Gastric Cancer Risk in Patients with Intestinal Metaplasia: Long-Term Outcomes from a Large Single-Center Cohort in Türkiye
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population and Data Sources
2.3. Inclusion and Exclusion Criteria
2.4. Primary and Secondary Endpoints
2.5. Final Cohort
2.6. Variables and Definitions
- GIM extent: classified as confined to the antrum, confined to the corpus, or involving both antrum and corpus.
- Additional risk factors: presence of H. pylori infection at baseline (determined histologically), family history of gastric cancer in a first-degree relative, residence in a region of Türkiye endemic for gastric cancer (Eastern Anatolian region) [9], demographic characteristics (age, sex), and other documented risk modifiers.
- İntestinal metaplasia risk groups: as outlined in the AJG guidelines, patients with GIM were stratified into two risk groups: the low-risk group, comprising individuals with GIM confined to the antrum; and the high-risk group, which included patients with GIM involving both the antrum and corpus, those with a first-degree relative diagnosed with gastric cancer, and individuals residing in endemic regions [3].
- Outcome: development of histologically confirmed gastric adenocarcinoma during follow-up. Follow-up outcomes of GIM were categorized into three groups: Regression, defined as the histological absence of GIM in follow-up biopsies. Unchanged, defined as the continued presence of GIM without histological progression. Progressed, defined as the development of dysplasia, adenocarcinoma, or other neoplastic lesions during follow-up.
2.7. Ethical Approval
2.8. Statistical Analysis
3. Results
3.1. Patient Flow and Baseline Characteristics
3.2. Follow-Up Outcomes of GIM Patients
3.3. Disease Progression and Survival Outcomes by Risk Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Morgan, D.R.; Corral, J.E.; Li, D.; Montgomery, E.A.; Riquelme, A.; Kim, J.J.; Sauer, B.; Shah, S.C. ACG Clinical Guideline: Diagnosis and Management of Gastric Premalignant Conditions. Am. J. Gastroenterol. 2025, 120, 709–737. [Google Scholar] [CrossRef]
- Plummer, M.; Franceschi, S.; Vignat, J.; Forman, D.; de Martel, C. Global Burden of Gastric Cancer Attributable to Helicobacter pylori. Int. J. Cancer 2015, 136, 487–490. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, N. Review of Atrophic Gastritis and Intestinal Metaplasia as a Premalignant Lesion of Gastric Cancer. J. Cancer Prev. 2015, 20, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.J.; Choi, A.Y.; Truong, C.D.; Yeh, M.M.; Hwang, J.H. Diagnosis and Management of Gastric Intestinal Metaplasia: Current Status and Future Directions. Gut Liver 2019, 13, 596–603. [Google Scholar] [CrossRef]
- Song, H.; Ekheden, I.G.; Zheng, Z.; Ericsson, J.; Nyrén, O.; Ye, W. Incidence of Gastric Cancer among Patients with Gastric Precancerous Lesions: Observational Cohort Study in a Low-Risk Western Population. BMJ 2015, 351, h3867. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Yalcin, S. Gastric Cancer in Turkey—A Bridge between West and East. Gastrointest. Cancer Res. 2009, 3, 29–32. [Google Scholar] [PubMed]
- Eriksson, N.K.; Kärkkäinen, P.A.; Färkkilä, M.A.; Arkkila, P.E. Prevalence and Distribution of Gastric Intestinal Metaplasia and Its Subtypes. Dig. Liver Dis. 2008, 40, 355–360. [Google Scholar] [CrossRef]
- Olmez, S.; Aslan, M.; Erten, R.; Sayar, S.; Bayram, I. The Prevalence of Gastric Intestinal Metaplasia and Distribution of Helicobacter pylori Infection, Atrophy, Dysplasia, and Cancer in Its Subtypes. Gastroenterol. Res. Pract. 2015, 2015, 434039. [Google Scholar] [CrossRef]
- O’Connor, A.; McNamara, D.; O’Moráin, C.A. Surveillance of Gastric Intestinal Metaplasia for the Prevention of Gastric Cancer. Cochrane Database Syst. Rev. 2013, 9, CD009322. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, F.; Wu, C.Y.; Leung, W.K. Prevalence and temporal trend of gastric preneoplastic lesions in Asia: A systematic review with meta-analysis. United Eur. Gastroenterol. J. 2024, 12, 139–151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, J.C.; Schnirer, I.I.; Reddy, S.; Chiang, S.; Najam, A.; Yu, C.; Giacco, G.; Hess, K.; Rashid, A.; Xie, K.; et al. Effects of sex and racial/ethnic group on the pattern of gastric cancer localization. Gastric Cancer 2002, 5, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, M.H.; Liptrot, S.; Paul, J.; Brown, I.L.; Morrison, D.; McColl, K.E. Oesophageal and gastric intestinal-type adenocarcinomas show the same male predominance due to a 17 year delayed development in females. Gut 2009, 58, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.C.; Goto, Y.; Zabaleta, J.; Morgan, D.R.; Correa, P.; Rabkin, C.S. Sex Hormones, Hormonal Interventions, and Gastric Cancer Risk: A Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2012, 21, 20–38. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Hu, W.; Ouyang, Q.; Zhang, S.; He, L.; Chen, W.; Li, X.; Hu, C. Helicobacter pylori Infection Induces Stem Cell-Like Properties in Correa Cascade of Gastric Cancer. Cancer Lett. 2022, 542, 215764. [Google Scholar] [CrossRef]
- Parkin, D.M.; Boyd, L.; Walker, L.C. The Fraction of Cancer Attributable to Lifestyle and Environmental Factors in the UK in 2010. Br. J. Cancer 2011, 105 (Suppl. 2), S77–S81. [Google Scholar] [CrossRef]
- Fan, C.; Xu, K.; Huang, Y.; Liu, S.; Wang, T.; Wang, W.; Hu, W.; Liu, L.; Xing, M.; Yang, S. Viscosity and Degradation Controlled Injectable Hydrogel for Esophageal Endoscopic Submucosal Dissection. Bioact. Mater. 2021, 6, 1150–1162, Correction in Bioact. Mater. 2021, 6, 4161–4162. [Google Scholar] [CrossRef]
- Tokat, M.; van Tilburg, L.; Koch, A.D.; Spaander, M.C.W. Artificial Intelligence in Upper Gastrointestinal Endoscopy. Dig. Dis. 2022, 40, 395–408. [Google Scholar] [CrossRef]
- González, C.A.; Pardo, M.L.; Ruiz Liso, J.M.; Alonso, P.; Bonet, C.; Garcia, R.M.; Sala, N.; Capella, G.; Sanz-Anquela, J.M. Gastric Cancer Occurrence in Preneoplastic Lesions: A Long-Term Follow-Up in a High-Risk Area in Spain. Int. J. Cancer 2010, 127, 2654–2660. [Google Scholar] [CrossRef]
- Spicer, S.S. Diamine Methods for Differentiating Mucosubstances Histochemically. J. Histochem. Cytochem. 1965, 13, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Filipe, M.I.; Muñoz, N.; Matko, I.; Kato, I.; Pompe-Kirn, V.; Jutersek, A.; Teuchmann, S.; Benz, M.; Prijon, T. Intestinal Metaplasia Types and the Risk of Gastric Cancer: A Cohort Study in Slovenia. Int. J. Cancer 1994, 57, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Jass, J.R.; Filipe, M.I. The Mucin Profiles of Normal Gastric Mucosa, Intestinal Metaplasia and Its Variants and Gastric Carcinoma. Histochem. J. 1981, 13, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Filipe, M.I.; Potet, F.; Bogomoletz, W.V.; Dawson, P.A.; Fabiani, B.; Chauveinc, P.; Fenzy, A.; Gazzard, B.; Goldfain, D.; Zeegen, R. Incomplete Sulphomucin-Secreting Intestinal Metaplasia for Gastric Cancer: Preliminary Data from a Prospective Study from Three Centres. Gut 1985, 26, 1319–1326. [Google Scholar] [CrossRef]
- Conchillo, J.M.; Houben, G.; de Bruïne, A.; Stockbrügger, R.W. Is Type III Intestinal Metaplasia an Obligatory Precancerous Lesion in Intestinal-Type Gastric Carcinoma? Eur. J. Cancer Prev. 2001, 10, 307–312. [Google Scholar] [CrossRef]
- Shah, S.C.; Gawron, A.J.; Mustafa, R.A.; Piazuelo, M.B. Histologic Subtyping of Gastric Intestinal Metaplasia: Overview and Considerations for Clinical Practice. Gastroenterology 2020, 158, 745–750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choudhuri, J.; Hall, S.; Castrodad-Rodriguez, C.A.; Westerhoff, M.; El Jabbour, T.; Jain, S.; Panarelli, N.C. Features That Aid Identification of Autoimmune Gastritis in a Background of Active Helicobacter pylori Infection. Arch. Pathol. Lab. Med. 2021, 145, 1536–1543. [Google Scholar] [CrossRef]
- Park, J.Y.; Cornish, T.C.; Lam-Himlin, D.; Shi, C.; Montgomery, E. Gastric Lesions in Patients with Autoimmune Metaplastic Atrophic Gastritis (AMAG) in a Tertiary Care Setting. Am. J. Surg. Pathol. 2010, 34, 1591–1598. [Google Scholar] [CrossRef]
- Coati, I.; Fassan, M.; Farinati, F.; Graham, D.Y.; Genta, R.M.; Rugge, M. Autoimmune Gastritis: Pathologist’s Viewpoint. World J. Gastroenterol. 2015, 21, 12179–12189. [Google Scholar] [CrossRef]
- Borch, K.; Ahrén, B.; Ahlman, H.; Falkmer, S.; Granérus, G.; Grimelius, L. Gastric Carcinoids: Biologic Behavior and Prognosis after Differentiated Treatment in Relation to Type. Ann. Surg. 2005, 242, 64–73. [Google Scholar] [CrossRef]
- Vannella, L.; Lahner, E.; Annibale, B. Risk for Gastric Neoplasias in Patients with Chronic Atrophic Gastritis: A Critical Reappraisal. World J. Gastroenterol. 2012, 18, 1279–1285. [Google Scholar] [CrossRef]
- Murphy, G.; Dawsey, S.M.; Engels, E.A.; Ricker, W.; Parsons, R.; Etemadi, A.; Lin, S.-W.; Abnet, C.C.; Freedman, N.D. Cancer Risk After Pernicious Anemia in the US Elderly Population. Clin. Gastroenterol. Hepatol. 2015, 13, 2282–2289.e4. [Google Scholar] [CrossRef]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and Grading of Gastritis: The Updated Sydney System. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Piazuelo, M.B.; Wilson, K.T. Pathology of Gastric Intestinal Metaplasia: Clinical Implications. Am. J. Gastroenterol. 2010, 105, 493–498. [Google Scholar] [CrossRef] [PubMed]
- de Vries, A.C.; Haringsma, J.; de Vries, R.A.; ter Borg, F.; Nagtzaam, N.M.; Steyerberg, E.W.; van Dekken, H.; Kuipers, E.J. The use of clinical, histologic, and serologic parameters to predict the intragastric extent of intestinal metaplasia: A recommendation for routine practice. Gastrointest. Endosc. 2009, 70, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Capelle, L.G.; de Vries, A.C.; Haringsma, J.; Ter Borg, F.; de Vries, R.A.; Bruno, M.J.; van Dekken, H.; Meijer, J.; van Grieken, N.C.; Kuipers, E.J. The Staging of Gastritis with the OLGA System by Using Intestinal Metaplasia as an Accurate Alternative for Atrophic Gastritis. Gastrointest. Endosc. 2010, 71, 1150–1158. [Google Scholar] [CrossRef]
- Cassaro, M.; Rugge, M.; Gutierrez, O.; Leandro, G.; Graham, D.Y.; Genta, R.M. Topographic Patterns of Intestinal Metaplasia and Gastric Cancer. Am. J. Gastroenterol. 2000, 95, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.K.; Wu, M.-S.; Kakugawa, Y.; Kim, J.J.; Yeoh, K.-G.; Goh, K.L.; Wu, K.-C.; Wu, D.-C.; Sollano, J.; Kachintorn, U.; et al. Screening for Gastric Cancer in Asia: Current Evidence and Practice. Lancet Oncol. 2008, 9, 279–287. [Google Scholar] [CrossRef]
- Hamashima, C.; Goto, R. Potential Capacity of Endoscopic Screening for Gastric Cancer in Japan. Cancer Sci. 2017, 108, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Ribeiro, M.; Areia, M.; de Vries, A.C.; Marcos-Pinto, R.; Monteiro-Soares, M.; O’connor, A.; Pereira, C.; Pimentel-Nunes, P.; Correia, R.; Ensari, A.; et al. Management of Precancerous Conditions and Lesions in the Stomach (MAPS): Guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Virchows Arch. 2012, 460, 19–46. [Google Scholar] [CrossRef] [PubMed]

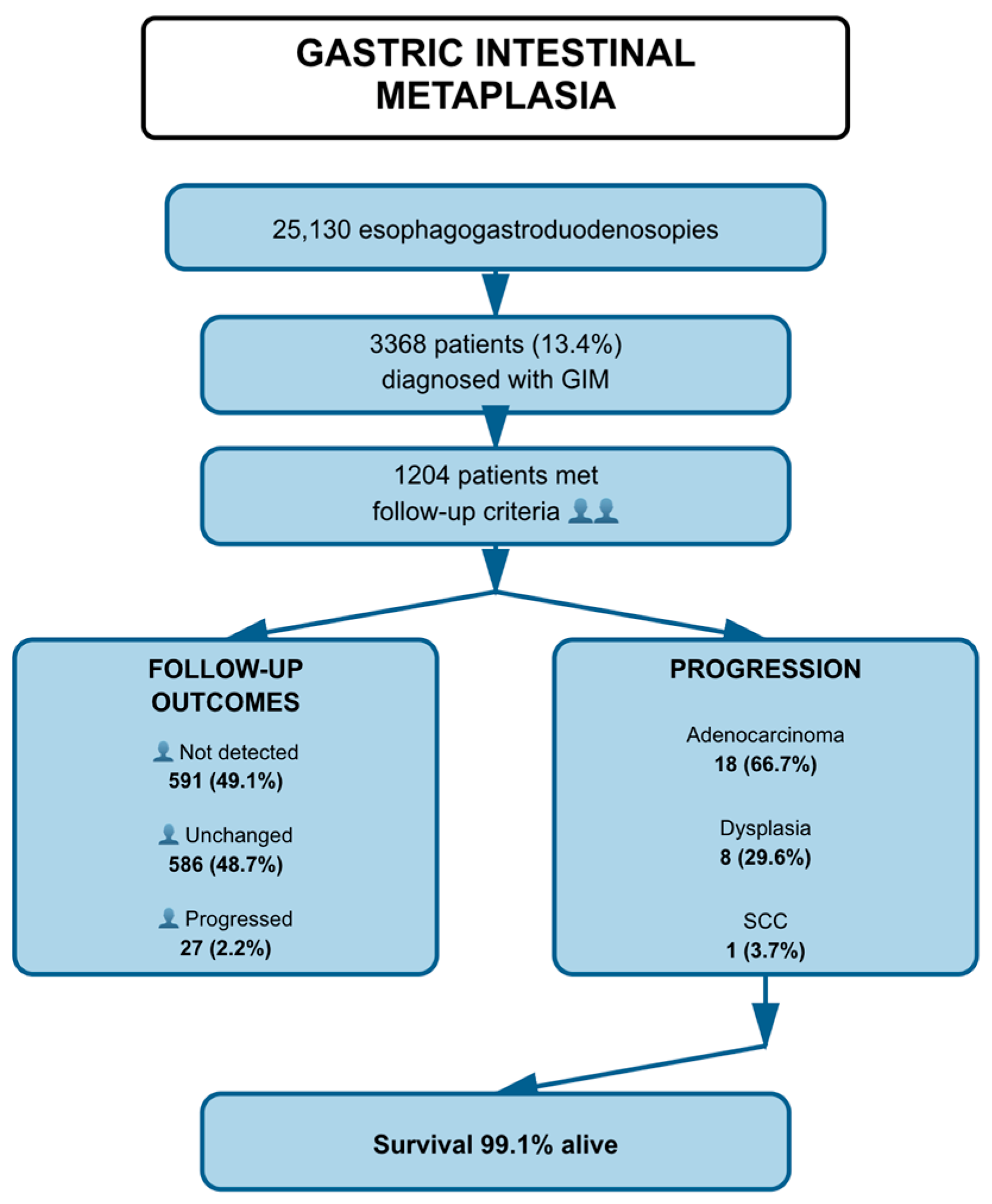
| Category | Variable | n | % |
|---|---|---|---|
| Follow-up Outcome (n = 1204) | Not detected | 591 | 49.1 |
| Unchanged | 586 | 48.7 | |
| Progressed | 27 | 2.2 | |
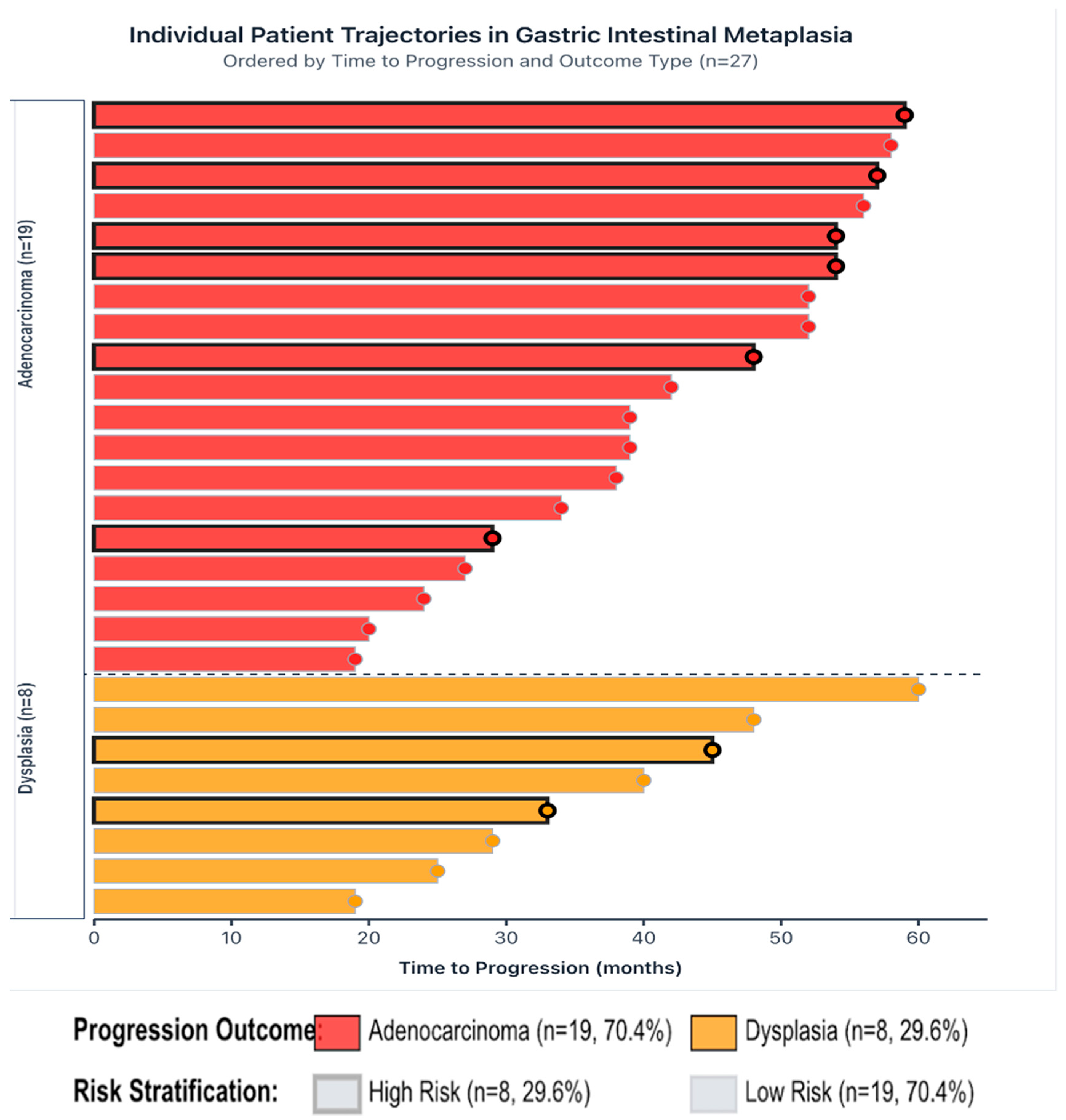
| Pathology Results in Progressed Cases (n = 27) | Adenocarcinoma | 18 | 66.7 |
| Dysplasia | 8 | 29.6 | |
| SCC | 1 | 3.7 | |
| Survival Status (n = 1204) | Alive | 1193 | 99.1 |
| Deceased | 11 | 0.9 |
| Variable | Category | Not Detected n (%)/Mean ± SD | Unchanged n (%)/Mean ± SD | Progressed n (%)/Mean ± SD | p |
|---|---|---|---|---|---|
| Gender | Female | 372 (54.0) | 299 (43.4) | 18 (2.6) | <0.001 |
| Male | 219 (42.5) | 279 (54.2) | 17 (3.3) | ||
| Location of Involvement | Antrum | 540 (52.8) | 455 (44.5) | 28 (2.7) | <0.001 |
| Antrum + Corpus | 51 (28.2) | 123 (68.0) | 7 (3.9) | ||
| ECL Hyperplasia | Present | 12 (44.4) | 15 (55.6) | 0 (0.0) | 0.747 |
| Absent | 579 (49.2) | 563 (47.8) | 35 (3.0) | ||
| H. pylori Presence | Present | 162 (52.6) | 110 (45.5) | 6 (1.9) | 0.233 |
| Absent | 429 (47.9) | 438 (48.9) | 29 (3.2) | ||
| Age (years) | — | 54.8 ± 12.4 | 58.3 ± 11.0 | 60.5 ± 11.6 | <0.001 |
| Follow-up Duration (months) | — | 39.3 ± 26.2 | 38.3 ± 27.2 | 31.2 ± 24.9 | 0.111 |
| Variable | Category | Alive n (%) | Deceased n (%) | p-Value |
|---|---|---|---|---|
| Survival by Follow-up Status (n = 1204) | Not Detected | 587 (99.3) | 4 (0.7) | <0.001 |
| Unchanged | 583 (99.5) | 3 (0.5) | ||
| Progressed | 23 (85.2) | 4 (14.8) |
| Risk Group | Not Detected | Unchanged | Progressed | Total | p Value |
|---|---|---|---|---|---|
| High | 215 (44.0%) | 266 (54.4%) | 8 (1.6%) | 489 (40.6%) | 0.004 |
| Low | 376 (52.6%) | 320 (44.8%) | 19 (2.7%) | 715 (59.4%) | |
| Total | 591 (49.1%) | 586 (48.7%) | 27 (2.2%) | 1204 (100.0%) |
| Pathology Diagnosis | Total n (%) | High Risk n (%) | Low Risk n (%) |
|---|---|---|---|
| Adenocarcinoma | 19 (52.8%) | 6 (31.6%) | 13 (68.4%) |
| NET (Neuroendocrine Tumor) | 5 (13.9%) | 4 (80.0%) | 1 (20.0%) |
| Dysplasia | 8 (22.2%) | 2 (25.0%) | 6 (75.0%) |
| GIST (Gastrointestinal Stromal Tumor) | 3 (8.3%) | 0 (0.0%) | 3 (100.0%) |
| Lymphoma | 1 (2.8%) | 0 (0%) | 1 (100.0%) |
| Total | 36 (100%) | 12 (100%) | 24 (100%) |
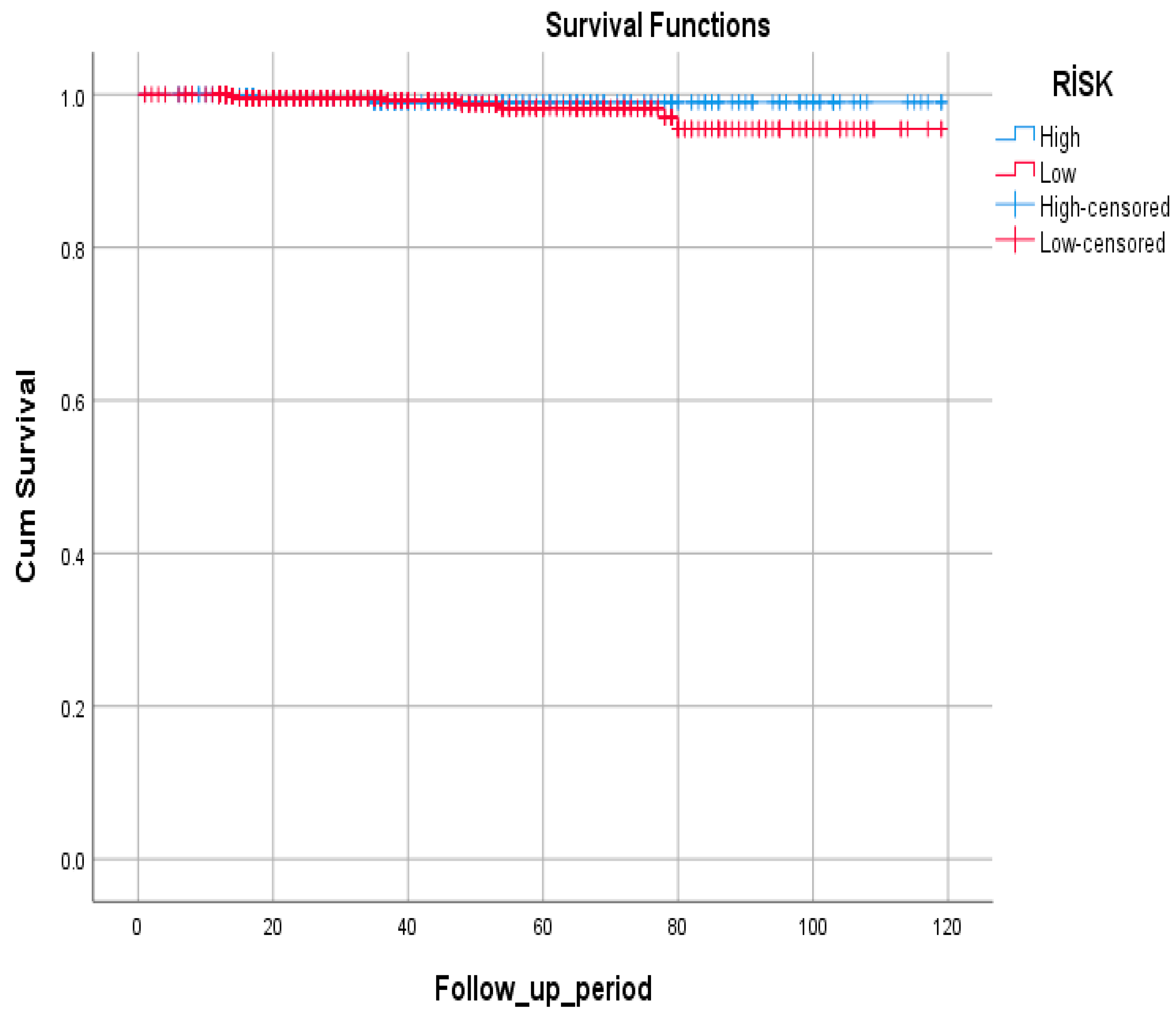
| Risk Group | Alive | Deceased | Total |
|---|---|---|---|
| High | 486 (99.4%) | 3 (0.6%) | 489 |
| Low | 707 (98.9%) | 8 (1.1%) | 715 |
| Total | 1193 (99.1%) | 11 (0.9%) | 1204 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomar, V.B.; Karataş, A.; Abiyev, A.; Albayrak, H.C.; Dumanlı, S.; Haliloğlu, S.; Öz, E.; İnan, M.A.; Cindoruk, M.; Karakan, T.; et al. Gastric Cancer Risk in Patients with Intestinal Metaplasia: Long-Term Outcomes from a Large Single-Center Cohort in Türkiye. J. Clin. Med. 2025, 14, 7662. https://doi.org/10.3390/jcm14217662
Tomar VB, Karataş A, Abiyev A, Albayrak HC, Dumanlı S, Haliloğlu S, Öz E, İnan MA, Cindoruk M, Karakan T, et al. Gastric Cancer Risk in Patients with Intestinal Metaplasia: Long-Term Outcomes from a Large Single-Center Cohort in Türkiye. Journal of Clinical Medicine. 2025; 14(21):7662. https://doi.org/10.3390/jcm14217662
Chicago/Turabian StyleTomar, Veysel Baran, Ali Karataş, Azar Abiyev, Haluk Cihad Albayrak, Serkan Dumanlı, Serhat Haliloğlu, Efkan Öz, Mehmet Arda İnan, Mehmet Cindoruk, Tarkan Karakan, and et al. 2025. "Gastric Cancer Risk in Patients with Intestinal Metaplasia: Long-Term Outcomes from a Large Single-Center Cohort in Türkiye" Journal of Clinical Medicine 14, no. 21: 7662. https://doi.org/10.3390/jcm14217662
APA StyleTomar, V. B., Karataş, A., Abiyev, A., Albayrak, H. C., Dumanlı, S., Haliloğlu, S., Öz, E., İnan, M. A., Cindoruk, M., Karakan, T., & Kekilli, M. (2025). Gastric Cancer Risk in Patients with Intestinal Metaplasia: Long-Term Outcomes from a Large Single-Center Cohort in Türkiye. Journal of Clinical Medicine, 14(21), 7662. https://doi.org/10.3390/jcm14217662







