Topical Application of SVF/PRF in Thermal Injuries—A Retrospective Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Selection
2.2. Debridement, Production, and Application of SVF and PRF
2.3. Outcome Assessment
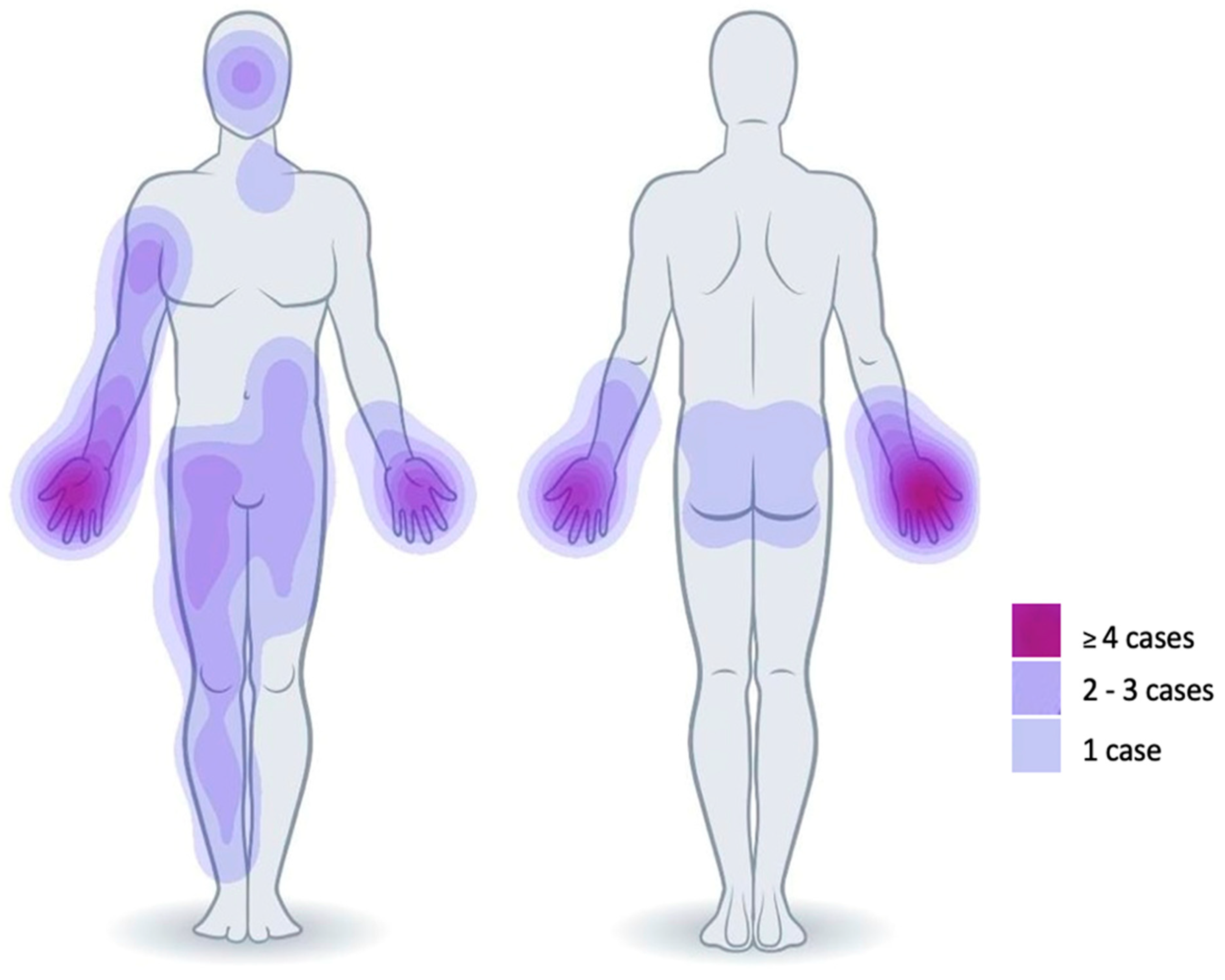
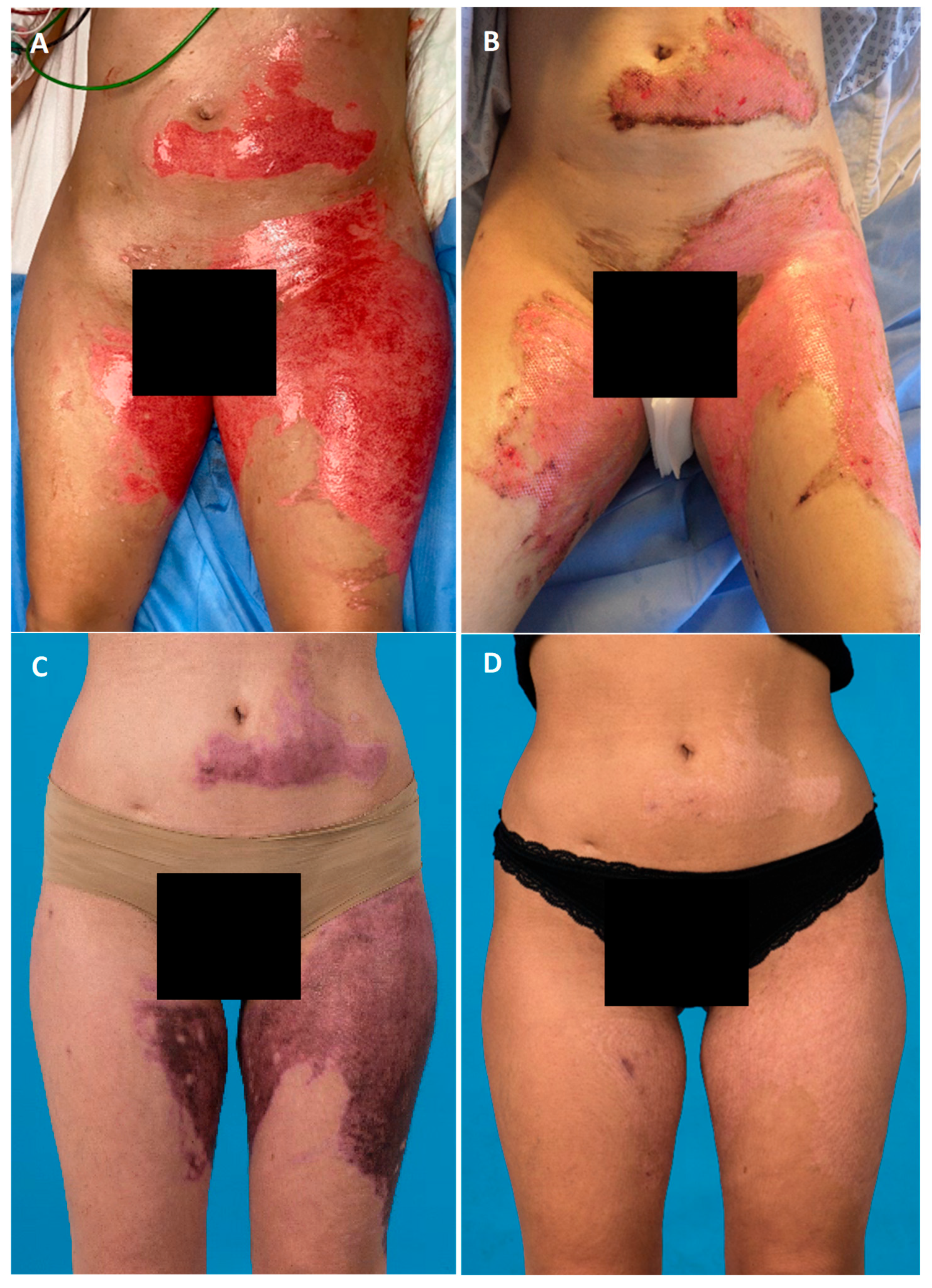
3. Results
3.1. Patients
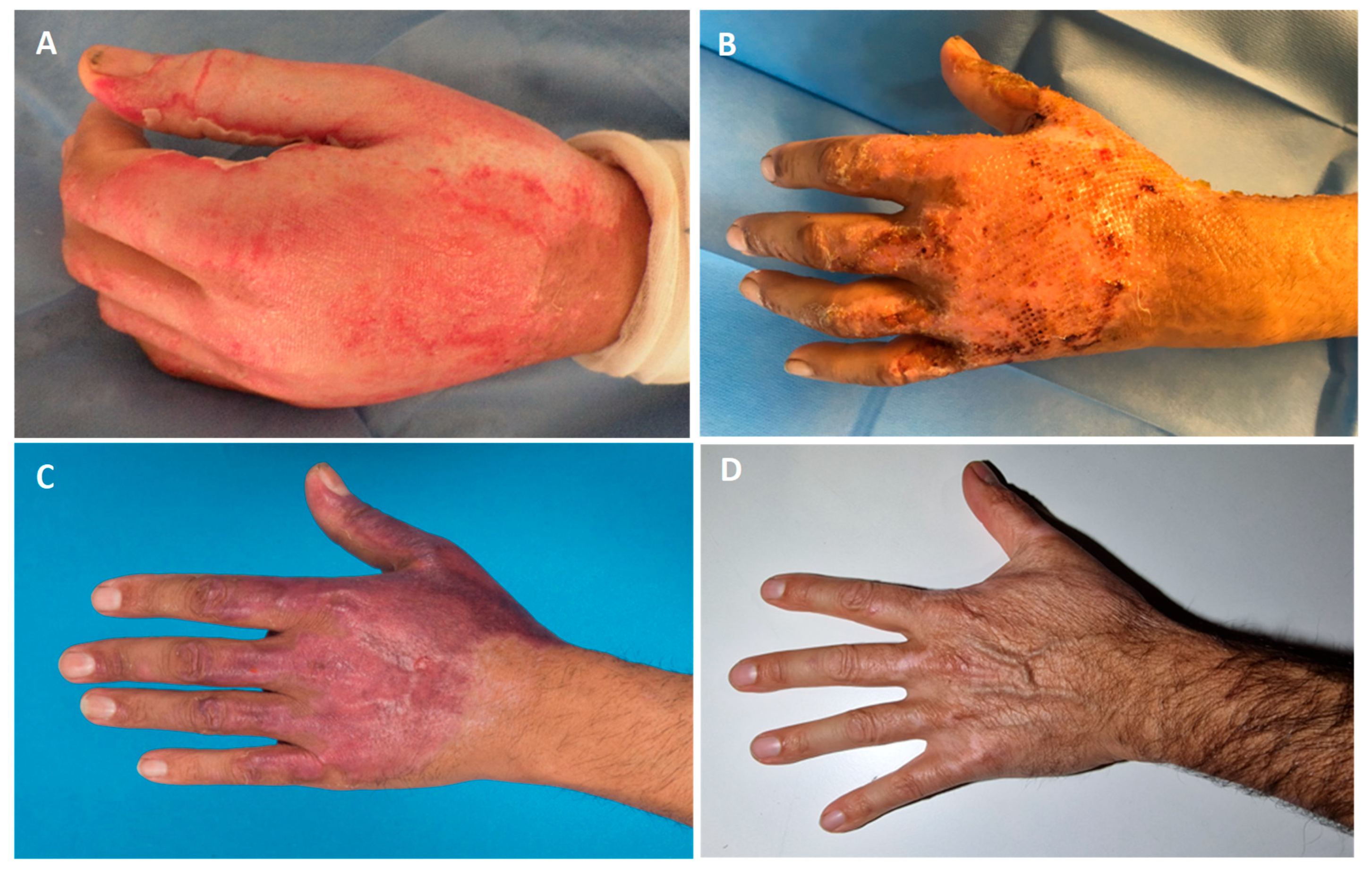
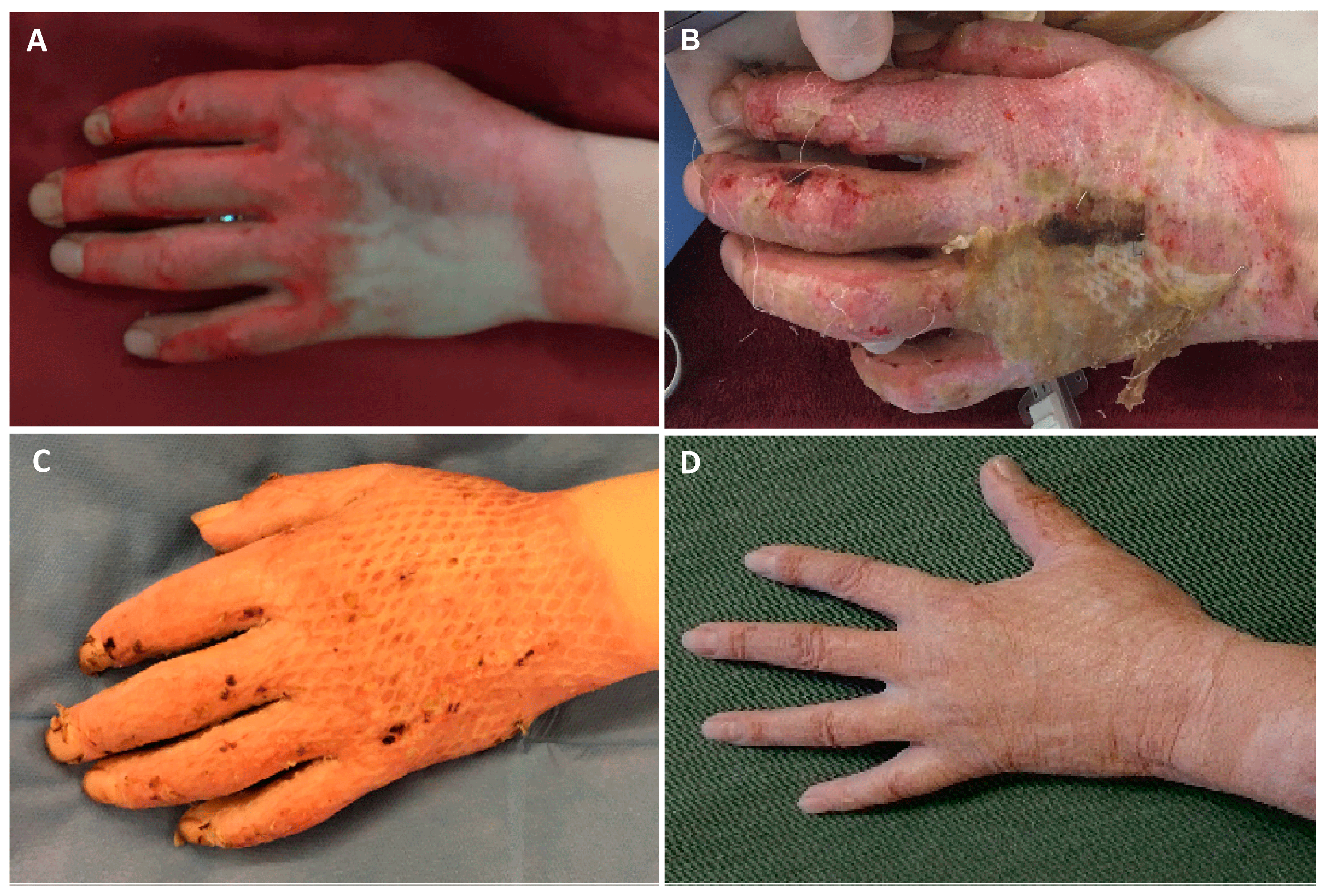
3.2. Debridement and Treatment with SVF and PRF
3.3. Residual Defects and Time-to-Heal
3.4. Hospitalization Time
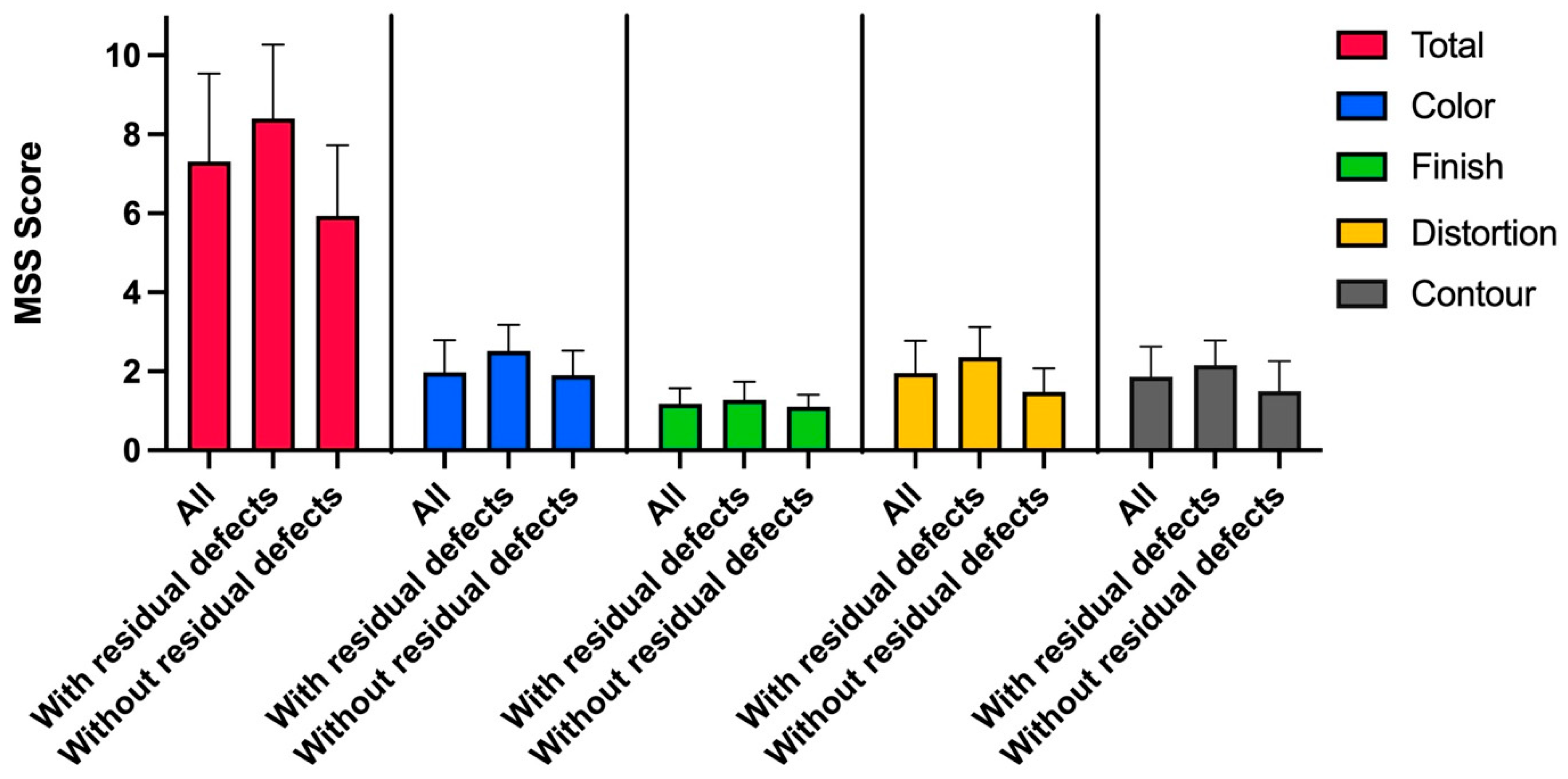
3.5. Aesthetic Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rennekampff, H.O.; Kremer, T. [Surgical management of burn injury patients: Comments on the guidelines on treatment of thermal injuries in adults]. Unfallchirurgie 2023, 127, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Bolton, L. Burn Debridement: Are We Optimizing Outcomes? Wounds 2019, 31, 298–300. [Google Scholar] [PubMed]
- Balakrishnan, C.; Hashim, M.; Gao, D. The effect of partial-thickness facial burns on social functioning. J. Burn. Care Rehabil. 1999, 20, 224–225. [Google Scholar] [CrossRef] [PubMed]
- Braza, M.E.; Fahrenkopf, M.P. Split-Thickness Skin Grafts. In StatPearls; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kearney, L.; Francis, E.C.; Clover, A.J. New technologies in global burn care—A review of recent advances. Int. J. Burns Trauma 2018, 8, 77–87. [Google Scholar]
- Guillamat-Prats, R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells 2021, 10, 1729. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef]
- Sorice, S.; Rustad, K.C.; Li, A.Y.; Gurtner, G.C. The Role of Stem Cell Therapeutics in Wound Healing: Current Understanding and Future Directions. Plast. Reconstr. Surg. 2016, 138 (Suppl. 3), 31s–41s. [Google Scholar] [CrossRef]
- Fakiha, K. Adipose stromal vascular fraction: A promising treatment for severe burn injury. Hum. Cell 2022, 35, 1323–1337. [Google Scholar] [CrossRef]
- Ichim, T.E.; Harman, R.J.; Min, W.-P.; Minev, B.; Solano, F.; Rodriguez, J.P.; Alexandrescu, D.T.; De Necochea-Campion, R.; Hu, X.; Marleau, A.M.; et al. Autologous stromal vascular fraction cells: A tool for facilitating tolerance in rheumatic disease. Cell Immunol. 2010, 264, 7–17. [Google Scholar] [CrossRef]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef]
- González, M.A.; Gonzalez-Rey, E.; Rico, L.; Büscher, D.; Delgado, M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009, 60, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. Concise Review: The Use of Adipose-Derived Stromal Vascular Fraction Cells and Platelet Rich Plasma in Regenerative Plastic Surgery. Stem Cells 2017, 35, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Trandafilovic, M.; Stojanovic, P. Platelet-rich fibrin: Basics of biological actions and protocol modifications. Open Med. 2021, 16, 446–454. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng. Part. B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef]
- Ratajczak, J.; Vangansewinkel, T.; Gervois, P.; Merckx, G.; Hilkens, P.; Quirynen, M.; Lambrichts, I.; Bronckaers, A. Angiogenic Properties of ‘Leukocyte- and Platelet-Rich Fibrin’. Sci. Rep. 2018, 8, 14632. [Google Scholar] [CrossRef]
- Tunalı, M.; Özdemir, H.; Küçükodacı, Z.; Akman, S.; Fıratlı, E. In vivo evaluation of titanium-prepared platelet-rich fibrin (T-PRF): A new platelet concentrate. Br. J. Oral. Maxillofac. Surg. 2013, 51, 438–443. [Google Scholar] [CrossRef]
- Yoshino, Y.; Ohtsuka, M.; Kawaguchi, M.; Sakai, K.; Hashimoto, A.; Hayashi, M.; Madokoro, N.; Asano, Y.; Abe, M.; Ishii, T.; et al. The wound/burn guidelines—6: Guidelines for the management of burns. J. Dermatol. 2016, 43, 989–1010. [Google Scholar] [CrossRef]
- Brown, J.C.; Shang, H.; Li, Y.; Yang, N.; Patel, N.; Katz, A.J. Isolation of Adipose-Derived Stromal Vascular Fraction Cells Using a Novel Point-of-Care Device: Cell Characterization and Review of the Literature. Tissue Eng. Part. C Methods 2017, 23, 125–135. [Google Scholar] [CrossRef]
- Beausang, E.; Floyd, H.; Dunn, K.W.; Orton, C.I.; Ferguson, M.W.J. A new quantitative scale for clinical scar assessment. Plast. Reconstr. Surg. 1998, 102, 1954–1961. [Google Scholar] [CrossRef]
- Chae, D.S.; Han, S.; Son, M.; Kim, S.-W. Stromal vascular fraction shows robust wound healing through high chemotactic and epithelialization property. Cytotherapy 2017, 19, 543–554. [Google Scholar] [CrossRef]
- Schulz, A.; Schiefer, J.; Fuchs, P.; Kanho, C.; Nourah, N.; Heitzmann, W. Does Platelet-Rich Fibrin Enhance Healing Of Burn Wounds? Our ofrst Experiences and Main Pitfalls. Ann. Burns Fire Disasters 2021, 34, 42–52. [Google Scholar] [PubMed]
- Lektemur Alpan, A.; Cin, G.T.; Kızıldağ, A.; Zavrak, N.; Özmen, Ö.; Arslan, Ş.; Mutlu, D. Evaluation of the effect of injectable platelet-rich fibrin (i-PRF) in wound healing and growth factor release in rats: A split-mouth study. Growth Factors 2024, 42, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Vorauer-Uhl, K.; Fürnschlief, E.; Wagner, A.; Ferko, B.; Katinger, H. Reepithelialization of experimental scalds effected by topically applied superoxide dismutase: Controlled animal studies. Wound Repair. Regen. 2002, 10, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Zor, F.; Ozturk, S.; Deveci, M.; Karacalioglu, O.; Sengezer, M. Saving the zone of stasis: Is glutathione effective? Burns 2005, 31, 972–976. [Google Scholar] [CrossRef]
- Eyuboglu, A.A.; Uysal, C.A.; Ozgun, G.; Coskun, E.; Ertas, N.M.; Haberal, M. The effect of adipose derived stromal vascular fraction on stasis zone in an experimental burn model. Burns 2018, 44, 386–396. [Google Scholar] [CrossRef]
- Yastı, A.Ç.; Akgün, A.E.; Akın, M. Use of stromal vascular fraction stem cell therapy for functional and cosmetic outcomes in a young female patient with deep dermal flame burns on the face. Burns Open 2022, 6, 116–119. [Google Scholar] [CrossRef]
- Giudice, G.; Filoni, A.; Maggio, G.; Bonamonte, D.; Maruccia, M.; Nacchiero, E.; Ribatti, D.; Annese, T.; Vestita, M. Use of the Stromal Vascular Fraction in Intermediate-Deep Acute Burns: A Case with Its Own Control. J. Burn. Care Res. 2018, 39, 846–849. [Google Scholar] [CrossRef]
- Jewell, L.; Guerrero, R.; Quesada, A.R.; Chan, L.S.; Garner, W.L. Rate of healing in skin-grafted burn wounds. Plast. Reconstr. Surg. 2007, 120, 451–456. [Google Scholar] [CrossRef]
- Di Lonardo, A.; Nardini, V.; De Rosa, M.; Pascone, C.; Graziano, A.; Criscuoli, A.M.; Ciappi, S. Enzymatic escharolysis with nexobrid® on partial thickness burn wounds: Pre- and post-debridement histological assessment. Ann. Burns Fire Disasters 2018, 31, 23–27. [Google Scholar]
- Li, A.; Yang, H.; Zhang, J.; Chen, S.; Wang, H.; Gao, Y. Additive effectiveness of autologous platelet-rich fibrin in the treatment of intrabony defects: A PRISMA-compliant meta-analysis. Medicine 2019, 98, e14759. [Google Scholar] [CrossRef]
- Shevchenko, S.N.; Rublenko, M. Histological Characteristics of Platelet-Rich Fibrin Clots Obtained Under Various Modes of Blood Centrifugation. Sci. Messenger Lnu Vet. Med. Biotechnol. 2020, 99, 84–93. [Google Scholar] [CrossRef]
- Diab, N.A.F.; Ibrahim, A.M.; Abdallah, A.M. Fluid Platelet-Rich Fibrin (PRF) Versus Platelet-Rich Plasma (PRP) in the Treatment of Atrophic Acne Scars: A Comparative Study. Arch. Dermatol. Res. 2023, 315, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Desai, C.B.; Mahindra, U.R.; Kini, Y.K.; Bakshi, M.K. Use of Platelet-Rich Fibrin over Skin Wounds: Modified Secondary Intention Healing. J. Cutan. Aesthet. Surg. 2013, 6, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2006, 101, e56–e60. [Google Scholar] [CrossRef]
- Josh, F.; Soekamto, T.H.; Adriani, J.R.; Jonatan, B.; Mizuno, H.; Faruk, M. The Combination of Stromal Vascular Fraction Cells and Platelet-Rich Plasma Reduces Malondialdehyde and Nitric Oxide Levels in Deep Dermal Burn Injury. J. Inflamm. Res. 2021, 14, 3049–3061. [Google Scholar] [CrossRef]
- Laidding, S.R.; Josh, F.; Battung, S.; Bukhari, A.; Warsinggih; Patellongi, I.J.; Massi, M.N.; Islam, A.A.; Dososaputro, I.; Faruk, M. Combination of platelet rich plasma and stromal vascular fraction on the level of vascular endothelial growth factor in rat subjects experiencing deep dermal burn injury. Ann. Med. Surg. 2021, 64, 102254. [Google Scholar] [CrossRef]
- Tiryaki, T.; Condé-Green, A.; Cohen, S.R.; Canikyan, S.B.; Kocak, P. A 3-step Mechanical Digestion Method to Harvest Adipose-derived Stromal Vascular Fraction. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2652. [Google Scholar] [CrossRef]
- Yaylacı, S.; Kaçaroğlu, D.; Hürkal, Ö.; Ulaşlı, A.M. An enzyme-free technique enables the isolation of a large number of adipose-derived stem cells at the bedside. Sci. Rep. 2023, 13, 8005. [Google Scholar] [CrossRef]
- Nguyen, A.; Guo, J.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 170–179. [Google Scholar] [CrossRef]

| Variable | Mean | SD |
|---|---|---|
| Age [years] | 45.3 | 13.5 |
| ABSI score | 6.5 | 3.5 |
| Total body surface area (TBSA) [%] | 29.6 | 25.8 |
| Body surface area (BSA) treated with SVF and PRF [%] | 6.3 | 4.7 |
| Time from trauma to initial debridement [days] | 1.6 | 0.7 |
| Time from initial debridement to SVF and PRF application [days] | 2.4 | 2.1 |
| Variable | Mean | SD |
|---|---|---|
| Patients with residual defects requiring further surgery [%] | 45 | - |
| BSA residual defects [%] | 6.6 | 6 |
| BSA residual defects/BSA-treated area in patients with residual defects [%] | 91 | - |
| Time-to-heal all patients [d] | 33 | 17.7 |
| Time-to-heal with residual defects [d] | 51 | 8.8 |
| Time-to-heal without residual defects [d] | 20 | 7.5 |
| Time-to-heal after residual defect coverage with STSG [d] | 13.4 | 4.6 |
| Patient | Age | Sex | Comorbidities | Burn Etiology | BSA | ABSI Score | A: BSA Treated with SVF and PRF B: Localization C: Burn Degree | Debridement of Area Treated with SVF and PRF | Time Trauma to Initial Debridement | Time Initial Debridement to SVF and PRF | Treatment | BSA of Residual Defects | Time SVF and PRF to Treatment of Residual Defects | Treatment of Residual Defects | Time-to-Heal | Hospitalization Time A: Total B: ICU C: Normal Ward | Follow-Up | Manchester Scar Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 41 | M | - | fire | Total: 65% SPTB: 15% DPTB: 46% FTB: 4% | 12 | A: 3% B: forearms/hands C: DPTB | enzymatic | 2 d | 2 d | SVF + PRF | 3% | 14 d | STSG | 57 d | A: 111 d B: 76 d C: 35 d | 66 m | 6 |
| 2 | 31 | F | Polytoxicomania | fire | Total: 36% SPTB: 2% DPTB: 11% FTB: 23% | 8 | A: 3% B: head C: SPTB/DPTB | manual (tangential) | 3 d | same day | SVF + PRF | 2% | 27 d | STSG | 60 d | A: 73 d B: 50 d C: 23 d | 30 m | 10 |
| 3 | 35 | M | Hypertonia | fire | Total: 26% SPTB: 5% DPTB: 10% FTB: 11% | 6 | A: 10% B: anterior torso C: DPTB/FTB | manual (epifascial) | 2 d | same day | SVF + PRF + STSG | - | - | - | 28 d | A: 39 d B: 29 d C: 10 d | n/a | n/a |
| 4 | 68 | M | Steatosis Hepatis | fire | Total: 18% SPTB: 16% DPTB: 2% | 6 | A: 4% B: hands C: DPTB | enzymatic | 1 d | 6 d | SVF + PRF | - | - | - | 25 d | A: 37 d B: 15 d C: 22 d | 15 m | 9 |
| 5 | 42 | M | - | fire | Total: 11.5% SPTB: 6.5% DPTB: 5% | 4 | A: 3% B: hand/upper arm C: SPTB/DPTB | enzymatic | 1 d | 6 d | SVF + PRF | - | - | - | 14 d | A: 16 d B: 0 d C: 16 d | 60 m | 4 |
| 6 | 52 | W | - | fire | Total: 17% SPTB: 2.5% DPTB: 5% FTB: 2.5% | 7 | A: 1% B: dorsal hand C: SPTB/DPTB | enzymatic | 1 d | 2 d | SVF + PRF | - | - | - | 20 d | A: 25 d B: 5 d C: 20 d | n/a | n/a |
| 7 | 51 | W | - | scald | Total: 13% SPTB: 2% DPTB: 11% | 5 | A: 11% B: gluteal area C: DPTB | enzymatic | 1 d | 4 d | SVF + PRF + STSG | 11% | 17 d | MEEK | 40 d | A: 46 d B: 33 d C: 13 d | 24 m | 8 |
| 8 | 54 | M | - | fire | Total: 11% SPTB: 4% DPTB: 7% | 3 | A: 3% B: hands/forearm C: SPTB/DPBT | enzymatic | 1 d | 2 d | SVF + PRF | 1% | 34 d | STSG | 50 d | A: 21 d B: 3 d C: 18 d | 24 m | 10 |
| 9 | 55 | W | Psoriasis | scald | Total: 6% SPTB: 4% DPTB: 2% | 3 | A: 3% B: hands, foot, neck C: SPTB/DPTB | enzymatic | 1 d | 3 d | SVF + PRF | - | - | - | 14 d | A: 10 d B: 0 d C: 10 d | 24 m | 6 |
| 10 | 35 | M | Polytoxicomania | scald | Total: 15% SPTB: 7% DPTB: 8% | 4 | A: 15% B: right leg, abdomen, forearm C. SPTB/DPTB | manual (tangential) | 3 d | same day | SVF + PRF | 15% | 11 d | STSG | n/a | A: 31 d B: 2 d C: 29 d | 45 m | 8 |
| 11 | 28 | W | - | scald | Total 15% SPTB: 10% DPTB: 5% | 3 | A: 15% B: thighs, abdomen C: SPTB/DPTB | enzymatic | 1 d | 4 d | SVF + PRF | - | - | - | 28 d | A: 12 d B: 0 d C: 12 d | 30 m | 5 |
| 12 | 30 | W | Depression | fire | Total: 85% SPTB: 10% DPTB: 25% FTB 50% | 13 | A: 3% B: face C: DPTB | enzymatic | 2 d | 3 d | SVF + PRF | n/a | n/a | n/a | n/a | Death after 11 d | n/a | n/a |
| 13 | 67 | M | - | fire | Total: 65% DPTB: 35% FTB: 30% | 11 | A: 4% B: right arm C: DPTB | manual (tangential) | 2 d | same day | SVF + PRF + STSG | n/a | n/a | n/a | n/a | Death after 19 d | n/a | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naef, L.; Vasella, M.; Watson, J.; Reid, G.; Breckwoldt, T.; Waldner, M.; Hofmann, L.; Pais, M.-A.; Buehler, P.; Plock, J.A.; et al. Topical Application of SVF/PRF in Thermal Injuries—A Retrospective Analysis. J. Clin. Med. 2025, 14, 4710. https://doi.org/10.3390/jcm14134710
Naef L, Vasella M, Watson J, Reid G, Breckwoldt T, Waldner M, Hofmann L, Pais M-A, Buehler P, Plock JA, et al. Topical Application of SVF/PRF in Thermal Injuries—A Retrospective Analysis. Journal of Clinical Medicine. 2025; 14(13):4710. https://doi.org/10.3390/jcm14134710
Chicago/Turabian StyleNaef, Lukas, Mauro Vasella, Jennifer Watson, Gregory Reid, Tabea Breckwoldt, Matthias Waldner, Luzie Hofmann, Michael-Alexander Pais, Philipp Buehler, Jan Alexander Plock, and et al. 2025. "Topical Application of SVF/PRF in Thermal Injuries—A Retrospective Analysis" Journal of Clinical Medicine 14, no. 13: 4710. https://doi.org/10.3390/jcm14134710
APA StyleNaef, L., Vasella, M., Watson, J., Reid, G., Breckwoldt, T., Waldner, M., Hofmann, L., Pais, M.-A., Buehler, P., Plock, J. A., & Kim, B.-S. (2025). Topical Application of SVF/PRF in Thermal Injuries—A Retrospective Analysis. Journal of Clinical Medicine, 14(13), 4710. https://doi.org/10.3390/jcm14134710







