Cutting Risk, Not Just Skin—An International Survey on the Role of Preoperative Lab Values in Risk Stratification for Plastic and Reconstructive Surgery
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Participant Recruitment
2.3. Data Handling and Statistical Analysis
3. Results
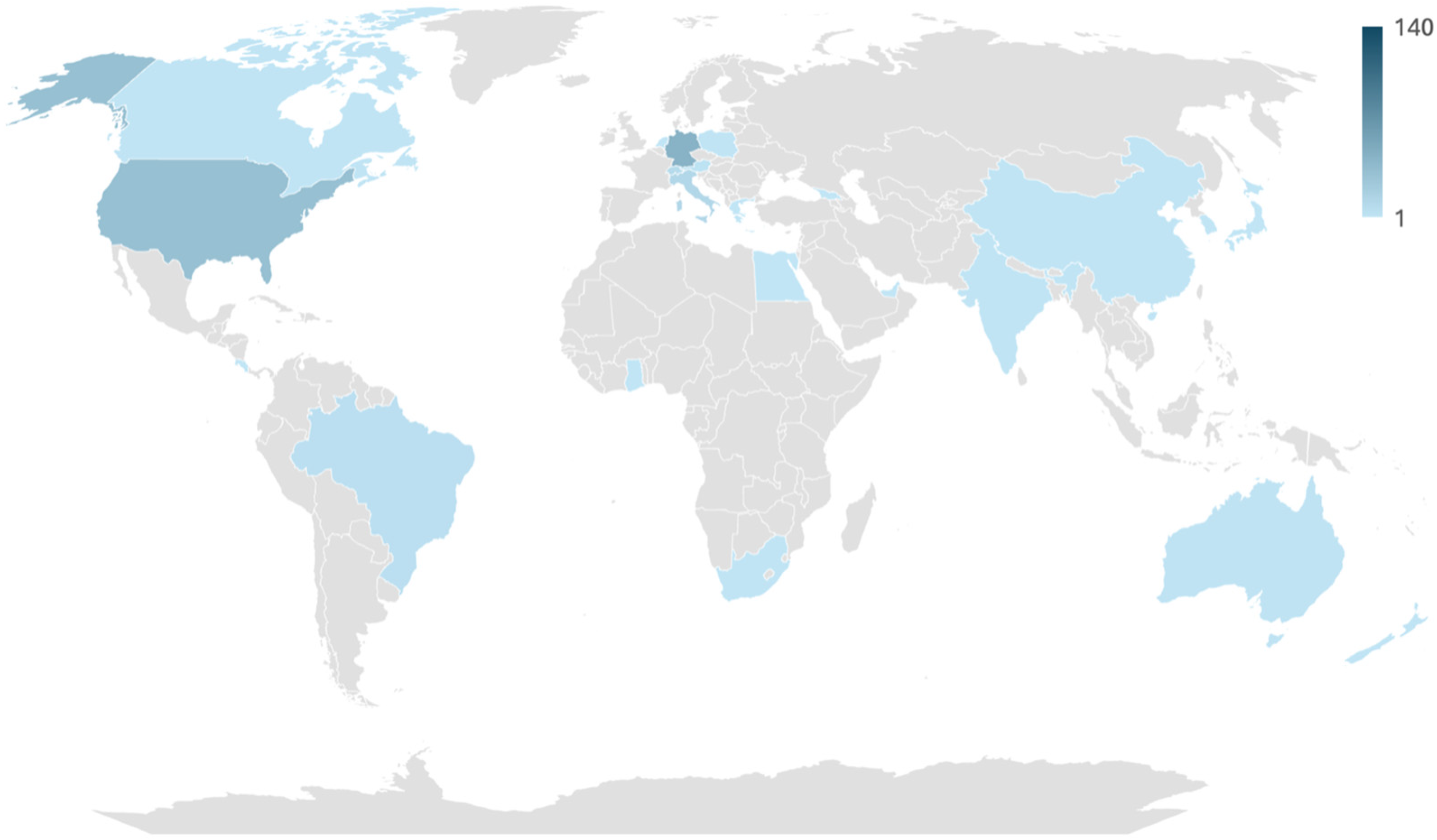
3.1. Participant Demographic Data
3.2. Preoperative Risk Assessment Practices
3.3. Preoperative Laboratory Testing
3.4. Differences by Practice Setting and PRS Subspecialization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alfertshofer, M.; Sherwani, K.; Machens, H.-G.; Broer, N.; Schenck, T.; Moellhoff, N.; Frank, K.; Knoedler, S.; Knoedler, L.; Cotofana, S. The landscape of current trends and procedures in plastic surgery practices across the D-A-CH region: An in-depth analysis of 511 websites. Eur. J. Plast. Surg. 2024, 47, 69. [Google Scholar] [CrossRef]
- Gutowski, K.S.B.; Chwa, E.S.B.; Weissman, J.P.B.; Garg, S.P.B.; Simmons, C.J.B.; Brandt, K.E.; Gosain, A.K. Practice Profile of Practicing Plastic Surgeons: A 20-Year Review of Plastic Surgery Statistics. Plast. Reconstr. Surg. Glob. Open 2023, 11, E5486. [Google Scholar] [CrossRef]
- Wan, M.; Zhang, J.X.; Ding, Y.; Jin, Y.; Bedford, J.; Nagarajan, M.; Bucevska, M.; Courtemanche, D.J.; Arneja, J.S. High-Risk Plastic Surgery: An Analysis of 108,303 Cases from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP). Plast. Surg. 2020, 28, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Sergesketter, A.R.; Shammas, R.L.; Geng, Y.; Levinson, H.; Matros, E.; Phillips, B.T. Tracking Complications and Unplanned Healthcare Utilization in Aesthetic Surgery: An Analysis of 214,504 Patients Using the TOPS Database. Plast. Reconstr. Surg. 2023, 151, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, A.B.; Wappler, F.; Zwilßer, B. Präoperative Risikoevaluation-Von der Routinediagnostik zur patientenorientierten Strategie. Dtsch. Arztebl. Int. 2014, 111, 437–445. [Google Scholar] [CrossRef]
- Firde, M.; Yetneberk, T. Preoperative investigation practices for elective surgical patients: Clinical audit. BMC Anesthesiol. 2024, 24, 184. [Google Scholar] [CrossRef] [PubMed]
- Zambouri, A. Preoperative evaluation and preparation for anesthesia and surgery. Hippokratia 2007, 11, 13–21. [Google Scholar] [PubMed] [PubMed Central]
- Hill, J.B.; Patel, A.; Del Corral, G.A.; Sexton, K.W.; Ehrenfeld, J.M.; Guillamondegui, O.D.; Shack, R.B. Preoperative anemia predicts thrombosis and free flap failure in microvascular reconstruction. Ann. Plast. Surg. 2012, 69, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Rondinelli, M.B.; Weltert, L.P.; Ruocco, G.; Ornelli, M.; Femmine, P.F.D.; De Rosa, A.; Pierelli, L.; Felici, N. Patient Blood Management in Microsurgical Procedures for Reconstructive Surgery. Diagnostics 2023, 13, 2758. [Google Scholar] [CrossRef]
- Torabi, S.; Overbeek, R.; Dusse, F.; Stoll, S.E.; Schroeder, C.; Zinser, M.; Zirk, M. Impact of perioperative anticoagulation management on free flap survival in reconstructive surgery: A retrospective analysis. BMC Anesthesiol. 2025, 25, 106. [Google Scholar] [CrossRef]
- Prowle, J.R.; Croal, B.; Abbott, T.E.F.; Cuthbertson, B.H.; Wijeysundera, D.N. Cystatin C or creatinine for pre-operative assessment of kidney function and risk of post-operative acute kidney injury: A secondary analysis of the METS cohort study. Clin. Kidney J. 2024, 17, sfae004. [Google Scholar] [CrossRef] [PubMed]
- Molinari, L.; Sakhuja, A.; Kellum, J.A. Perioperative Renoprotection: General Mechanisms and Treatment Approaches. Anesth. Analg. 2020, 131, 1679–1692. [Google Scholar] [CrossRef]
- Dortch, J.D.; Eck, D.L.; Ladlie, B.; TerKonda, S.P. Perioperative Glycemic Control in Plastic Surgery: Review and Discussion of an Institutional Protocol. Aesthet. Surg. J. 2016, 36, 821–830. [Google Scholar] [CrossRef]
- De Vries, F.E.E.; Gans, S.L.; Solomkin, J.S.; Allegranzi, B.; Egger, M.; Dellinger, E.P.; Boermeester, M.A. Meta-analysis of lower perioperative blood glucose target levels for reduction of surgical-site infection. Br. J. Surg. 2017, 104, e95–e105. [Google Scholar] [CrossRef]
- Martin, E.T.; Kaye, K.S.; Knott, C.; Nguyen, H.; Santarossa, M.; Evans, R.; Bertran, E.; Jaber, L. Diabetes and risk of surgical site infection: A systematic review and meta-analysis. Infect. Control Hosp. Epidemiol. 2016, 37, 88–99. [Google Scholar] [CrossRef]
- Loftus, T.J.; Brown, M.P.; Slish, J.H.; Rosenthal, M.D. Serum Levels of Prealbumin and Albumin for Preoperative Risk Stratification. Nutr. Clin. Pract. 2019, 34, 340–348. [Google Scholar] [CrossRef]
- van Stijn, M.F.; Korkic-Halilovic, I.; Bakker, M.S.; van der Ploeg, T.; van Leeuwen, P.A.; Houdijk, A.P. Preoperative nutrition status and postoperative outcome in elderly general surgery patients: A systematic review. JPEN J. Parenter. Enteral Nutr. 2013, 37, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Knoedler, S.; Matar, D.Y.; Knoedler, L.; Obed, D.; Haug, V.; Gorski, S.M.; Kim, B.-S.; Kauke-Navarro, M.; Kneser, U.; Panayi, A.C.; et al. Association of age with perioperative morbidity among patients undergoing surgical management of minor burns. Front. Surg. 2023, 10, 1131293. [Google Scholar] [CrossRef]
- Knoedler, S.; Klimitz, F.J.; Diatta, F.; Perozzo, F.A.; Sofo, G.; Alfertshofer, M.; Cherubino, M.; Mayer, H.; Panayi, A.C.; Kim, B.-S.; et al. Protein as a preoperative predictor–Impact of hypoalbuminemia on 30-day outcomes of breast reduction surgery. J. Plast. Reconstr. Aesthetic Surg. 2025, 100, 144–152. [Google Scholar] [CrossRef] [PubMed]
- American College of Surgeons National Surgical Quality Improvement Program. ACS NSQIP 2023 Participant Use File (PUF) User Guide. 2024. Available online: https://www.facs.org/media/ekmnc2ge/nsqip_puf_userguide_2023.pdf (accessed on 19 August 2025).
- Montrief, T.; Bornstein, K.; Ramzy, M.; Koyfman, A.; Long, B.J. Plastic Surgery Complications: A Review for Emergency Clinicians. West. J. Emerg. Med. 2020, 21, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Sahin, H.; Pirat, A.; Arslan, G. Anaesthesia and surgery in patients with abnormal preoperative liver enzymes. Eur. J. Anaesthesiol. 2007, 24, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.L.; Cunningham, J.L.; Rabinstein, A.A.; Wijdicks, E.F.M. Electrolyte orders in the neuroscience intensive care unit: Worth the value or waste? Neurocrit. Care 2011, 14, 216–221. [Google Scholar] [CrossRef]
- Kasprowicz, A.; Thomson, C. Pathology Price Changes for 2020–2021. 2020. Available online: https://practice365.co.uk/uploads/sites/225/2020/12/Private-Test-Costs-2020-21.pdf (accessed on 27 October 2025).
- Government of British Columbia. SCHEDULE OF FEES for the Laboratory Services Outpatient Payment Schedule. In SCHEDULE OF FEES (pp. 1–5) [Report]. 2015. Available online: https://www.phsa.ca/plms/Documents/Laboratory%20Services%20Outpatient%20Payment%20Schedule.pdf (accessed on 27 October 2025).
- Centers for Medicare & Medicaid Services. 25CLABQ1—CY 2025 Q1 Release: Clinical Laboratory Fee Schedule (CLFS) Files. CMS, 2025. Available online: https://www.cms.gov/medicare/payment/fee-schedules/clinical-laboratory-fee-schedule-clfs/files/25clabq1 (accessed on 27 October 2025).
- Griffin, F.S.; Stead, T.S.; Zeyl, V.G.; Mehrzad, R.; King, V.A.; Kalliainen, L.K. Low Preoperative Albumin Levels Significantly Associated with Increased Risk of Wound Infection and Bleeding After Panniculectomy. Plast. Surg. 2024, 22925503241292350. [Google Scholar] [CrossRef]
- Larson, D.W.; El Aziz, M.A.A.; Perry, W.; D’aNgelo, A.-L.; Behm, K.T.; Mathis, K.L.; Grass, F. Additional Value of Preoperative Albumin for Surgical Risk Stratification among Colorectal Cancer Patients. Ann. Nutr. Metab. 2021, 76, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.K.; Roy Chowdhury, S.; Aravindan, A.; Saha, S.; Rapaka, S. Medical and Surgical Care of Critical Burn Patients: A Comprehensive Review of Current Evidence and Practice. Cureus 2022, 14, e31550. [Google Scholar] [CrossRef]
- Haberal, M.; Abali, A.E.S.; Karakayali, H. Fluid management in major burn injuries. Indian J. Plast. Surg. 2010, 43 (Suppl. S1), S29–S36. [Google Scholar] [CrossRef]
- Biermann, N.; Chak, J.C.; Wiesmeier, A.; Klein, S.M.; Ruewe, M.; Spoerl, S.; Kruppa, P.; Prantl, L.; Anker, A.M. Evidence-Based Approaches to Anticoagulation in Reconstructive Microsurgery—A Systematic Literature Review. Life 2024, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Choudry, U.H.; Hyza, P.; Lane, J.; Petty, P. The importance of preoperative hemoglobin evaluation in large volume liposuction: Lessons learned from our 15-year experience. Ann. Plast. Surg. 2008, 61, 230–234. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Routine Preoperative Tests for Elective Surgery (NG45); National Institute for Health and Care Excellence: London, UK, 2016; Available online: https://www.nice.org.uk/guidance/ng45 (accessed on 19 August 2025).
- Kannaujia, A.K.; Gupta, A.; Verma, S.; Srivastava, U.; Haldar, R.; Jasuja, S. Importance of Routine Laboratory Investigations Before Elective Surgery. Discoveries 2020, 8, e114. [Google Scholar] [CrossRef] [PubMed]

| Subgroup | n = 140 | % | |
|---|---|---|---|
| Gender | Male | 103 | 73.6 |
| Female | 37 | 26.4 | |
| Age | Mean, SD | 52.3 ± 14.2 | |
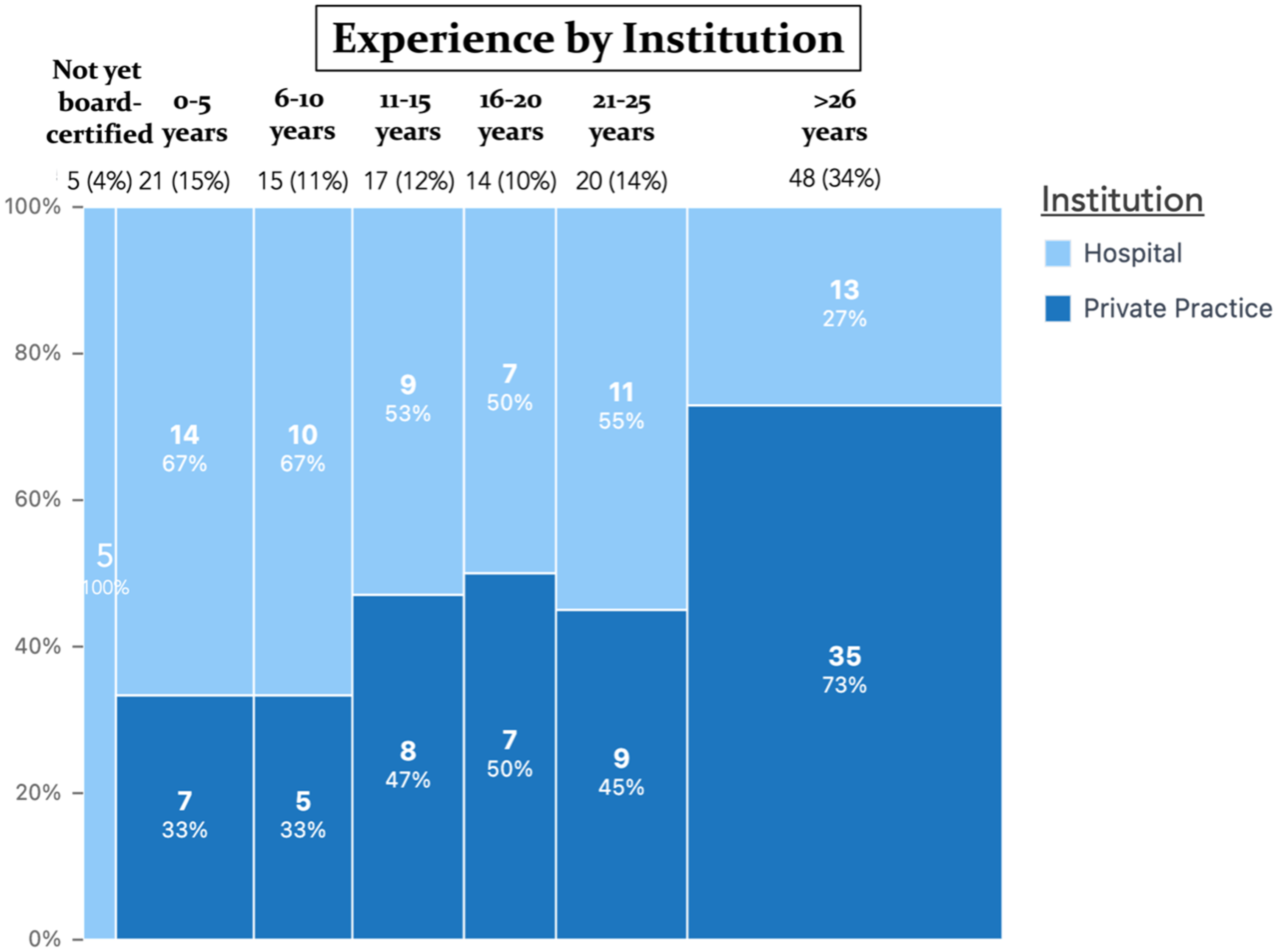
| Years of being a board-certified plastic surgeon | Not yet board certified | 5 | 3.6 |
| 0–5 years | 21 | 15.0 | |
| 6–10 years | 15 | 10.7 | |
| 11–15 years | 17 | 12.1 | |
| 16–20 years | 14 | 10 | |
| 21–25 years | 20 | 14.3 | |
| >26 years | 48 | 34.3 | |
| Work setting | Hospital (e.g., academic institution) | 69 | 49.3 |
| Private practice | 71 | 50.7 | |
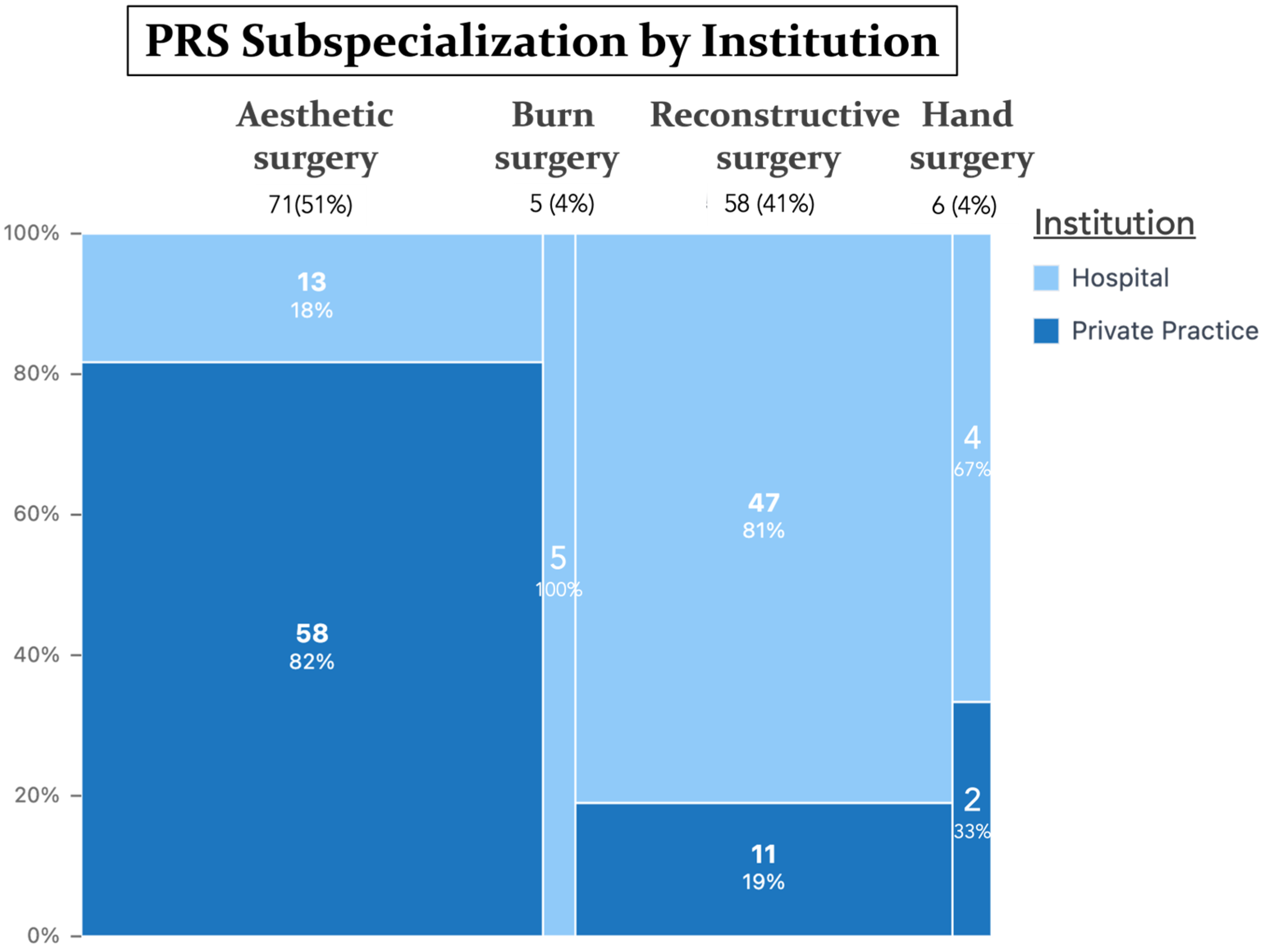
| Primary focus of surgery | Aesthetic surgery | 71 | 50.7 |
| Reconstructive surgery | 58 | 41.4 | |
| Hand surgery | 6 | 4.3 | |
| Burn surgery | 5 | 3.6 |
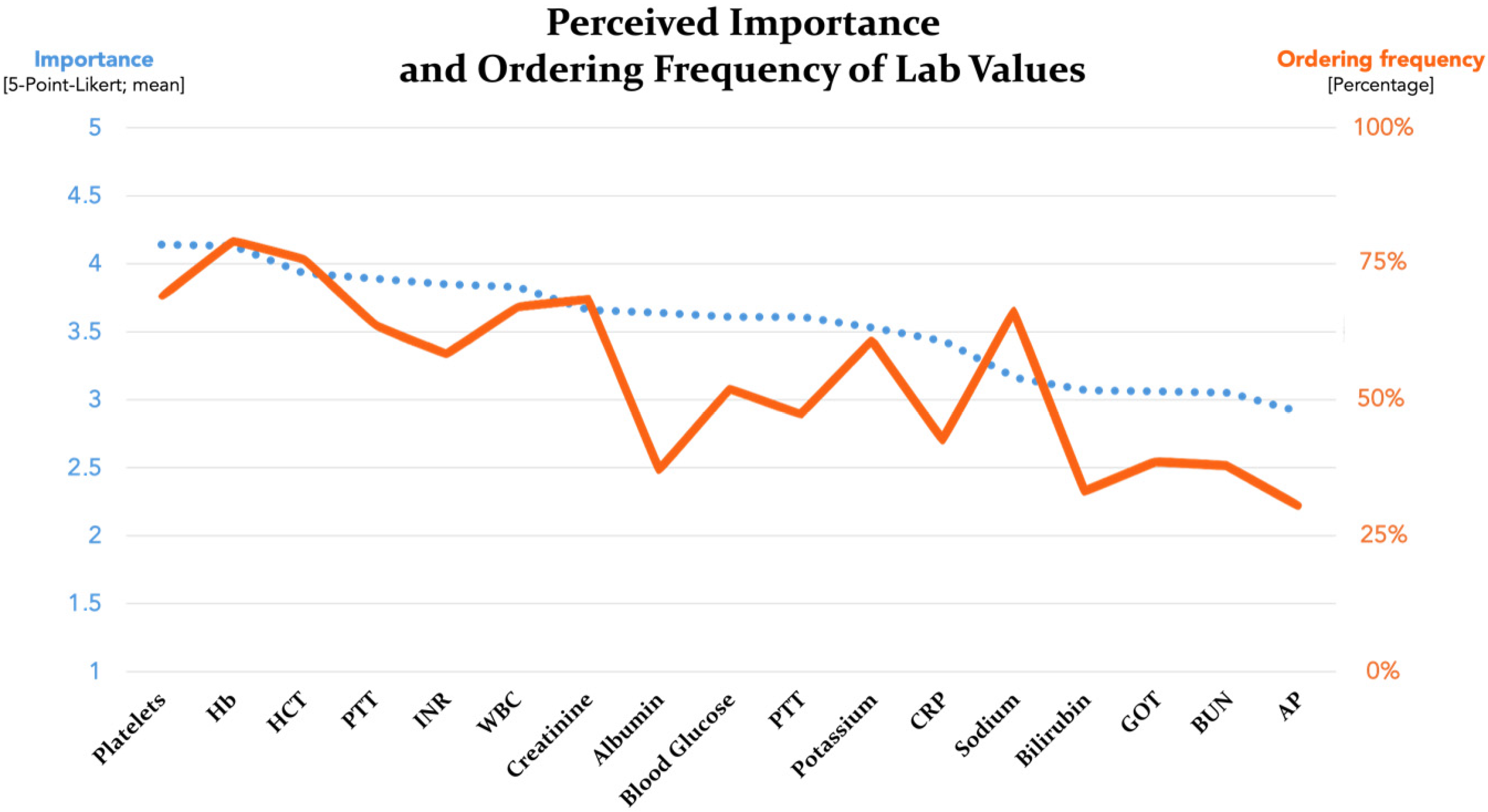
| Percentage of Study Participants Routinely Ordering the Laboratory Value | Perceived Importance of Laboratory Value for Preoperative Risk Stratification | ||
|---|---|---|---|
| Hb | 80.0% (112) | Platelets | 4.14 (1.06) |
| HCT | 76.4% (107) | Hb | 4.13 (1.08) |
| Platelets | 69.3% (97) | HCT | 3.93 (1.04) |
| Creatinine | 68.6% (96) | PTT | 3.89 (1.13) |
| WBC | 67.1% (94) | INR | 3.85 (1.16) |
| Sodium | 66.4% (93) | WBC | 3.83 (1.04) |
| PTT | 63.6% (89) | Creatinine | 3.66 (1.09) |
| Potassium | 60.7% (85) | Albumin | 3.64 (1.09) |
| INR | 57.9% (81) | Blood glucose | 3.61 (1.14) |
| Blood glucose | 51.4% (72) | PT | 3.61 (1.14) |
| PT | 46.4% (65) | Potassium | 3.53 (1.08) |
| CRP | 41.4% (58) | CRP | 3.43 (1.24) |
| GOT | 37.1% (52) | Sodium | 3.16 (1.08) |
| BUN | 36.4% (51) | Bilirubin | 3.07 (1.10) |
| Albumin | 35.7% (50) | GOT | 3.06 (1.10) |
| Bilirubin | 31.4% (44) | BUN | 3.05 (1.04) |
| AP | 28.6% (40) | AP | 2.91 (1.08) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfertshofer, M.; Kempa-Timler, J.; Moellhoff, N.; Knoedler, S.; Mert, S.; Knoedler, L.; Machens, H.-G.; Broer, P.N.; Hartmann, R.; Kasielska-Trojan, A.; et al. Cutting Risk, Not Just Skin—An International Survey on the Role of Preoperative Lab Values in Risk Stratification for Plastic and Reconstructive Surgery. J. Clin. Med. 2025, 14, 7686. https://doi.org/10.3390/jcm14217686
Alfertshofer M, Kempa-Timler J, Moellhoff N, Knoedler S, Mert S, Knoedler L, Machens H-G, Broer PN, Hartmann R, Kasielska-Trojan A, et al. Cutting Risk, Not Just Skin—An International Survey on the Role of Preoperative Lab Values in Risk Stratification for Plastic and Reconstructive Surgery. Journal of Clinical Medicine. 2025; 14(21):7686. https://doi.org/10.3390/jcm14217686
Chicago/Turabian StyleAlfertshofer, Michael, Joanna Kempa-Timler, Nicholas Moellhoff, Samuel Knoedler, Sinan Mert, Leonard Knoedler, Hans-Günther Machens, P. Niclas Broer, Robin Hartmann, Anna Kasielska-Trojan, and et al. 2025. "Cutting Risk, Not Just Skin—An International Survey on the Role of Preoperative Lab Values in Risk Stratification for Plastic and Reconstructive Surgery" Journal of Clinical Medicine 14, no. 21: 7686. https://doi.org/10.3390/jcm14217686
APA StyleAlfertshofer, M., Kempa-Timler, J., Moellhoff, N., Knoedler, S., Mert, S., Knoedler, L., Machens, H.-G., Broer, P. N., Hartmann, R., Kasielska-Trojan, A., Heiland, M., Koerdt, S., & Moog, P. (2025). Cutting Risk, Not Just Skin—An International Survey on the Role of Preoperative Lab Values in Risk Stratification for Plastic and Reconstructive Surgery. Journal of Clinical Medicine, 14(21), 7686. https://doi.org/10.3390/jcm14217686










