Abstract
Background and Objectives: People living with HIV (PLWH) have excess fragility fractures not fully explained by areal DXA. We reviewed bone “quality” in PLWH—microarchitecture, estimated strength, tissue-level properties—and vertebral fractures (VFs). Methods: PRISMA-conform systematic review (2000–2025) of randomized, cohort, and cross-sectional studies assessing HR-pQCT (±finite-element analysis), trabecular bone score (TBS), impact microindentation (BMSi), femoral QCT/MRI, and VF imaging (DXA-VFA or radiography). Risk of bias used ROBINS-I (non-randomized) and RoB 2 (randomized/switch). No meta-analysis was performed due to clinical/methodological heterogeneity; evidence was synthesized narratively per SWiM. Results: Fourteen studies met criteria. HR-pQCT showed cortical/trabecular deficits with lower finite-element–estimated strength in PLWH. BMSi was 3–4 units lower; it declined after ART initiation but improved after TDF→TAF switch. TBS was modestly lower and reclassified risk when BMD was non-osteoporotic. VF prevalence was 12–25% and frequently occurred at non-osteoporotic BMD. Signals aligned with modifiable risks (smoking, glucocorticoids) and specific ART exposures. Conclusions: Beyond DXA, PLWH exhibit quantifiable decrements in microarchitecture, estimated strength, and tissue-level properties alongside a meaningful VF burden. TBS and VFA are pragmatic, scalable adjuncts to refine risk; HR-pQCT/BMSi add mechanistic value in research/tertiary settings. Prospective studies linking these metrics to incident fractures are warranted.
1. Introduction
Fracture burden remains disproportionately high among people living with HIV (PLWH), even in the era of durable virologic suppression, and is only partially explained by areal bone mineral density (BMD) on DXA [1]. Contemporary guidance emphasizes systematic screening and risk modification in HIV care, reflecting both HIV-related and treatment-related skeletal liabilities [2]. In randomized evidence from the START BMD substudy, immediate antiretroviral therapy (ART) initiation accelerated BMD loss at hip and spine versus deferred therapy, highlighting a treatment-onset effect superimposed on background vulnerability [3]. Longer-term follow-up indicates that this early decline attenuates after the first 1–2 years, but does not eliminate the elevated fragility signal seen epidemiologically [4].
Therapeutic choices also matter. Multiple switch trials demonstrate skeletal safety advantages when replacing tenofovir disoproxil fumarate (TDF) with tenofovir alafenamide (TAF), with improvements in hip and spine BMD and tubular markers in diverse populations, including older adults and those with renal impairment [5,6,7]. These observations reinforce the need to look beyond areal BMD to capture dimensions of bone quality that may mediate residual fracture risk despite ART optimization.
Microstructure-oriented metrics offer that lens. Trabecular bone score (TBS)—a texture index derived from lumbar DXA—predicts osteoporotic fractures independently of BMD and can refine FRAX probabilities in general populations [8,9]. High-resolution peripheral quantitative CT (HR-pQCT) provides in vivo “virtual biopsy” of distal radius/tibia, quantifying trabecular number, separation, and cortical thickness; prospective cohorts show that HR-pQCT microarchitecture and estimated failure load independently forecast incident fractures beyond DXA [10,11]. Translating these insights into HIV cohorts, emerging data indicate that PLWH exhibit lower TBS than matched controls and that TBS can prognosticate vertebral fractures, suggesting a clinically actionable signal not captured by BMD alone [12].
Guideline frameworks are beginning to acknowledge these nuances. The European AIDS Clinical Society (EACS) v12.0 update integrates broader musculoskeletal risk assessment and supports judicious DXA use alongside ART selections that minimize bone and renal toxicity [13]. Still, a key evidence gap persists: standardized synthesis of HIV-specific findings from advanced phenotyping, TBS, HR-pQCT, femoral QCT/MRI, and tissue-level measures, linking them to fractures and modifiable drivers (TDF/TAF exposure, protease inhibitor duration, inflammation). Moreover, early ART-associated bone loss, well documented across trials, invites evaluation of whether microstructure/quality measures better capture recovery or persistent deficits than BMD alone [14].
Beyond epidemiology, sex and gender shape musculoskeletal biology and orthopedic outcomes through differences in bone/cartilage/ligament physiology, hormonal milieus, injury patterns, and care pathways. A recent narrative overview in orthopedics synthesizes these sex- and gender-specific mechanisms and highlights implications for personalized care—including the needs of sexual and gender minority patients and potential effects of gender-affirming hormones—reinforcing that sex/gender should be prespecified and reported in HIV skeletal phenotyping [11,12,13,14,15].
We hypothesized that PLWH exhibit measurable deficits in cortical and trabecular microarchitecture and in tissue-level mechanical properties beyond those detectable by areal DXA. Our objective was to synthesize modality-specific evidence (HR-pQCT with FE-strength, TBS, BMSi, femoral QCT/MRI, and vertebral fractures) and relate these to ART exposures and conventional risks.
2. Materials and Methods
2.1. Protocol, Registration, and Reporting Standards
This review was preregistered on the Open Science Framework (OSF vxkau) prior to screening; any protocol deviations (e.g., inclusion of femoral QCT/MRI encountered during scoping) were logged. The review adheres to PRISMA items on title/abstract, rationale, objectives, eligibility, information sources, search, selection, data items, risk of bias, effect measures, synthesis methods, and certainty assessment, and the PRISMA checklist and flow diagram will be provided in the supplements.
2.2. Research Question and PICO Statement
The research question was framed to evaluate bone “quality” in people living with HIV (PLWH) beyond areal BMD. The population comprised PLWH of any age, sex, treatment status, or care setting. The index exposures were HIV infection and antiretroviral therapy (ART) characteristics assessed with bone quality modalities beyond areal DXA, namely HR-pQCT with or without finite-element analysis, trabecular bone score (TBS), impact microindentation yielding Bone Material Strength index (BMSi), femoral QCT, and MRI-based microarchitectural or marrow adiposity measures, as well as vertebral fracture imaging by VFA or standard radiography. Comparators were HIV-negative controls, within-person pre/post contrasts around ART initiation or drug switches (e.g., TDF to TAF), or HIV subgroups such as immunologic responders versus non-responders. Primary outcomes were microarchitectural and material properties, including cortical thickness, trabecular number and separation, volumetric BMD, estimated failure load, TBS, BMSi, and femoral QCT/MRI metrics and marrow adiposity; secondary outcomes were prevalent or incident fragility fractures—especially vertebral fractures—and associations with ART classes, inflammatory indices, immune recovery, and conventional DXA where co-reported.
2.3. Eligibility Criteria
Eligible designs included randomized or quasi-experimental trials, prospective or retrospective cohorts, and cross-sectional studies with original quantitative data. We excluded case reports, case series with fewer than 10 participants, narrative reviews, editorials, and conference abstracts without extractable data. Studies had to include PLWH and report at least one bone quality endpoint beyond areal DXA (DXA-only studies without TBS were excluded because our aim was to synthesize quality-oriented metrics beyond areal BMD). No language restrictions were imposed, the time window spanned 1 January 2000 through 1 August 2025, and non-English full texts were translated when feasible. Full-text exclusions were recorded with explicit reasons, including absence of a bone quality endpoint, insufficient data, or overlap with a more complete publication. Vertebral fracture (VF) studies using VFA (DXA-based) were synthesized separately from standard radiography; definitions and thresholds were recorded.
2.4. Information Sources
Primary information sources were PubMed/MEDLINE, Web of Science Core Collection (Science Citation Index Expanded, Social Sciences Citation Index, Emerging Sources Citation Index), and Scopus. To enhance completeness, we performed backward citation chasing of all included articles, forward citation tracking in Google Scholar, hand-searched key journals in HIV and skeletal research (AIDS, HIV Medicine, JAIDS, Journal of Bone and Mineral Research, Bone, Osteoporosis International), and screened trial registries (ClinicalTrials.gov and EU-CTR) for relevant completed studies with published bone quality endpoints. Search strategies were PRESS-checked to balance sensitivity and specificity across platforms.
2.5. Search Strategy
Searches combined controlled vocabulary and free-text terms for HIV and bone quality modalities, limiting only by publication date and excluding non-human studies where supported. In PubMed/MEDLINE, we used the following string executed on 23 September 2025: “(“HIV”[Mesh] OR “HIV Infections”[Mesh] OR HIV[tiab] OR “human immunodeficiency virus”[tiab]) AND (“high-resolution peripheral quantitative computed tomography”[tiab] OR HR-pQCT[tiab] OR “trabecular bone score”[tiab] OR TBS[tiab] OR microindentation[tiab] OR “bone material strength”[tiab] OR BMSi[tiab] OR “finite element”[tiab] OR FEA[tiab] OR “quantitative computed tomography”[tiab] OR QCT[tiab] OR “magnetic resonance imaging”[tiab] OR MRI[tiab] OR microarchitectur*[tiab] OR micro-architectur*[tiab] OR “bone marrow adiposity”[tiab] OR “marrow fat”[tiab] OR “vertebral fracture assessment”[tiab] OR VFA[tiab]) AND (bone[tiab] OR skeletal[tiab] OR cortical[tiab] OR trabecular[tiab]) NOT (animals[mh] NOT humans[mh]) AND (“1 January 2000”[Date—Publication]: “23 September 2025”[Date—Publication])”. In the Web of Science Core Collection, the topic query was “TS = ((HIV OR “human immunodeficiency virus”) AND (“high-resolution peripheral quantitative computed tomography” OR HR-pQCT OR “trabecular bone score” OR TBS OR microindentation OR “bone material strength” OR BMSi OR “finite element” OR FEA OR “quantitative computed tomography” OR QCT OR “magnetic resonance imaging” OR MRI OR microarchitectur* OR “bone marrow adiposity” OR “marrow fat” OR “vertebral fracture assessment” OR VFA) AND (bone OR skeletal OR cortical OR trabecular))”, with a timespan of 2000–2025, indexes SCI-EXPANDED, SSCI, ESCI, and document type Article. In Scopus, the TITLE-ABS-KEY string was “TITLE-ABS-KEY((HIV OR “human immunodeficiency virus”) AND (“high-resolution peripheral quantitative computed tomography” OR HR-pQCT OR “trabecular bone score” OR TBS OR microindentation OR “bone material strength” OR BMSi OR “finite element” OR FEA OR “quantitative computed tomography” OR QCT OR “magnetic AND resonance AND imaging” OR MRI OR microarchitectur* OR “bone marrow adiposity” OR “marrow fat” OR “vertebral fracture assessment” OR VFA) AND (bone OR skeletal OR cortical OR trabecular)) AND (PUBYEAR > 1999 AND PUBYEAR < 2026) AND DOCTYPE(ar)”. Synonyms and hyphenation variants were tested and retained when they improved recall without excessive noise.
2.6. Study Selection and PRISMA Flow
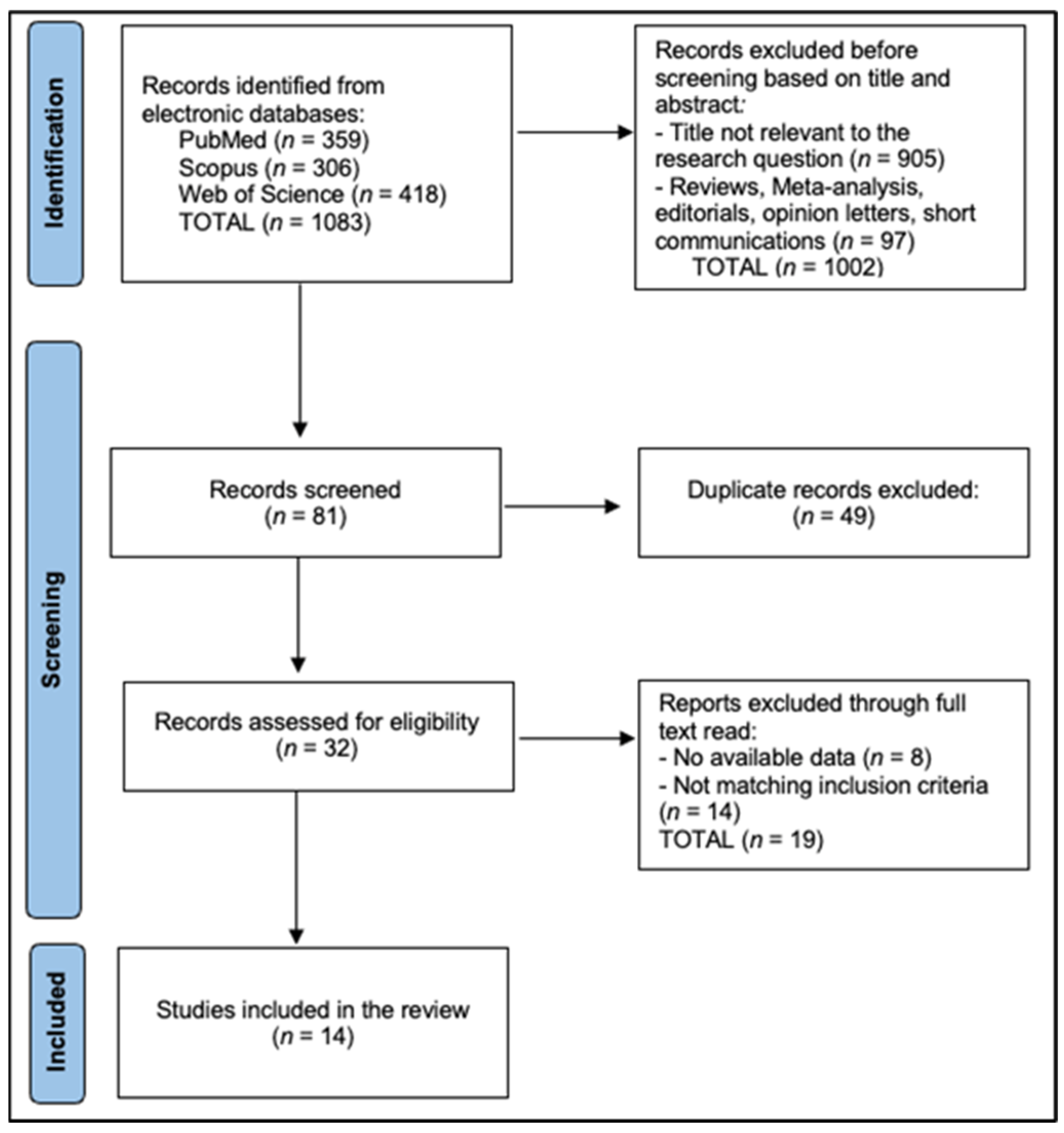
All records were exported and de-duplicated in EndNote X20 using DOI, title, author, and journal fields with manual verification of near duplicates, then imported to Rayyan QCRI for blinded screening. Two reviewers independently screened titles/abstracts and full texts and independently extracted data (two extractors). Inter-rater agreement was quantified with Cohen’s κ: κ_title/abstract = 0.82 and κ_full-text = 0.86. The reviewers recorded standardized exclusion reasons at full text (DXA-only without TBS, no bone quality endpoint, non-original study, insufficient extractable data, or overlapping cohort superseded elsewhere). Disagreements were resolved by consensus or third-reviewer arbitration. The PRISMA flow diagram is described in Figure 1.
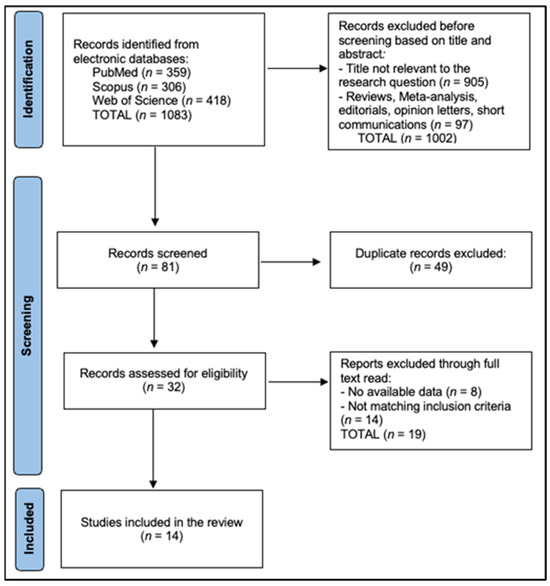
Figure 1.
PRISMA Flowchart Diagram.
2.7. Data Items and Extraction Procedures
Extraction used a calibrated, pilot-tested template capturing study design; setting and country; sample size and participant characteristics; HIV and ART history with emphasis on TDF, TAF, and protease inhibitor exposure; immune and inflammatory markers including nadir and current CD4 counts and viral suppression; bone quality modality and device generation, acquisition sites, and analysis pipelines including finite-element parameters and TBS software version; outcomes including cortical thickness, trabecular number and separation, volumetric BMD, estimated failure load, TBS values, BMSi units, femoral QCT/MRI microstructure and marrow adiposity; vertebral fracture definitions and ascertainment; and statistical adjustments. Corresponding authors were contacted when critical numeric endpoints were missing. When multiple articles reported the same cohort, the most complete dataset or longest follow-up was prioritized.
For HR-pQCT harmonization, we extracted device generation (XtremeCT vs. XtremeCT II), nominal voxel size (measured in µm), in-line vs. manufacturer FE solvers, and segmentation approaches. To enhance comparability, between-group differences were expressed as percent change where possible and synthesized by skeletal site (distal radius vs. tibia) and device generation. Findings mixing generations are marked in tables/captions.
2.8. Risk of Bias Assessment
Risk of bias was appraised independently by two reviewers at the outcome level. Non-randomized studies were assessed with ROBINS-I across confounding, selection, exposure classification, deviations, missing data, outcome measurement, and selective reporting. Randomized or randomized-like comparisons (e.g., drug-switch trials) were evaluated with RoB 2. Judgments and rationales were harmonized by discussion with third-reviewer adjudication when needed.
Because of heterogeneity and limited comparable metrics, we applied a narrative synthesis consistent with SWiM guidance. During synthesis, outcomes judged to have serious RoB (ROBINS-I) or high RoB (RoB 2) were down-weighted: effect directions were reported but not allowed to dominate conclusions; where mixed-quality evidence informed a statement, we explicitly flagged certainty and downgraded in GRADE for risk of bias, as presented in Table 1 and Table 2 [16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Across non-randomized comparisons (ROBINS-I), most studies were moderate risk; three were serious for confounding and/or selection; randomized/switch trials were generally low to some concerns (RoB 2). A domain-level summary is provided in Table 1 (ROBINS-I) and Table 2 (RoB 2).

Table 1.
ROBINS-I summary (non-randomized studies; n = 13).

Table 2.
RoB 2 summary (randomized/switch evidence; n = 1).
2.9. Synthesis Without Meta-Analysis
Due to heterogeneity across study designs, populations, skeletal sites, imaging platforms (HR-pQCT vendors/generations), outcome definitions, and incomplete variance reporting, we prespecified a narrative synthesis per SWiM guidance. We grouped results by modality (HR-pQCT/FE, BMSi, TBS, femoral QCT/MRI, VFs), expressed effects as absolute or percent differences where extractable, and summarized consistency in direction and approximate magnitude. When numeric values were not tabulated, we abstracted effect sizes from figures or text and, where necessary, converted them to percent differences to improve comparability. Where risk of bias was serious/high or inconsistency was marked, we qualified conclusions and reflected this in certainty statements.
3. Results
Across 14 studies [16,17,18,19,20,21,22,23,24,25,26,27,28,29] from Europe, North America, and China, cohorts were sizable and modality diverse, enabling triangulation of bone quality in PLWH beyond DXA. HR-pQCT studies consistently enrolled balanced PLWH/control samples: Calmy (92 vs. 95 premenopausal women; radius/tibia) [16], Biver (70 vs. 61 elderly men; radius/tibia) [17], Macdonald (103 vs. 102 women; radius/tibia) [18], and Foreman (103 vs. 77 mixed adults; radius/tibia) [19]. Tissue-level microindentation (BMSi) was examined in cross-sectional and longitudinal Spanish cohorts—Güerri-Fernández (85 vs. 79; tibia) [20], Lerma-Chippirraz (44 starting ART; tibia) [21], Soldado-Folgado 2022 (59 switching TDF→TAF; tibia) [22], Soldado-Folgado 2023 (85 vs. 60; tibia) [23], and Rins-Lozano (82 immunologic responders vs. non-responders; tibia) [24]. Texture-based TBS studies included McGinty (201 PLWH vs. 262 controls; lumbar DXA) [25], Sharma (319 PLWH women vs. 118 controls) [26], and Guan (233 ART-naïve followed 48 weeks post-ART) [27]. Vertebral fracture imaging cohorts (without non-HIV controls) were substantial—Llop (n = 199, ≥50 years) [28] and Gazzola (n = 194) [29]. ART exposure was long-standing in HR-pQCT cohorts [16,17,18,19], BMSi cohorts captured ART initiation or TDF→TAF switching [21,22,23], and TBS cohorts spanned ART-naïve through long-term therapy [25,26,27], collectively supporting evaluation of modality-specific sensitivity to HIV/ART factors (Table 3).

Table 3.
Study characteristics (populations, modality, skeletal site).
Microarchitecture and tissue-level quality were consistently worse in PLWH versus controls or reference groups. In HR-pQCT, PLWH showed a lower estimated failure load with trabecular thinning and cortical deficits; Calmy reported ~5–15% decrements across Tb.N, Tb.Sp, and Ct.Th with reduced FEA-derived strength [16], corroborated directionally by Biver in elderly men on long-term ART [17], Macdonald in mid-life women (reduced failure load; cortical deficits linked to TDF history) [18], and Foreman (lower failure load/stiffness associated with PI exposure and smoking) [19]. Tissue-level BMSi was lower in PLWH: Güerri-Fernández 77.2 ± 6.9 vs. 80.6 ± 6.2 (Δ = −3.4; p < 0.01) [20]; Soldado-Folgado (2023) 78.4 ± 7.1 vs. 82.0 ± 6.4 (Δ = −3.6; p < 0.01) [23]; and immunologic non-responders in Rins-Lozano 76.7 ± 6.3 vs. 80.2 ± 6.1 (Δ = −3.5; p = 0.001) [24]. Longitudinally, BMSi declined after ART initiation (−2.1 units by ~6–12 months; p = 0.02) [21] but improved after switching from TDF to TAF (+2.5 units at ~6–12 months; p < 0.05) [22]. TBS differences were modest but significant: McGinty median 1.349 [1.263–1.436] vs. 1.380 [1.301–1.453] (unadjusted Δ = −0.031; p = 0.009; adjusted β for HIV = −0.037; p = 0.002) [25]; Sharma observed a 64% higher adjusted prevalence of degraded TBS (<1.35) among HIV-positive women [26]. Vertebral fracture burden remained notable despite often non-osteoporotic BMD—Llop reported ~25% subclinical VFs in older PLWH [28], and Gazzola found 12.4% with predictors including age (aOR 1.09/year) and steroids (aOR 3.64) [29], as seen in Table 4.

Table 4.
Microarchitecture and Tissue Quality.
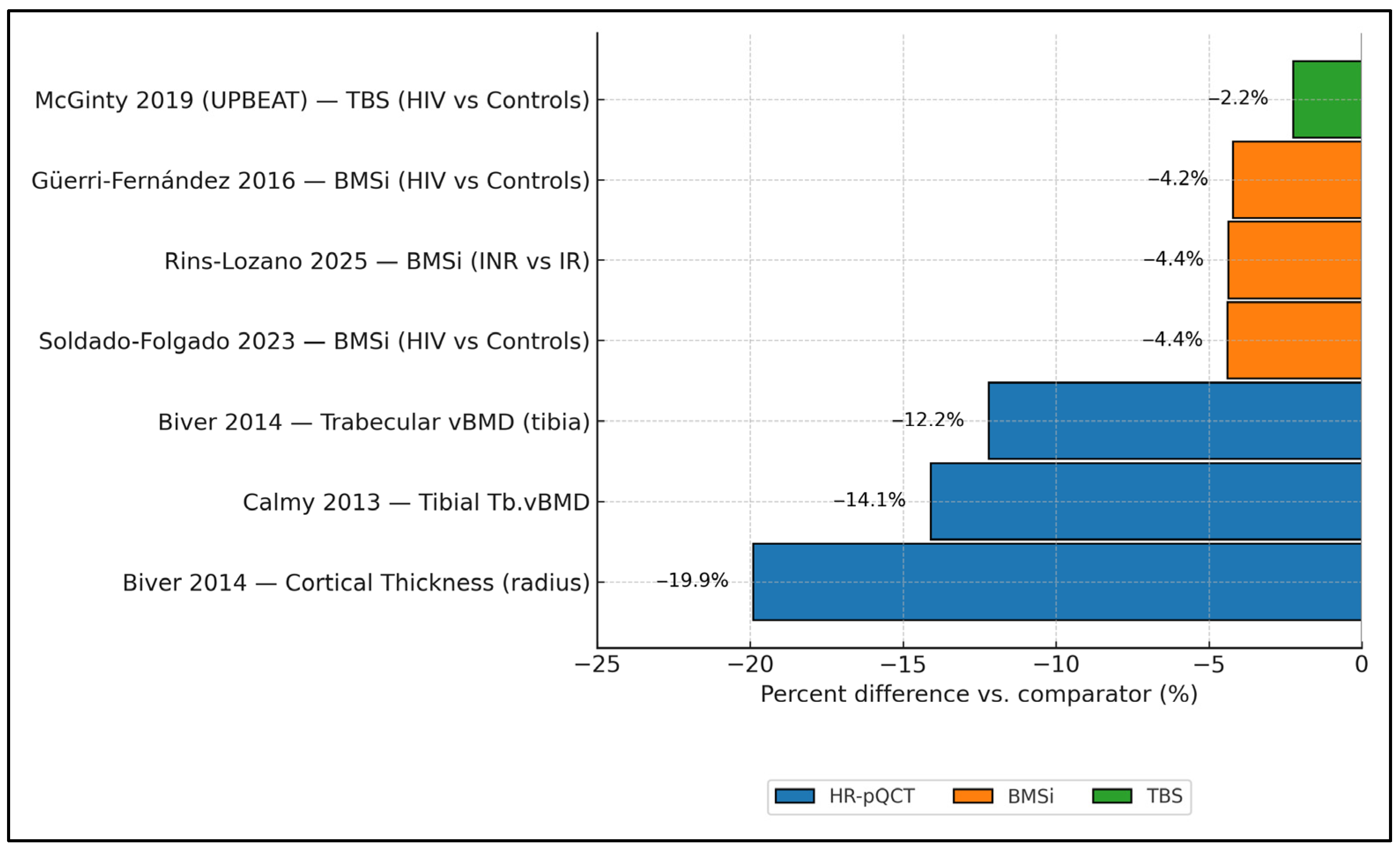
Figure 2 synthesizes cross-modality effect magnitudes in PLWH, showing consistently negative percent differences versus comparators. On HR-pQCT, cortical thickness at the radius was ≈−19.9% and tibial trabecular vBMD ≈−12.2% to −14.1% (Biver 2014 [17]; Calmy 2013 [16]). Tissue-level strength (BMSi) was also lower: Güerri-Fernández 2016 [20] showed −3.4% vs. controls (77.2 vs. 80.6 units), Soldado-Folgado 2023 [23] −4.4% (78.4 vs. 82.0), and Rins-Lozano 2025 [24] −4.4% when comparing immunologic non-responders to responders (−3.5 units on an ≈80.2-unit reference). For microarchitecture by TBS, UPBEAT (McGinty 2019 [25]) reported a −2.2% difference (1.349 vs. 1.380). Taken together, impairments are largest for cortical thickness and trabecular density by HR-pQCT, and clearly detectable—although smaller in magnitude—by BMSi and TBS.
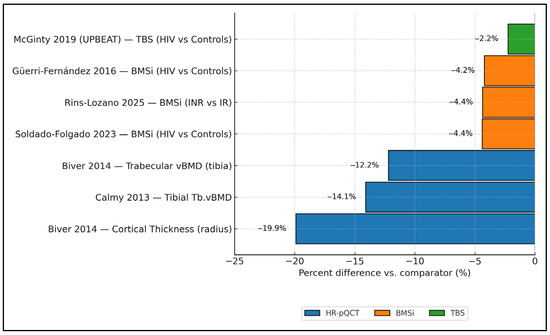
Figure 2.
Cross-modality structural impairment in PLWH is expressed as percent differences versus comparators. HR-pQCT results are grouped by skeletal site and device generation (XtremeCT vs. XtremeCT II) where reported; mixed-generation estimates are flagged. Error bars represent reported precision (SD, SE, or 95% CI) when available; otherwise, point estimates only. Data collected from studies included in the final analysis [16,17,20,23,24,25].
Predictor analyses aligned across modalities: Exposure to PIs and current smoking were associated with lower HR-pQCT strength indices (failure load/stiffness) in Foreman [19], while prior TDF were related to worse cortical parameters and reduced failure load in Macdonald [18]. On TBS, HIV status itself carried an adjusted β of −0.037 (p = 0.002) in McGinty, with further reductions linked to PI exposure and lower nadir CD4 [25], and HIV-positive women had a 64% higher likelihood of degraded TBS in Sharma [26]. Longitudinally, ART initiation produced early declines with partial recovery by 48 weeks for both TBS and BMD in Guan, and 19.3% had normal BMD but abnormal TBS at baseline, underscoring added diagnostic yield [27]. Tissue-level effects were dynamic: BMSi fell after ART start (−2.1 units; p = 0.02) [21] and improved after TDF→TAF switch (+2.5 units; p < 0.05) [22]; immunologic non-responders had −3.5 BMSi units vs. responders (p = 0.001) [24]; and cross-sectionally PLWH showed −3.6 BMSi units vs. controls (p < 0.01) [23]. Fracture-oriented predictors were consistent: older age and steroid exposure increased VF risk—Gazzola aOR 1.09 per year and 3.64 for steroids, with ~70% of VFs occurring at non-osteoporotic BMD [29]—and Llop highlighted ~25% asymptomatic VFs in PLWH over 50 years [28]. Early HR-pQCT studies (Calmy [16]; Biver [17]) already signaled lower Tb.N/Ct.Th and higher Tb.Sp, which are consistent with reduced estimated failure load in PLWH, as described in Table 5.

Table 5.
Predictors and longitudinal effects.
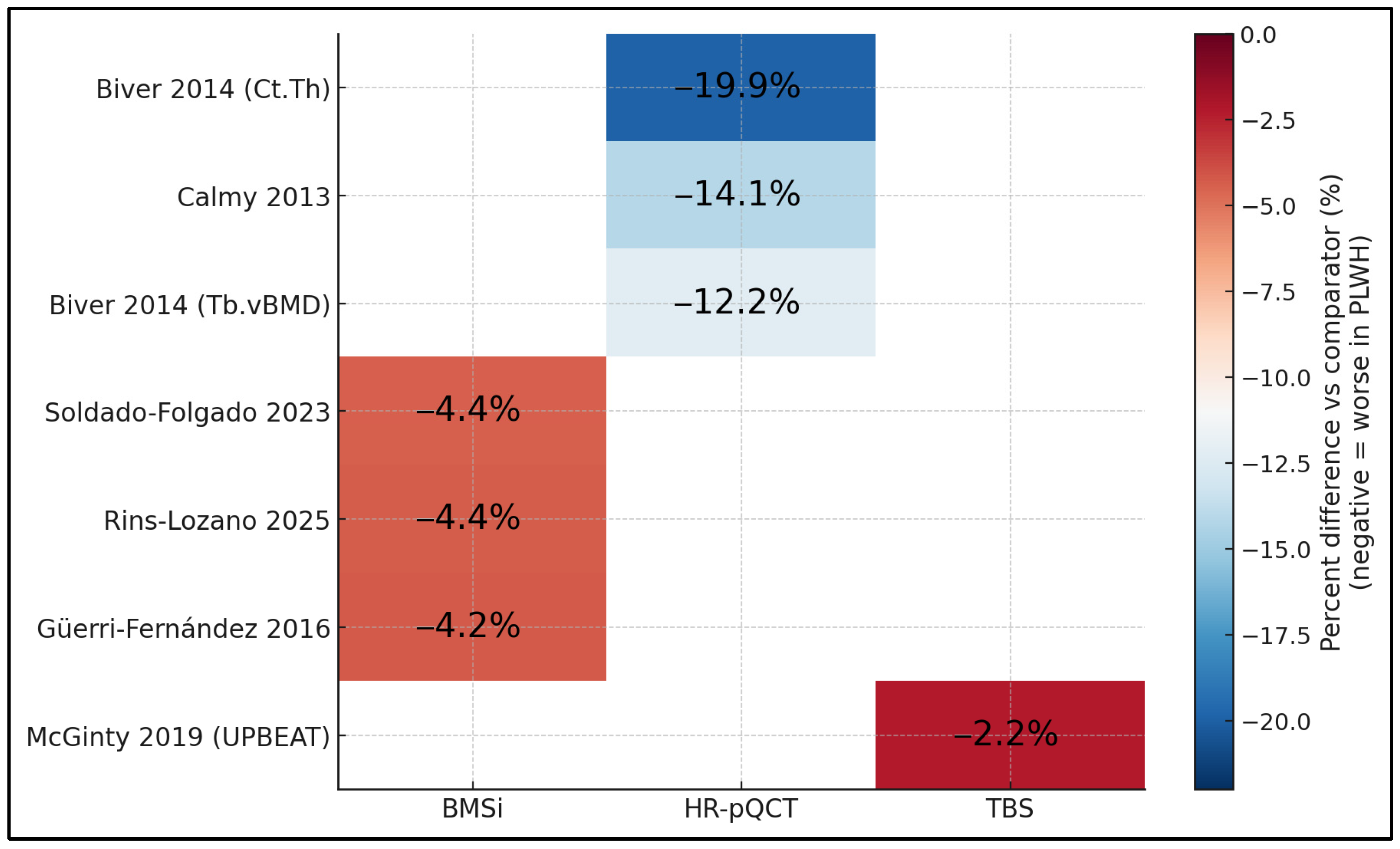
Figure 3 compares percent differences vs. comparators across modalities and studies on a common color scale. HR-pQCT shows the largest deficits, with cortical thickness reduced by around −19.9% and trabecular vBMD by −12.2% to −14.1% (Biver 2014 [17]; Calmy 2013 [16]). BMSi results are consistently moderately reduced by about −4.2% to −4.4% across studies (Güerri-Fernández 2016 [20]; Soldado-Folgado 2023 [23]; Rins-Lozano 2025 [24]), while TBS reveals a smaller yet directionally consistent reduction of −2.2% (UPBEAT, McGinty 2019 [25]). The grid makes clear that, regardless of modality, PLWH exhibit uniformly negative deviations, with effect magnitudes greatest for cortical and trabecular metrics captured by HR-pQCT and smaller (but present) for tissue-level (BMSi) and texture-based (TBS) measures.
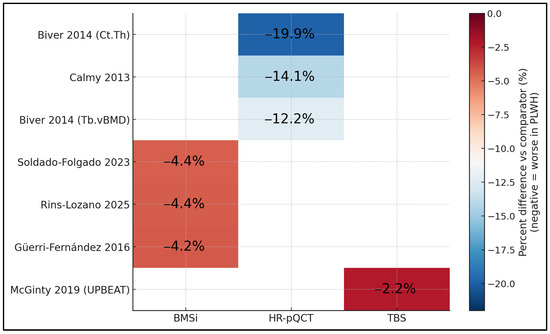
Figure 3.
Labeled heatmap of percent differences by study × modality [16,17,20,23,24,25].
4. Discussion
4.1. Summary of Evidence
Our findings of impaired microarchitecture and estimated strength in PLWH align with earlier HR-pQCT work showing disproportionately cortical deficits. In postmenopausal minority women, Yin et al. reported ~11–12% lower tibial cortical thickness/area in HIV vs. controls despite broadly similar trabecular vBMD—an anatomic pattern we also observed at peripheral sites and that plausibly contributes to lower FE-estimated failure load even when areal BMD is not frankly osteoporotic [30]. Methodologically, these strength estimates are robust at the radius/tibia: FE outcomes based on HR-pQCT demonstrate low in vivo precision errors (CV% typically ~1–3% for key parameters), supporting their use for between-group comparisons and longitudinal follow-up in HIV cohorts [30,31,32,33,34]. More recent multimodal imaging at the proximal femur further indicates trabecular-predominant compromise in fracture-prone regions, reinforcing the concept that site-specific quality losses may not be captured by DXA alone [35,36,37,38,39]. Where serious/high RoB or inconsistency arose, we refrained from strength-of-effect language and downgraded certainty accordingly. As a result, overall certainty for vertebral fracture prevalence was low to moderate, and for modality-specific quality metrics, low to moderate, primarily due to residual confounding and imprecision.
The TBS signal we detected (lower values and higher prevalence of degraded TBS in PLWH) is consistent with independent cohorts and extends beyond density per se. In middle-aged HIV-positive men on treatment, Sellier et al. demonstrated disrupted trabecular micro-architecture with lower volumetric trabecular density at both tibia and radius (≈16–17% decrements), consonant with degraded texture metrics and elevated bone fragility risk [31]. Narrative and scoping syntheses focused on “bone quality” in HIV conclude that texture, microarchitecture, and material properties deteriorate in PLWH, with likely contributions from chronic immune activation and antiretroviral exposures—again matching our cross-modality pattern that TBS and HR-pQCT each add information beyond DXA [33].
Tissue-level mechanics by impact microindentation (BMSi) showed HIV-associated decrements in our pooled analysis, and the broader literature supports the clinical relevance of BMSi as a complementary risk signal. While most BMSi studies in HIV remain single-center, general population data and technical reviews indicate that microindentation captures bone material properties not reflected in density or macro-architecture and can discriminate fracture status independent of BMD; this helps contextualize the consistent −3 to −4 BMSi gaps we observed between PLWH and comparators [8]. Taken together with HR-pQCT/TBS, these results argue for a multidimensional framework for skeletal assessment in HIV, particularly in patients whose BMD appears “non-osteoporotic” but who accumulate other quality deficits.
Clinically, the vertebral fracture (VF) burden we observed despite frequently non-osteoporotic BMD is concordant with meta-analytic evidence. Ilha et al. reported that HIV-positive individuals have more than twice the odds of VFs compared with HIV-negative controls, with high between-study heterogeneity reflecting differences in age, treatment era, and imaging methods [34]. Our findings that age and glucocorticoid exposure predict VFs echo the broader HIV literature synthesizing classic risk factors with HIV-specific drivers (e.g., inflammation, ART exposure) [38]. Additionally, vitamin-D insufficiency appears common and clinically meaningful in HIV: in a case–control series of 100 PLWH vs. 100 controls, Atteritano et al. found markedly higher VF prevalence in PLWH and a strong association between low 25-OH-vitamin-D and VFs, underscoring an actionable cofactor that may amplify microarchitectural fragility [35].
Drug-specific patterns provide mechanistic plausibility for our modality-specific signals. Randomized phase-3 trials showed that TAF-based initial regimens preserve BMD substantially better than otherwise-identical TDF-based backbones through 48 weeks (spine −1.30% vs. −2.86%; hip −0.66% vs. −2.95%), without compromising virologic efficacy [36]. Reviews focusing on protease inhibitors similarly implicate PI-containing regimens in greater bone loss and possibly fracture risk, likely via effects on osteoclastogenesis, vitamin D metabolism, and systemic inflammation [37]. These pharmacologic signatures dovetail with our observations that prior TDF and PI exposure tracked with worse cortical indices and lower FE-strength, suggesting that quality-oriented metrics may be especially sensitive to cumulative ART effects.
Finally, emerging proximal-femur imaging using QCT and MRI integrates the story: PLWH exhibit lower trabecular vBMD, poorer trabecular morphology, and higher marrow adiposity at the hip compared to matched controls—changes that imply lower femoral strength even when DXA T-scores are not frankly osteoporotic [39]. Coupled with methodologic data supporting reproducibility of FE-derived strength estimates [32] and narrative syntheses linking HIV pathobiology and ART to bone quality deterioration [33], these results strengthen the case for incorporating TBS and, where available, HR-pQCT/QCT into research protocols and for targeting modifiable risks (vitamin D insufficiency, smoking, glucocorticoids, and TDF/PI exposure when alternatives exist) in clinical care.
In routine HIV care—especially where resources are constrained—a feasibility-first pathway can embed bone health into standard visits without new infrastructure. Begin with structured triage using clinical history (prior low-trauma fracture, falls, and low BMI), medication review (glucocorticoids and ART exposures), and simple labs already common in HIV clinics (vitamin D, where locally available). Provide brief counseling and written prompts targeting modifiable risks (smoking cessation, weight-bearing/impact exercise, and sunlight/dietary strategies for vitamin D/calcium) and schedule reassessment at 12–24 months or sooner after major ART or glucocorticoid changes. Where densitometry is unavailable, apply validated clinical risk tools and contemporaneously review ART to preferentially select bone-sparing options when clinically interchangeable; when densitometry is available, incorporate adjunct measures per local protocols without extending visit length. Task shifting to nurses and pharmacists (checklist-driven “Practice Box”) and integrating order sets into the EHR can standardize delivery with minimal added time.
For service design and quality improvement, we propose explicit referral triggers and audit metrics rather than technology expansion. Referral to specialist evaluation should be prompted by discordant phenotypes (e.g., fragility fracture despite “non-osteoporotic” BMD), unexplained height loss/back pain suggestive of silent vertebral fracture, or repeated falls. Program-level dashboards can track the following: (i) the proportion of eligible patients with documented fracture-risk assessment; (ii) completion of medication review with an ART bone-sparing consideration when appropriate; (iii) delivery of brief lifestyle counseling; and (iv) timely reassessment after treatment changes. Sites can adopt a minimal “bone bundle” (risk checklist, counseling script, order set, follow-up interval) and iteratively PDSA-cycle it for flow efficiency. In parallel, pragmatic research should prioritize implementation outcomes (reach, fidelity, and cost/time per patient) and equity (uptake in high-risk subgroups), ensuring that any added measurements translate into earlier prevention or treatment decisions, not just additional testing.
4.2. Strengths and Limitations
Strengths include protocol registration, comprehensive multimodal capture (HR-pQCT/FE, TBS, BMSi, femoral QCT/MRI, VFs), dual independent screening and extraction with good agreement, and explicit risk-of-bias integration with SWiM-based synthesis.
This review also has several limitations that should temper interpretation. First, heterogeneity across studies was substantial in design (cross-sectional vs. longitudinal), populations (age, sex distribution, duration of HIV, and nadir/current CD4), and imaging platforms (HR-pQCT vendors, acquisition protocols, and finite-element pipelines; different TBS software/thresholds), which constrained meta-analytic pooling and likely widened between-study variance. For HR-pQCT, vendor/generation, voxel size, and segmentation/FE pipelines varied across studies; although we harmonized to percent differences and stratified by site and generation, residual technical heterogeneity likely inflated between-study variance. Secondly, most cohorts were from high-income settings with long-term ART exposure, limiting generalizability to resource-limited contexts, ART-naïve individuals, or populations with different comorbidity burdens (tuberculosis and undernutrition). Thirdly, residual confounding is probable: smoking, glucocorticoids, hypogonadism, vitamin D status, and physical activity were inconsistently measured or adjusted for and may partly account for observed gaps in microarchitecture and BMSi. Fourthly, several HR-pQCT reports provided incomplete numerical data for key parameters, and BMSi studies were single-center with modest sample sizes, increasing the risk of imprecision and small-study effects. Fifthly, fracture ascertainment relied predominantly on vertebral fracture assessment or radiographs without systematic adjudication of non-vertebral outcomes, limiting causal inference between “bone quality” metrics and incident, patient-important fractures in PLWH. Finally, publication bias cannot be excluded given the predominance of positive directional findings and the relative novelty of advanced phenotyping modalities in HIV research.
5. Conclusions
Across modalities, PLWH demonstrate consistent reductions in cortical and trabecular microarchitecture (HR-pQCT/FE-strength) and tissue-level properties (BMSi), with modest TBS decrements and a clinically relevant vertebral-fracture burden. While biologically plausible and aligning with ART exposure profiles, causality cannot be inferred from observational evidence. In practice, TBS and VFA are scalable adjuncts to refine risk when BMD is not overtly osteoporotic; HR-pQCT/BMSi add mechanistic value in research or complex cases. Prospective and interventional studies linking these measures to incident fractures are needed to translate bone “quality” profiling into reduced fragility in PLWH.
Author Contributions
Conceptualization, D.V.R.; methodology, D.V.R.; software, O.R. and F.B.; validation, O.R. and F.B.; formal analysis, O.R. and F.B.; investigation, V.P. and S.V.V.; resources, V.P. and S.V.V.; data curation, V.P. and S.V.V.; writing—original draft preparation, D.V.R.; writing—review and editing, A.V.; visualization, A.V.; supervision, A.V.; project administration, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to acknowledge the Victor Babes University of Medicine and Pharmacy, Timisoara, for paying the APC. The funder had no role in study design, data collection/analysis, decision to publish, or manuscript preparation.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors used ChatGPT v4.0, an AI language model developed by OpenAI (San Francisco, CA, USA), to exclusively improve the manuscript’s language and readability. AI assistance did not generate or alter data, analyses, or interpretations. All the scientific content, interpretations, and conclusions are the original work of the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pramukti, I.; Lindayani, L.; Chen, Y.C.; Yeh, C.Y.; Tai, T.W.; Fetzer, S.; Ko, N.Y. Bone fracture among people living with HIV: A systematic review and meta-regression of prevalence, incidence, and risk factors. PLoS ONE 2020, 15, e0233501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, T.T.; Hoy, J.; Borderi, M.; Guaraldi, G.; Renjifo, B.; Vescini, F.; Yin, M.T.; Powderly, W.G. Recommendations for evaluation and management of bone disease in HIV. Clin. Infect. Dis. 2015, 60, 1242–1251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoy, J.F.; Grund, B.; Roediger, M.; Schwartz, A.V.; Shepherd, J.; Avihingsanon, A.; Badal-Faesen, S.; de Wit, S.; Jacoby, S.; La Rosa, A.; et al. Immediate Initiation of Antiretroviral Therapy for HIV Infection Accelerates Bone Loss Relative to Deferring Therapy: Findings from the START Bone Mineral Density Substudy, a Randomized Trial. J. Bone Miner. Res. 2017, 32, 1945–1955. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carr, A.; Grund, B.; Schwartz, A.V.; Avihingsanon, A.; Badal-Faesen, S.; Bernadino, J.I.; Estrada, V.; La Rosa, A.; Mallon, P.; Pujari, S.; et al. The rate of bone loss slows after 1-2 years of initial antiretroviral therapy: Final results of the Strategic Timing of Antiretroviral Therapy (START) bone mineral density substudy. HIV Med. 2020, 21, 64–70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orkin, C.; DeJesus, E.; Ramgopal, M.; Crofoot, G.; Ruane, P.; LaMarca, A.; Mills, A.; Vandercam, B.; de Wet, J.; Rockstroh, J.; et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide coformulated with rilpivirine and emtricitabine in virally suppressed adults with HIV-1 infection: A randomised, double-blind, multicentre, phase 3b, non-inferiority study. Lancet HIV 2017, 4, e195–e204. [Google Scholar] [CrossRef] [PubMed]
- Post, F.A.; Tebas, P.; Clarke, A.; Cotte, L.; Short, W.R.; Abram, M.E.; Jiang, S.; Cheng, A.; Das, M.; Fordyce, M.W. Brief Report: Switching to Tenofovir Alafenamide, Coformulated With Elvitegravir, Cobicistat, and Emtricitabine, in HIV-Infected Adults With Renal Impairment: 96-Week Results From a Single-Arm, Multicenter, Open-Label Phase 3 Study. J. Acquir. Immune Defic. Syndr. 2017, 74, 180–184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moore, A.E.B.; Burns, J.E.; Sally, D.; Milinkovic, A.; Krokos, G.; John, J.; Rookyard, C.; Borca, A.; Pool, E.R.M.; Tostevin, A.; et al. Bone turnover change after randomized switch from tenofovir disoproxil to tenofovir alafenamide fumarate in men with HIV. AIDS 2024, 38, 521–529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCloskey, E.V.; Odén, A.; Harvey, N.C.; Leslie, W.D.; Hans, D.; Johansson, H.; Barkmann, R.; Boutroy, S.; Brown, J.; Chapurlat, R.; et al. A Meta-Analysis of Trabecular Bone Score in Fracture Risk Prediction and Its Relationship to FRAX. J. Bone Miner. Res. 2016, 31, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Palomo, T.; Muszkat, P.; Weiler, F.G.; Dreyer, P.; Brandão, C.M.A.; Silva, B.C. Update on trabecular bone score. Arch. Endocrinol. Metab. 2022, 66, 694–706. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sornay-Rendu, E.; Boutroy, S.; Duboeuf, F.; Chapurlat, R.D. Bone Microarchitecture Assessed by HR-pQCT as Predictor of Fracture Risk in Postmenopausal Women: The OFELY Study. J. Bone Miner. Res. 2017, 32, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P.; Dufour, A.B.; Hannan, M.T.; Kiel, D.P.; Chapurlat, R.; Sornay-Rendu, E.; Merle, B.; Boyd, S.K.; Whittier, D.E.; Hanley, D.A.; et al. Fracture risk based on high-resolution peripheral quantitative computed tomography measures does not vary with age in older adults-the bone microarchitecture international consortium prospective cohort study. J. Bone Miner. Res. 2024, 39, 561–570. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mannarino, T.; D’Antonio, A.; Mercinelli, S.; Falzarano, M.; Volpicelli, F.; Mainolfi, C.G.; Zappulo, E.; Di Filippo, G.; Cotugno, M.R.; Gentile, I.; et al. Trabecular bone score assessed by dual-energy X ray absorption predicts vertebral fractures in HIV infected young adults. Bone Rep. 2024, 22, 101797. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ambrosioni, J.; Levi, L.; Alagaratnam, J.; Van Bremen, K.; Mastrangelo, A.; Waalewijn, H.; Molina, J.M.; Guaraldi, G.; Winston, A.; Boesecke, C.; et al. Major revision version 12.0 of the European AIDS Clinical Society guidelines 2023. HIV Med. 2023, 24, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Biz, C.; Khamisy-Farah, R.; Puce, L.; Szarpak, L.; Converti, M.; Ceylan, H.İ.; Crimì, A.; Bragazzi, N.L.; Ruggieri, P. Investigating and Practicing Orthopedics at the Intersection of Sex and Gender: Understanding the Physiological Basis, Pathology, and Treatment Response of Orthopedic Conditions by Adopting a Gender Lens: A Narrative Overview. Biomedicines 2024, 12, 974. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calmy, A.; Chevalley, T.; Delhumeau, C.; Toutous-Trellu, L.; Spycher-Elbes, R.; Ratib, O.; Zawadynski, S.; Rizzoli, R. Long-term HIV infection and antiretroviral therapy are associated with bone microstructure alterations in premenopausal women. Osteoporos. Int. 2013, 24, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Biver, E.; Calmy, A.; Delhumeau, C.; Durosier, C.; Zawadynski, S.; Rizzoli, R. Microstructural alterations of trabecular and cortical bone in long-term HIV-infected elderly men on successful antiretroviral therapy. AIDS 2014, 28, 2417–2427. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, H.M.; Maan, E.J.; Berger, C.; Dunn, R.A.; Côté, H.C.F.; Murray, M.C.M.; Pick, N.; Prior, J.C. Deficits in bone strength, density and microarchitecture in women living with HIV: A cross-sectional HR-pQCT study. Bone 2020, 138, 115509. [Google Scholar] [CrossRef] [PubMed]
- Foreman, S.C.; Wu, P.H.; Kuang, R.; John, M.D.; Tien, P.C.; Link, T.M.; Krug, R.; Kazakia, G.J. Factors associated with bone microstructural alterations assessed by HR-pQCT in long-term HIV-infected individuals. Bone 2020, 133, 115210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Güerri-Fernández, R.; Molina, D.; Villar-García, J.; Prieto-Alhambra, D.; Mellibovsky, L.; Nogués, X.; González-Mena, A.; Guelar, A.; Trenchs-Rodríguez, M.; Herrera-Fernández, S.; et al. Brief Report: HIV Infection Is Associated with Worse Bone Material Properties, Independently of Bone Mineral Density. J. Acquir. Immune Defic. Syndr. 2016, 72, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Lerma-Chippirraz, E.; Pineda-Moncusí, M.; González-Mena, A.; Soldado-Folgado, J.; Knobel, H.; Trenchs-Rodríguez, M.; Díez-Pérez, A.; Brown, T.T.; García-Giralt, N.; Güerri-Fernández, R. Inflammation status in HIV-positive individuals correlates with changes in bone tissue quality after initiation of ART. J. Antimicrob. Chemother. 2019, 74, 1381–1388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soldado-Folgado, J.; Chillarón, J.J.; Cañas-Ruano, E.; Arrieta-Aldea, I.; González-Mena, A.; Blasco-Hernando, F.; Knobel, H.; Garcia-Giralt, N.; Güerri-Fernández, R. An Abnormal Inflammatory Pattern Associated with Long-Term Non-Progression of HIV Infection Impacts Negatively on Bone Quality. J. Clin. Med. 2022, 11, 2927. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soldado-Folgado, J.; Rins-Lozano, O.; Arrieta-Aldea, I.; Gonzále-Mena, A.; Cañas-Ruano, E.; Knobel, H.; Garcia-Giralt, N.; Güerri-Fernández, R. Changes in bone quality after switching from a TDF to a TAF based ART: A pilot randomized study. Front. Endocrinol. 2023, 14, 1076739. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rins-Lozano, O.; Rodríguez-Morera, J.; Arrieta-Aldea, I.; González-Mena, A.; Rodríguez-Mercader, S.; Suaya, L.; Pascual-Aranda, M.; Cañas-Ruano, E.; Fernandez-Quiroga, M.J.; Canepa, C.; et al. Impaired Bone Tissue Quality Associated With Inflammation in HIV-immunological Nonresponders: A Cross-sectional Analysis. J. Clin. Endocrinol. Metab. 2025, 110, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- McGinty, T.; Cotter, A.G.; Sabin, C.A.; Macken, A.; Kavanagh, E.; Compston, J.; Sheehan, G.; Lambert, J.; Mallon, P.W.G.; HIV UPBEAT (Understanding the Pathology of Bone Diseases in HIV-infected Subjects) Study Group. Assessment of trabecular bone score, an index of bone microarchitecture, in HIV positive and HIV negative persons within the HIV UPBEAT cohort. PLoS ONE 2019, 14, e0213440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, A.; Ma, Y.; Tien, P.C.; Scherzer, R.; Anastos, K.; Cohen, M.H.; Hans, D.; Yin, M.T. HIV Infection Is Associated With Abnormal Bone Microarchitecture: Measurement of Trabecular Bone Score in the Women’s Interagency HIV Study. J. Acquir. Immune Defic. Syndr. 2018, 78, 441–449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guan, W.M.; Pan, W.; Yu, W.; Cao, W.; Lin, Q.; Zhang, Z.Z.; Song, X.J.; Li, Y.L.; Tian, J.P.; Xu, Y.; et al. Changes in trabecular bone score and bone mineral density in Chinese HIV-Infected individuals after one year of antiretroviral therapy. J. Orthop. Translat. 2021, 29, 72–77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Llop, M.; Sifuentes, W.A.; Bañón, S.; Macia-Villa, C.; Perez-Elías, M.J.; Rosillo, M.; Moreno, S.; Vázquez, M.; Casado, J.L. Increased prevalence of asymptomatic vertebral fractures in HIV-infected patients over 50 years of age. Arch. Osteoporos. 2018, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Gazzola, L.; Savoldi, A.; Bai, F.; Magenta, A.; Dziubak, M.; Pietrogrande, L.; Tagliabue, L.; Del Sole, A.; Bini, T.; Marchetti, G.; et al. Assessment of radiological vertebral fractures in HIV-infected patients: Clinical implications and predictive factors. HIV Med. 2015, 16, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.T.; Shu, A.; Zhang, C.A.; Boutroy, S.; McMahon, D.J.; Ferris, D.C.; Colon, I.; Shane, E. Trabecular and cortical microarchitecture in postmenopausal HIV-infected women. Calcif. Tissue Int. 2013, 92, 557–565. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sellier, P.; Ostertag, A.; Collet, C.; Trout, H.; Champion, K.; Fernandez, S.; Lopes, A.; Morgand, M.; Clevenbergh, P.; Evans, J.; et al. Disrupted trabecular bone micro-architecture in middle-aged male HIV-infected treated patients. HIV Med. 2016, 17, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Kawalilak, C.E.; Kontulainen, S.A.; Amini, M.A.; Lanovaz, J.L.; Olszynski, W.P.; Johnston, J.D. In vivo precision of three HR-pQCT-derived finite element models of the distal radius and tibia in postmenopausal women. BMC Musculoskelet. Disord. 2016, 17, 389. [Google Scholar] [CrossRef] [PubMed]
- Olali, A.Z.; Carpenter, K.A.; Myers, M.; Sharma, A.; Yin, M.T.; Al-Harthi, L.; Ross, R.D. Bone Quality in Relation to HIV and Antiretroviral Drugs. Curr. HIV/AIDS Rep. 2022, 19, 312–327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ilha, T.A.S.H.; Comim, F.V.; Copes, R.M.; Compston, J.E.; Premaor, M.O. HIV and Vertebral Fractures: A Systematic Review and Metanalysis. Sci. Rep. 2018, 8, 7838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Atteritano, M.; Mirarchi, L.; Venanzi-Rullo, E.; Santoro, D.; Iaria, C.; Catalano, A.; Lasco, A.; Arcoraci, V.; Lo Gullo, A.; Bitto, A.; et al. Vitamin D Status and the Relationship with Bone Fragility Fractures in HIV-Infected Patients: A Case Control Study. Int. J. Mol. Sci. 2018, 19, 119. [Google Scholar] [CrossRef]
- Sax, P.E.; Wohl, D.; Yin, M.T.; Post, F.; DeJesus, E.; Saag, M.; Pozniak, A.; Thompson, M.; Podzamczer, D.; Molina, J.M.; et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate coformulated with elvitegravir cobicistat emtricitabine for initial treatment of HIV-1 infection: Two randomised double-blind phase 3, non-inferiority trials. Lancet 2015, 385, 2606–2615. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.A.; Weitzmann, M.N.; Ofotokun, I. The protease inhibitors and HIV-associated bone loss. Curr. Opin. HIV AIDS 2016, 11, 333–342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Premaor, M.O.; Compston, J.E. The Hidden Burden of Fractures in People Living with HIV. JBMR Plus. 2018, 2, 247–256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carballido-Gamio, J.; Posadzy, M.; Wu, P.H.; Kenny, K.; Saeed, I.; Link, T.M.; Tien, P.C.; Krug, R.; Kazakia, G.J. People living with HIV have low trabecular bone mineral density, high bone marrow adiposity, and poor trabecular bone microarchitecture at the proximal femur. Osteoporos. Int. 2022, 33, 1739–1753. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).