [11,12]. In the PARTNER trial, pre-existing conduction disturbances were independently associated with higher PPI rates, longer hospital stays, and a greater incidence of adverse clinical events [11]. In this context, balloon expandable valves are often preferred in patients with baseline conduction disease, as their greater implantation precision and reduced radial force on the conduction system are associated with lower PPI rates. Registry data suggest PPI rates as low as 4–8% with newer-generation balloon-expandable valves, compared with 10–20% for self-expanding valves [4], supporting their use as a strategy to mitigate conduction-related complications in higher-risk patients.
Despite advances in procedural techniques and patient selection, the inflammatory and fibrotic response triggered by prosthesis deployment remains a significant contributor to conduction system injury after TAVR. Therefore, attention has shifted toward strategies aimed at attenuating the inflammatory and fibrotic cascades responsible for post-TAVR conduction abnormalities.
4. Pharmacologic Strategies
Pharmacologic interventions aimed at modulating this inflammatory cascade have been proposed to reduce the incidence of conduction abnormalities and, consequently, the need for permanent pacemaker implantation. Although still in the exploratory phase, several therapeutic agents have been evaluated or are currently under investigation for this purpose.
- Corticosteroids: Corticosteroids were initially investigated for their potential to attenuate the post-TAVR inflammatory response and limit conduction tissue edema. Early reports were encouraging; however, subsequent larger studies failed to demonstrate a consistent reduction in PPI incidence, and concerns persist regarding systemic side effects and the lack of clear patient selection criteria [13,14,15].
- Colchicine: Colchicine, a well-established anti-inflammatory agent, is currently being evaluated in the Co-STAR trial (NCT04870424), a randomized, double-blind, placebo-controlled study. The trial aims to determine colchicine’s efficacy in preventing fibrosis-related conduction disorders and atrial arrhythmias after TAVR by dampening the inflammatory process. This trial is ongoing, with primary results expected in mid-2025. If successful, it could establish the first pharmacologic approach specifically designed to reduce post-procedural conduction disturbances.
5. Post-Procedural Monitoring
Monitoring after TAVR is essential for the prompt detection of conduction disturbances and to guide the need for permanent pacemaker implantation (PPI). Risk stratification based on early post-procedural ECG changes enables clinicians to differentiate between patients requiring immediate intervention and those who can be safely observed.
- Electrocardiographic and Electrophysiological Surveillance: Continuous ECG monitoring for up to 7 days is recommended by the ESC/EHRA guidelines, supported by evidence showing that more than 50% of high-grade atrioventricular block events occur within the first 72 h after TAVR, although a relevant proportion may still develop later during hospitalization. For this reason, ambulatory monitoring with external systems or implantable loop recorders may be extended up to 30 days in selected cases [16]. In addition, invasive electrophysiological study (EPS) may be considered from the third day after TAVR, particularly in patients with new-onset left bundle branch block (LBBB), PR interval > 240 ms, QRS duration > 150 ms, or marked prolongation (>20 ms) in those with pre-existing conduction disease. An HV interval ≥ 70 ms is widely regarded as predictive of high-grade atrioventricular (AV) block, although some studies have proposed alternative thresholds, such as HV ≥ 65 ms or a delta HV ≥ 13 ms when comparing pre- and post-procedural measurements [16,17]. Together, these strategies highlight the importance of combining extended non-invasive monitoring with targeted invasive evaluation to refine risk stratification and guide timely pacemaker implantation.
6. Telemonitoring and Early Discharge Programs
The integration of remote monitoring technologies has emerged as a valuable component in post-TAVR care, particularly as healthcare systems strive for safe early discharge strategies. Telemonitoring allows for real-time detection of delayed conduction disturbances while enhancing resource utilization and patient satisfaction.
- TeleTAVI Study: Demonstrated that early discharge supported by structured telemonitoring using artificial intelligence (AI) is both feasible and safe. The study included stratified discharge timing—very early (<24 h), early (24–48 h), and standard (>48 h)—coupled with daily follow-up through a virtual voice assistant using natural language processing. Patients in the early discharge arms had comparable 30-day event rates to those discharged later, while reporting high adherence and satisfaction with monitoring [18].
- Additionally, several studies are focused on pre-, intra-, and post-TAVR electrocardiographic monitoring to validate the performance of conduction disturbance risk scales (NCT05657912), estimate a reduction in disturbances through notifications during the procedure (NCT05465655), and monitor patients with pre-existing conduction disturbances or those that develop intra- or peri-procedurally after discharge [19,20].
7. Preventive Pacemaker Implantation
Although not routinely performed, preventive permanent pacemaker implantation (PPI) prior to TAVR has been proposed in selected high-risk patients as a strategy to avoid periprocedural complications and prolonged hospitalization. This approach is particularly considered in patients with baseline right bundle branch block (RBBB), a short membranous septum, or advanced conduction system disease, in whom the likelihood of developing high-grade atrioventricular block is significantly increased. In this subgroup, the risk of requiring urgent pacing support or emergent PPI is high, and anticipating this scenario with a planned implantation may improve safety and streamline management. Retrospective analyses from high-volume centers have reported that prophylactic PPI in such patients can shorten hospital stay and reduce the incidence of secondary complications associated with prolonged immobilization, including phlebitis, urinary tract infections, and delirium. Moreover, preventive PPI may simplify procedural logistics by reducing reliance on temporary pacing and minimizing the need for emergent pacemaker implantation, both of which are associated with greater procedural complexity, higher resource utilization, and potential hemodynamic compromise [21,22]. While this strategy remains controversial and is not currently endorsed by international guidelines, its role continues to be explored as part of a broader effort to optimize conduction management in high-risk patients undergoing TAVR.
8. Conclusions
Ongoing advancements in our understanding of clinical, anatomical, and procedural contributors to post-TAVR conduction disturbances have led to significant improvements in patient management. Predictive imaging, refined implantation techniques, and strategic valve selection now enable tailored interventions for patients at the highest risk. In parallel, innovations in ambulatory monitoring, artificial intelligence-based follow-up, and emerging anti-inflammatory therapies offer new opportunities for the timely detection and prevention of PPI. A multifaceted, evidence-based approach—incorporating these insights—has the potential not only to reduce PPI rates but also to enhance the safety, efficiency, and broader adoption of TAVR in increasingly younger and lower-risk populations.
Author Contributions
Conceptualization, A.R.M., C.E.G., V.A.J.D. and E.M.A.G.; investigation, A.R.M., V.A.J.D. and D.O.M.; writing—original draft preparation, A.R.M., C.E.G. and H.L.d.l.G.; writing—review and editing, A.R.M., V.A.J.D., P.J.S. and J.A.B.A.; supervision, V.A.J.D., J.A.B.A. and A.I.R. All authors have read and agreed to the published version of the manuscript.
Funding
This This report has been granted by the Galician Innovation Agency (GAIN) through program code IN607B-2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TAVR | Transcatheter Aortic Valve Implantation |
| PPI | Permanent Pacemaker Implantation |
| RBBB | Right Bundle Branch Block |
| LAFB | Left Anterior Fascicular Block |
| LBBB | Left Bundle Branch Block |
| EPS | Electrophysiological Study |
Appendix A
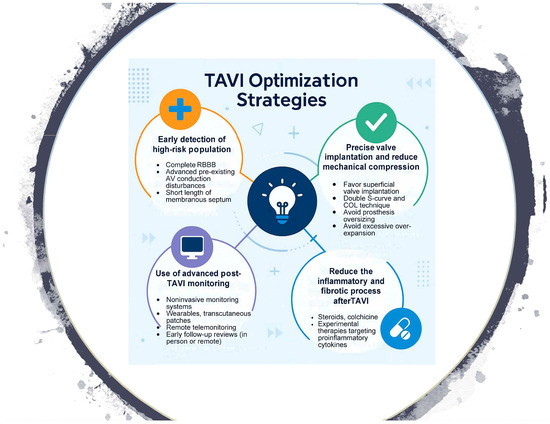
Figure A1.
Central Image.
Figure A1.
Central Image.
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Fu, Q.; Xia, Y.; Wu, Y. Predictors, clinical impact, and management strategies for conduction abnormalities after transcatheter aortic valve replacement: An updated review. Front. Cardiovasc. Med. 2024, 11, 1370244. [Google Scholar] [CrossRef]
- Hamdan, A.; Guetta, V.; Klempfner, R.; Konen, E.; Raanani, E.; Glikson, M.; Goitein, O.; Segev, A.; Barbash, I.; Fefer, P.; et al. Inverse relationship between membranous septal length and the risk of atrioventricular block in patients undergoing transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 2015, 8, 1218–1228. [Google Scholar] [CrossRef]
- Novelli, L.; Jamie, G.; Regazzoli, D.; Reimers, B.; Frontera, A.; Mangieri, A. How to predict conduction disturbances after transcatheter aortic valve replacement. Kardiol. Pol. 2023, 81, 330–337. [Google Scholar] [CrossRef]
- Tang, G.H.L.; Zaid, S.; Michev, I.; Ahmad, H.; Kaple, R.; Undemir, C.; Cohen, M.; Lansman, S.L. “cusp-overlap” view simplifies fluoroscopy-guided implantation of self-expanding valve in transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 2018, 11, 1663–1665. [Google Scholar] [CrossRef]
- Ben-Shoshan, J.; Alosaimi, H.; Lauzier, P.T.; Pighi, M.; Talmor-Barkan, Y.; Overtchouk, P.; Martucci, G.; Spaziano, M.; Finkelstein, A.; Gada, H.; et al. Double S-curve versus cusp-overlap technique. JACC Cardiovasc. Interv. 2021, 14, 185–194. [Google Scholar] [CrossRef]
- Rawish, E.; Macherey, S.; Jurczyk, D.; Pätz, T.; Jose, J.; Stiermaier, T.; Eitel, I.; Frerker, C.; Schmidt, T. Reduction of permanent pacemaker implantation by using the cusp overlap technique in transcatheter aortic valve replacement: A meta-analysis. Clin. Res. Cardiol. 2023, 112, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Bouzas-Mosquera, A.; Barbeito-Caamaño, C.; Martínez-Sapiña, M.J.; Otero-Muinelo, S.; Vázquez-Rodríguez, J.M. Noninvasive imaging techniques in transcatheter aortic valve implantation. Cir. Cardiovasc. 2024, 32, 58–64. [Google Scholar] [CrossRef]
- Jørgensen, T.J.; Hansson, N.; De Backer, O.; Bieliauskas, G.; Terkelsen, C.T.; Wang, X.; Jensen, J.J.; Christiansen, E.C.; Piazza, N.; Svendsen, J.S.; et al. Membranous septum morphology and risk of conduction abnormalities after transcatheter aortic valve implantation. EuroIntervention 2022, 17, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Baraka, M.; Kamal, D.; Mostafa, A.E. Depth of implantation in relation to membranous septum as a predictor of conduction disturbances after transcatheter aortic valve implantation. Indian Pacing Electrophysiol. J. 2024, 24, 133–139. [Google Scholar] [CrossRef]
- Gabbieri, D.; Ghidoni, I.; Mascheroni, G.; Chiarabelli, M.; D’aNniballe, G.; Pisi, P.; Meli, M.; Labia, C.; Barbieri, A.; Spina, F.; et al. Pacemaker implantation after surgical aortic valve replacement and balloon-expandable transcatheter aortic valve implantation: Incidence, predictors and prognosis. Heart Rhythm O2 2025, 6, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Nazif, T.M.; Dizon, J.M.; Hahn, R.T.; Xu, K.; Babaliaros, V.; Douglas, P.S.; El-Chami, M.F.; Herrmann, H.C.; Mack, M.; Makkar, R.R.; et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: The PARTNER (Placement of AoRtic TraNscathetER Valves) trial and registry. JACC Cardiovasc. Interv. 2015, 8 Pt A, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Oestreich, B.; Gurevich, S.; Adabag, S.; Kelly, R.; Helmer, G.; Raveendran, G.; Yannopoulos, D.; Biring, T.; Garcia, S. Exposure to glucocorticoids prior to transcatheter aortic valve replacement is associated with reduced incidence of high-degree AV block and pacemaker. Cardiovasc. Revasc. Med. 2019, 20, 328–331. [Google Scholar] [CrossRef]
- Barone, L.; Muscoli, S.; Belli, M.; Di Luozzo, M.; Sergi, D.; Marchei, M.; Prandi, F.R.; Uccello, G.; Romeo, F.; Barillà, F. Effect of acute CORticosteroids on conduction defects after Transcatheter Aortic Valve Implantation: The CORTAVI study. J. Cardiovasc. Med. 2023, 24, 676–679. [Google Scholar] [CrossRef]
- Tiago, C.; Dias Vaz, M.; Marques, A.; Barata, M.; Braga, J.P.; Boa, A.; Carvalho, A.F. Intraoperative corticosteroids and pacemaker implantation after transcatheter aortic valve replacement. Cureus. 2024, 16, e56824. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) with the special contribution of the European Heart Rhythm Association (EHRA). Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Rivard, L.; Schram, G.; Asgar, A.; Khairy, P.; Andrade, J.G.; Bonan, R.; Dubuc, M.; Guerra, P.G.; Ibrahim, R.; Macle, L.; et al. Electrocardiographic and electrophysiological predictors of atrioventricular block after transcatheter aortic valve replacement. Heart Rhythm 2015, 12, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Samper, R.; Riquelme, J.; Pineda, J.; Bordes, P.; Torres-Mezcua, F.; Valencia, J.; Torres-Saura, F.; Manso, M.G.; Ajo, R.; et al. Early discharge programme after transcatheter aortic valve implantation based on close follow-up supported by telemonitoring using artificial intelligence: The TeleTAVI study. Eur. Heart J.-Digit. Health 2024, 6, ztae089. [Google Scholar] [CrossRef]
- Muntané-Carol, G.; Okoh, A.K.; Chen, C.; Nault, I.; Kassotis, J.; Mohammadi, S.; Coromilas, J.; Lee, L.Y.; Alperi, A.; Philippon, F.; et al. Ambulatory electrocardiographic monitoring following minimalist transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 2021, 14, 2711–2722. [Google Scholar] [CrossRef]
- Scotti, A.; Sturla, M.; Coisne, A.; Assafin, M.; Chau, M.; Ho, E.C.; Granada, J.F.; Ferrick, K.J.; Di Biase, L.; Latib, A. Monitoring conduction disturbances following TAVR: Feasibility study of early discharge using a novel telemetry patch. Int. J. Cardiol. 2022, 364, 35–37. [Google Scholar] [CrossRef]
- Zorman, M.; Bamford, P.; Coronelli, M.; Barnes, C.; Saunderson, C.; Gamble, J.; Dawkins, S.; Kharbanda, R.K.; Newton, J.; Banning, A.P.; et al. Prophylactic permanent pacemaker implantation for baseline right bundle branch block in patients undergoing transcatheter aortic valve replacement: Clinical efficacy, safety, and long-term pacing requirement. Struct. Heart 2024, 8, 100326. [Google Scholar] [CrossRef] [PubMed]
- Fukutomi, M.; Hokken, T.; Wong, I.; Bieliauskas, G.; Daemen, J.; de Jaegere, P.; Van Mieghem, N.; Søndergaard, L.; De Backer, O. Prophylactic permanent pacemaker strategy in patients with right bundle branch block undergoing transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2021, 98, E1017–E1025. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
