Artificial Intelligence in Postmenopausal Health: From Risk Prediction to Holistic Care
Abstract
1. Introduction
Impact on Quality of Life
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
- Studies not employing AI or computational models, including standard clinical trials.
- Animal or in vitro studies.
- Conference abstracts, editorials, commentaries, or opinion pieces without original AI data or analysis.
- Studies published before 2010, unless they were foundational AI papers directly relevant to menopausal care.
2.3. Literature Search Strategy
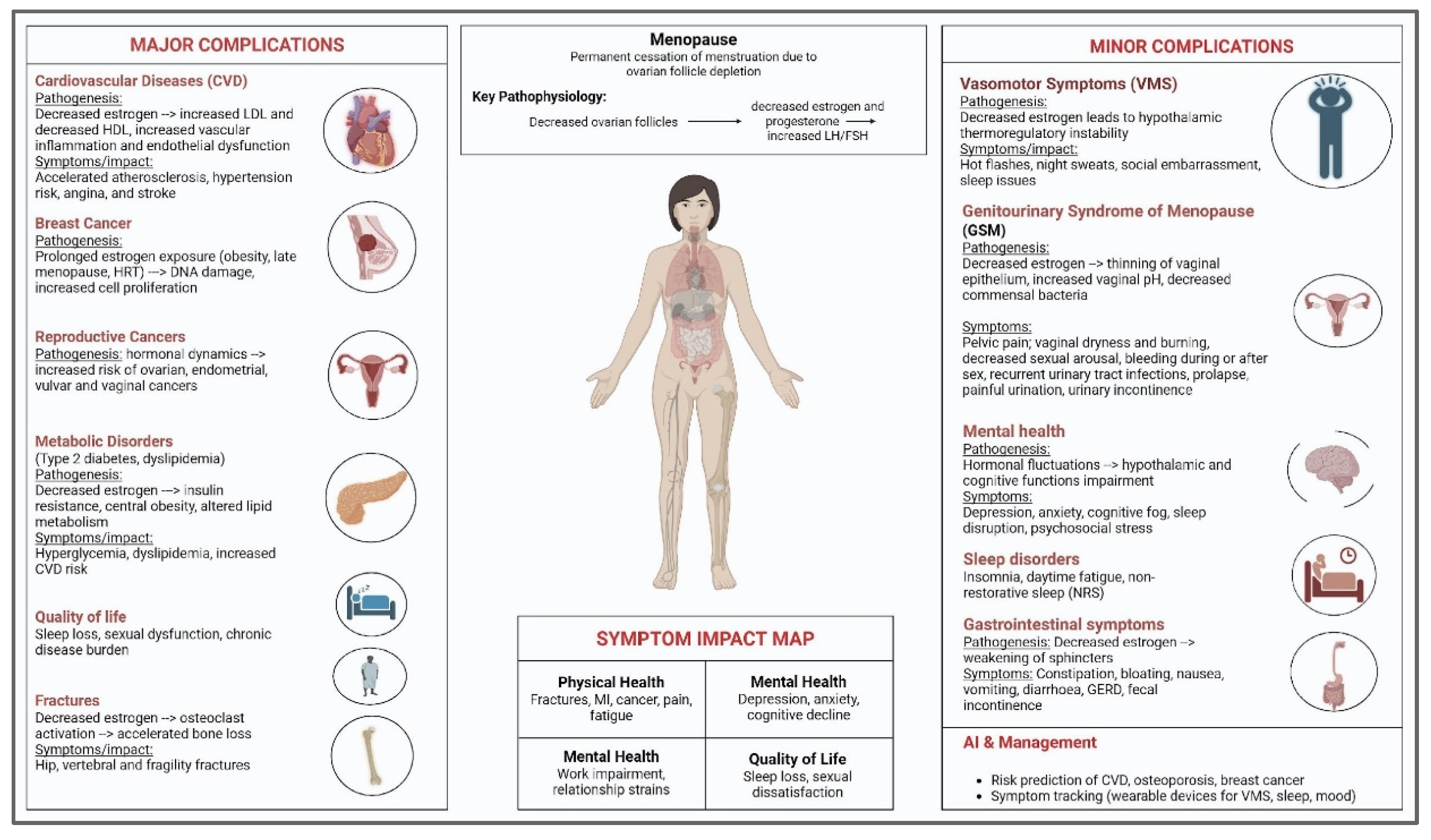
3. Postmenopausal Complications: A Spectrum of Major Complications
3.1. Cardiovascular Disease: Pathophysiology
3.1.1. Atherosclerosis
3.1.2. Angina
3.1.3. Hypertension
3.1.4. Heart Failure
3.1.5. Stroke
3.1.6. Palpitations
3.1.7. Screening, Prevention, and Management
3.2. Cancers
3.2.1. Breast Cancer
3.2.2. Ovarian Cancer
3.2.3. Endometrial Cancer
3.2.4. Vulvar and Vaginal Cancers
3.3. Osteoporosis and Fractures: Hip, Vertebral, and Fragility Fractures
3.4. Metabolic Disorders: Type 2 Diabetes, Lipid Abnormalities
4. Minor Complications
4.1. Vasomotor Symptoms
4.2. Ocular Dysfunctions
4.3. Urological Dysfunctions
4.4. Nephrolithiasis
4.5. Sexual Dysfunction
4.6. Elevation in Blood Pressure
4.7. Sleep Disturbance
4.8. Mood Disorders
4.9. Gastrointestinal Complications
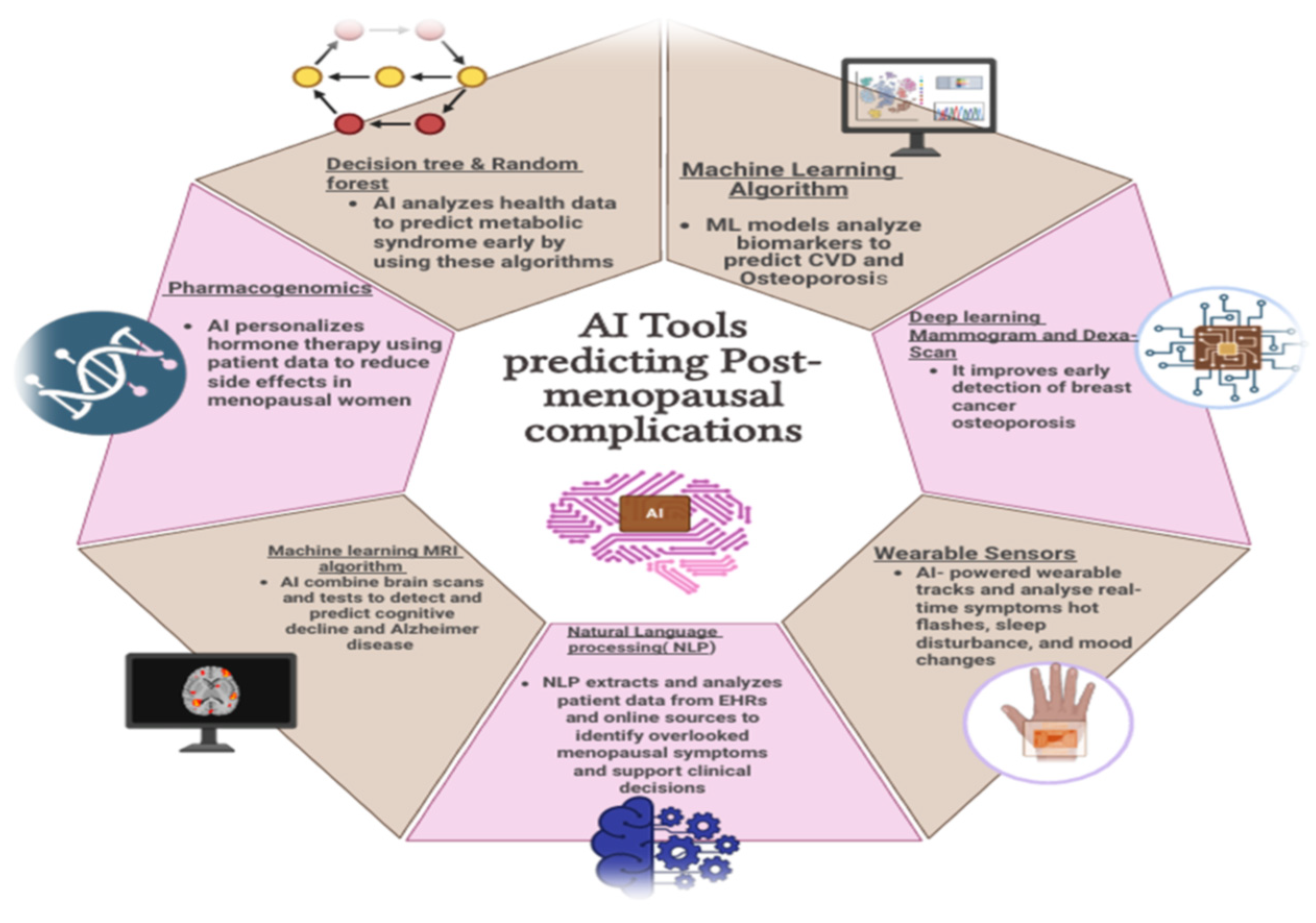
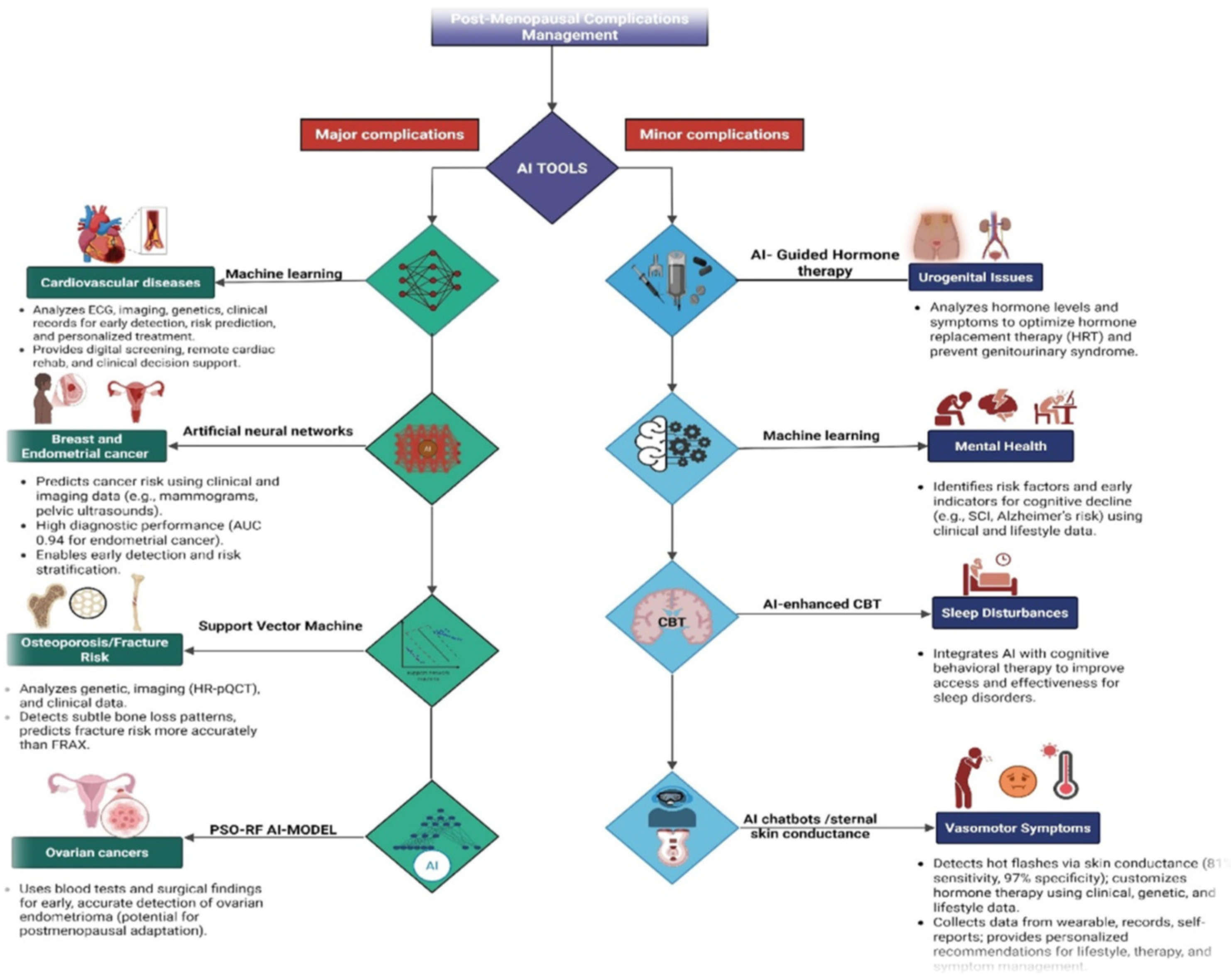
5. Artificial Intelligence (AI) in Risk Prediction
5.1. AI Models Analyzing Biomarkers for Cardiovascular Disease and Osteoporosis
5.2. Imaging AI for Mammography and DEXA Scans
5.3. Metabolic-Syndrome Risk Prediction
5.4. Identifying Minor Complications
5.4.1. Wearable Sensors and AI for Tracking Hot Flashes, Sleep, and Mood
5.4.2. NLP Tools Mining EHRs and Patient Journals for Underreported Symptoms
5.4.3. AI for Cognitive Decline and Alzheimer’s Risk
5.4.4. Pharmacogenomics for Hormone-Therapy Response Prediction
5.5. Comparative Summary and Emerging Directions
6. Discussion
6.1. AI in Managing Major Complications
6.2. AI Managing Minor Complications and Quality of Life Domains
6.3. Critical Analysis and Future Directions
6.4. Practical Integration with Healthcare Systems
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greendale, G.A.; Lee, N.P.; Arriola, E.R. The menopause. Lancet 1999, 353, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Mckinlay, S.M. The normal menopause transition: An overview. Maturitas 1996, 23, 137–145. [Google Scholar] [CrossRef]
- Hill, K. The demography of menopause. Maturitas 1996, 23, 113–127. [Google Scholar] [CrossRef]
- Burger, H.G.; Dudley, E.C.; Robertson, D.M.; Dennerstein, L. Hormonal changes in the menopause transition. Recent Prog. Horm. Res. 2002, 57, 257–276. [Google Scholar] [CrossRef]
- Bruce, D.; Rymer, J. Symptoms of the menopause. Best Pract. Res. Clin. Obstet. Gynaecol. 2009, 23, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.T. Epidemiology of the menopause. Br. Med. Bull. 1992, 48, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Timmana, V.G.; Mattapalli, G.; Gujju, E.; Boddu, P. Understanding menopause-postmenopausal complications, management, and quality of life. Int. J. Pharm. Drug Anal. 2021, 9, 109–125. [Google Scholar] [CrossRef]
- Santoro, N.; Roeca, C.; Peters, B.A.; Neal-Perry, G. The menopause transition: Signs, symptoms, and management options. J. Clin. Endocrinol. Metab. 2021, 106, 1–15. [Google Scholar] [CrossRef]
- McKinlay, S.M.; Brambilla, D.J.; Posner, J.G. The normal menopause transition. Maturitas 1992, 14, 103–115. [Google Scholar] [CrossRef]
- Harlow, B.L.; Signorello, L.B. Factors associated with early menopause. Maturitas 2000, 35, 3–9. [Google Scholar] [CrossRef]
- Sarri, G.; Davies, M.; Lumsden, M.A. Diagnosis and management of menopause: Summary of NICE guidance. BMJ 2015, 351, h5746. [Google Scholar] [CrossRef]
- Lumsden, M.A. The NICE Guideline–Menopause: Diagnosis and management. Climacteric 2016, 19, 426–429. [Google Scholar] [CrossRef]
- Zahn, K.; Pittman, A.; Conklin, J.; Knittel, A.; Neal-Perry, G. Disparities in menopausal care in the United States: A systematic review. Maturitas 2024, 186, 108021. [Google Scholar] [CrossRef]
- Delanerolle, G.; Phiri, P.; Elneil, S.; Talaulikar, V.; Eleje, G.U.; Kareem, R.; Shetty, A.; Saraswath, L.; Kurmi, O.; Benetti-Pinto, C.L.; et al. Menopause: A global health and wellbeing issue that needs urgent attention. Lancet Glob. Health 2025, 13, e196–e198. [Google Scholar] [CrossRef]
- Sharp, C.A.; Dennis, N.; Hobson, G.; Hamilton-Kirkwood, M.; Hughes, K. Where Are the Knowledge Gaps in Menopause Across a Population? A National Cross-Sectional Survey in Wales. Int. J. Environ. Res. Public Health 2025, 22, 287. [Google Scholar] [CrossRef] [PubMed]
- Marlatt, K.L.; Beyl, R.A.; Redman, L.M. A qualitative assessment of health behaviors and experiences during menopause: A cross-sectional, observational study. Maturitas 2018, 116, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Constantine, G.D.; Graham, S.; Clerinx, C.; Bernick, B.A.; Krassan, M.; Mirkin, S.; Currie, H. Behaviours and attitudes influencing treatment decisions for menopausal symptoms in five European countries. Post Reprod. Health 2016, 22, 112–122. [Google Scholar] [CrossRef]
- Berga, S.L.; Garovic, V.D. Barriers to the care of menopausal women. Mayo Clin. Proc. 2019, 94, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Swanson, L.; Stoddard, K.; Fritz, J.; Anderson, B.; Cortez, M.; Conboy, L.; Sheng, X.; Flake, N.; Sanchez-Birkhead, A.; Stark, L.A.; et al. Midlife Women’s Menopausal Transition Symptom Experience and Access to Medical and Integrative Health Care: Informing the Development of MENOGAP. Glob. Adv. Integr. Med. Health 2024, 13, 27536130241268355. [Google Scholar] [CrossRef]
- Li, X.; Chen, F.; Sun, D.; Tao, M. Predicting menopausal symptoms with artificial neural network. Expert Syst. Appl. 2015, 42, 8698–8706. [Google Scholar] [CrossRef]
- Rostami-Moez, M.; Masoumi, S.Z.; Otogara, M.; Farahani, F.; Alimohammadi, S.; Oshvandi, K. Examining the health-related needs of females during menopause: A systematic review study. J. Menopausal Med. 2023, 29, 1. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.P.G.; Birkhäuser, M. Quality of life in climacteric women. Climacteric 2017, 20, 187–194. [Google Scholar] [CrossRef]
- Secinaro, S.; Calandra, D.; Secinaro, A.; Muthurangu, V.; Biancone, P. The role of artificial intelligence in healthcare: A structured literature review. BMC Med. Inform. Decis. Mak. 2021, 21, 125. [Google Scholar] [CrossRef] [PubMed]
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Healthc. J. 2019, 6, 94–98. [Google Scholar] [CrossRef]
- Hamet, P.; Tremblay, J. Artificial intelligence in medicine. Metabolism 2017, 69, S36–S40. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Mascagni, G.; Giannini, A.; Genazzani, A.R.; Simoncini, T. Symptoms of menopause—Global prevalence, physiology and implications. Nat. Rev. Endocrinol. 2018, 14, 199–215. [Google Scholar] [CrossRef]
- Ryczkowska, K.; Adach, W.; Janikowski, K.; Banach, M.; Bielecka-Dabrowa, A. Menopause and women’s cardiovascular health: Is it really an obvious relationship? Arch. Med. Sci. 2022, 19, 458. [Google Scholar] [CrossRef]
- Peters, H.W.; Westendorp, I.C.D.; Hak, A.E.; Grobbee, D.E.; Stehouwer, C.D.A.; Hofman, A.; Witteman, J.C.M. Menopausal status and risk factors for cardiovascular disease. J. Intern. Med. 1999, 246, 521–528. [Google Scholar] [CrossRef]
- Atsma, F.; Bartelink, M.L.E.; Grobbee, D.E.; Van Der Schouw, Y.T. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: A meta-analysis. Menopause 2006, 13, 265–279. [Google Scholar] [CrossRef]
- van der Schouw, Y.T.; van der Graaf, Y.; Steyerberg, E.W.; Eijkemans, M.J.; Banga, J.D. Age at menopause as a risk factor for cardiovascular mortality. Lancet 1996, 347, 714–718. [Google Scholar] [CrossRef]
- Zhu, D.; Chung, H.F.; Dobson, A.J.; Pandeya, N.; Giles, G.G.; Bruinsma, F.; Brunner, E.; Kuh, D.; Hardy, R.; Avis, N.; et al. Age at natural menopause and risk of incident cardiovascular disease: A pooled analysis of individual patient data. Lancet Public Health 2019, 4, e553–e564. [Google Scholar] [CrossRef]
- Bittner, V. Menopause, age, and cardiovascular risk: A complex relationship. J. Am. Coll. Cardiol. 2009, 54, 2374–2375. [Google Scholar] [CrossRef]
- Kannel, W.B.; Hjortland, M.C.; Mcnamara, P.M.; Gordon, T. Menopause and risk of cardiovascular disease: The Framingham study. Ann. Intern. Med. 1976, 85, 447–452. [Google Scholar] [CrossRef]
- Wellons, M.; Ouyang, P.; Schreiner, P.J.; Herrington, D.M.; Vaidya, D. Early menopause predicts future coronary heart disease and stroke: The Multi-Ethnic Study of Atherosclerosis. Menopause 2012, 19, 1081–1087. [Google Scholar] [CrossRef]
- Muka, T.; Oliver-Williams, C.; Kunutsor, S.; Laven, J.; Fauser, B.; Chowdhury, R.; Kavousi, M.; Franco, O. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: A systematic review and meta-analysis. JAMA Cardiol. 2016, 1, 767–776. [Google Scholar] [CrossRef]
- Nappi, R.E.; Cucinella, L. Long-term consequences of menopause. In Female Reproductive Dysfunction; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–13. [Google Scholar]
- Lobo, R.A.; Davis, S.R.; De Villiers, T.J.; Gompel, A.; Henderson, V.W.; Hodis, H.N.; Lumsden, M.A.; Mack, W.J.; Shapiro, S.; Baber, R.J. Prevention of diseases after menopause. Climacteric 2014, 17, 540–556. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.C.; Vitale, C.; Marazzi, G.; Volterrani, M. Menopause and cardiovascular disease: The evidence. Climacteric 2007, 10, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Lambrinoudaki, I.; Stevenson, J.C.; Goulis, D.G. Menopause-associated risk of cardiovascular disease. Endocr. Connect. 2022, 11, e210537. [Google Scholar] [CrossRef] [PubMed]
- Dosi, R.; Bhatt, N.; Shah, P.; Patell, R. Cardiovascular disease and menopause. J. Clin. Diagn. Res. 2014, 8, 62. [Google Scholar] [CrossRef]
- Van der Graaf, Y.; de Kleijn, M.J.; Schouw, Y.V.D. Menopause and cardiovascular disease. J. Psychosom. Obstet. Gynecol. 1997, 18, 113–120. [Google Scholar] [CrossRef]
- Samaan, S.A.; Crawford, M.H. Estrogen and cardiovascular function after menopause. J. Am. Coll. Cardiol. 1995, 26, 1403–1410. [Google Scholar] [CrossRef]
- Nair, A.R.; Pillai, A.J.; Nair, N. Cardiovascular changes in menopause. Curr. Cardiol. Rev. 2021, 17, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Faubion, S.S.; Kuhle, C.L.; Shuster, L.T.; Rocca, W.A. Long-term health consequences of premature or early menopause and considerations for management. Climacteric 2015, 18, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Newson, L. Menopause and cardiovascular disease. Post Reprod. Health 2018, 24, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Bonithon-Kopp, C.; Scarabin, P.Y.; Darne, B.; Malmejac, A.; Guize, L. Menopause-related changes in lipoproteins and some other cardiovascular risk factors. Int. J. Epidemiol. 1990, 19, 42–48. [Google Scholar] [CrossRef]
- Dunneram, Y.; Greenwood, D.C.; Cade, J.E. Diet, menopause and the risk of ovarian, endometrial and breast cancer. Proc. Nutr. Soc. 2019, 78, 438–448. [Google Scholar] [CrossRef]
- Trichopoulos, D.; MacMahon, B.; Cole, P. Menopause and breast cancer risk. J. Natl. Cancer Inst. 1972, 48, 605–613. [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: Individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012, 13, 1141–1151. [Google Scholar] [CrossRef]
- Berrino, F.; Muti, P.; Micheli, A.; Bolelli, G.; Krogh, V.; Sciajno, R.; Secreto, G. Serum sex hormone levels after menopause and subsequent breast cancer. J. Natl. Cancer Inst. 1996, 88, 291–297. [Google Scholar] [CrossRef]
- Paffenbarger Jr, R.S.; Kampert, J.B.; Chang, H.G. Characteristics that predict risk of breast cancer before and after the menopause. Am. J. Epidemiol. 1980, 112, 258–268. [Google Scholar] [CrossRef]
- Byrne, C.; Schairer, C.; Wolfe, J.; Parekh, N.; Salane, M.; Brinton, L.A.; Hoover, R.; Haile, R. Mammographic features and breast cancer risk: Effects with time, age, and menopause status. J. Natl. Cancer Inst. 1995, 87, 1622–1629. [Google Scholar] [CrossRef]
- La Vecchia, C.; Brinton, L.A.; McTiernan, A. Menopause, hormone replacement therapy and cancer. Maturitas 2001, 39, 97–115. [Google Scholar] [CrossRef]
- Fiorica, J. Association of breast cancer and its therapy with menopause-related symptoms. Menopause 2004, 11, 502–504. [Google Scholar] [CrossRef]
- Reeves, G.K.; Beral, V.; Green, J.; Gathani, T.; Bull, D. Hormonal therapy for menopause and breast-cancer risk by histological type: A cohort study and meta-analysis. Lancet Oncol. 2006, 7, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, R.A.; Fireman, B.H. Smoking, menopause, and breast cancer. J. Natl. Cancer Inst. 1986, 76, 833–838. [Google Scholar]
- Ganz, P.A. Breast cancer, menopause, and long-term survivorship: Critical issues for the 21st century. Am. J. Med. 2005, 118, 136–141. [Google Scholar] [CrossRef]
- Britt, K. Menarche, menopause, and breast cancer risk. Lancet Oncol. 2012, 13, 1071–1072. [Google Scholar] [CrossRef]
- Sprague, B.L.; Trentham-Dietz, A.; Egan, K.M.; Titus-Ernstoff, L.; Hampton, J.M.; Newcomb, P.A. Proportion of invasive breast cancer attributable to risk factors modifiable after menopause. Am. J. Epidemiol. 2008, 168, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Einstein, M.H.; Levine, N.F.; Nevadunsky, N.S. Menopause and cancers. Endocrinol. Metab. Clin. 2015, 44, 603–617. [Google Scholar] [CrossRef]
- Hartge, P.; Hoover, R.; Mcgowan, L.; Lesher, L.; Norris, H.J. Menopause and ovarian cancer. Am. J. Epidemiol. 1988, 127, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sun, W.; Liu, H.; Zhang, D. Age at menopause and risk of developing endometrial cancer: A meta-analysis. BioMed Res. Int. 2019, 2019, 8584130. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Hampson, G. The pathogenesis, diagnosis, investigation and management of osteoporosis. J. Clin. Pathol. 2011, 64, 1042–1050. [Google Scholar] [CrossRef]
- Ji, M.X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar] [CrossRef]
- Cummings, S.R.; Ettinger, B.; Delmas, P.D.; Kenemans, P.; Stathopoulos, V.; Verweij, P.; Mol-Arts, M.; Kloosterboer, L.; Mosca, L.; Christiansen, C.; et al. The effects of tibolone in older postmenopausal women. N. Engl. J. Med. 2008, 359, 697–708. [Google Scholar] [CrossRef]
- Yong, E.L.; Logan, S. Menopausal osteoporosis: Screening, prevention and treatment. Singap. Med. J. 2021, 62, 159. [Google Scholar] [CrossRef]
- Jeong, H.G.; Park, H. Metabolic disorders in menopause. Metabolites 2022, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Kim, H.S. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Petrillo, T.; Semprini, E.; Aio, C.; Foschi, M.; Ambrosetti, F.; Sponzilli, A.; Ricciardiello, F.; Battipaglia, C. Metabolic syndrome, insulin resistance, and menopause: The changes in body structure and the therapeutic approach. Gynecol. Reprod. Endocrinol. Metab. 2024, 4, 86–91. [Google Scholar]
- Mounier-Vehier, C.; Madika, A.L. Post-menopausal hypertension: Detecting, treating, accompany, prevent. Presse Medicale 2019, 48, 1288–1294. [Google Scholar] [CrossRef]
- Altura, B.M. Sex as a factor influencing the responsiveness of arterioles to catecholamines. Eur. J. Pharmacol. 1972, 20, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, I.A. Ocular hypertensive effect of menopause with and without systemic hypertension. Acta Obstet. Gynecol. Scand. 1996, 75, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Ziemanski, J.F.; Wolters, L.R.; Jones-Jordan, L.; Nichols, J.J.; Nichols, K.K. Relation between dietary essential fatty acid intake and dry eye disease and meibomian gland dysfunction in postmenopausal women. Am. J. Ophthalmol. 2018, 189, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Gębka, N.; Głogowska-Szeląg, J.; Adamczyk, J.; Gębka-Kępińska, B.; Szeląg, M.; Kępiński, M. The most common urological conditions in postmenopausal women. Wiad. Lek. 2022, 75, 2026–2030. [Google Scholar] [CrossRef] [PubMed]
- Sitini, B.; Ntihinyurwa, P.; Ntirushwa, D.; Mafende, L.; Small, M.; Rulisa, S. Prevalence, impact and management of postmenopausal symptoms among postmenopausal women in Rwanda. Climacteric 2023, 26, 613–618. [Google Scholar] [CrossRef]
- Proserpio, P.; Marra, S.; Campana, C.; Agostoni, E.C.; Palagini, L.; Nobili, L.; Nappi, R.E. Insomnia and menopause: A narrative review on mechanisms and treatments. Climacteric 2020, 23, 539–549. [Google Scholar] [CrossRef]
- Stone, A.B.; Pearlstein, T.B. Evaluation and treatment of changes in mood, sleep, and sexual functioning associated with menopause. Obstet. Gynecol. Clin. N. Am. 1994, 21, 391–403. [Google Scholar] [CrossRef]
- Shaw, N.; Pettinger, C. Gastrointestinal Symptoms in the Peri-and Postmenopause: A Protocol for a Scoping Review; University of Plymouth: Plymouth, UK, 2024. [Google Scholar]
- Lenhart, A.; Naliboff, B.; Shih, W.; Gupta, A.; Tillisch, K.; Liu, C.; Mayer, E.A.; Chang, L. Postmenopausal women with irritable bowel syndrome (IBS) have more severe symptoms than premenopausal women with IBS. Neurogastroenterol. Motil. 2020, 32, e13913. [Google Scholar] [CrossRef]
- Lurati, A.R. Effects of menopause on appetite and the gastrointestinal system. Nurs. Women’s Health 2018, 22, 499–505. [Google Scholar] [CrossRef]
- Xie, X.; Song, J.; Wu, Y.; Li, M.; Guo, W.; Li, S.; Li, Y. Study on gut microbiota and metabolomics in postmenopausal women. BMC Women’s Health 2024, 24, 608. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, H.; Liu, M.; Gao, J.; Xie, L.; Zhang, C.; Wei, L.; Ding, Y.; Jiang, H. Detecting cardiovascular diseases using unsupervised machine learning clustering based on electronic medical records. BMC Med. Res. Methodol. 2024, 24, 309. [Google Scholar] [CrossRef]
- Ahn, J.S.; Shin, S.; Yang, S.-A.; Park, E.K.; Kim, K.H.; Cho, S.I.; Ock, C.-Y.; Kim, S. Artificial intelligence in breast cancer diagnosis and personalized medicine. J. Breast Cancer 2023, 26, 405. [Google Scholar] [CrossRef]
- Han, G.-R.; Goncharov, A.; Eryilmaz, M.; Ye, S.; Palanisamy, B.; Ghosh, R.; Lisi, F.; Rogers, E.; Guzman, D.; Yigci, D.; et al. Machine learning in point-of-care testing: Innovations, challenges, and opportunities. Nat. Commun. 2025, 16, 3165. [Google Scholar] [CrossRef]
- Ramesh, A.; Nayak, T.; Beestrum, M.; Quer, G.; Pandit, J.A. Heart rate variability in psychiatric disorders: A systematic review. Neuropsychiatr. Dis. Treat. 2023, 19, 2217–2239. [Google Scholar] [CrossRef]
- Carlson, K.; Nguyen, H. Genitourinary Syndrome of Menopause. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Khatiwada, P.; Yang, B.; Lin, J.C.; Blobel, B. Patient-generated health data (PGHD): Understanding, requirements, challenges, and existing techniques for data security and privacy. J. Pers. Med. 2024, 14, 282. [Google Scholar] [CrossRef] [PubMed]
- Vrudhula, A.; Kwan, A.C.; Ouyang, D.; Cheng, S. Machine learning and bias in medical imaging: Opportunities and challenges. Circ. Cardiovasc. Imaging 2024, 17, e015495. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Graffy, P.M.; Zea, R.; Lee, S.J.; Liu, J.; Sandfort, V.; Summers, R.M. Automated CT biomarkers for opportunistic prediction of future cardiovascular events and mortality in an asymptomatic screening population: A retrospective cohort study. Lancet Digit. Health 2020, 2, e192–e200. [Google Scholar] [CrossRef]
- Ong, W.; Liu, R.W.; Makmur, A.; Low, X.Z.; Sng, W.J.; Tan, J.H.; Kumar, N.; Hallinan, J.T.P.D. Artificial intelligence applications for osteoporosis classification using computed tomography. Bioengineering 2023, 10, 1364. [Google Scholar] [CrossRef]
- Davis, S.R.; Pinkerton, J.; Santoro, N.; Simoncini, T. Menopause—Biology, consequences, supportive care, and therapeutic options. Cell 2023, 186, 4038–4058. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.E.; Ahmed, S.G.; Ellatif, M.A.B.A. Effectiveness of Health Promoting Based Program versus Artificial Intelligence Based Program on Quality of Life among Menopausal Women. Egypt. J. Health Care 2024, 15, 1411–1432. [Google Scholar] [CrossRef]
- Adedinsewo, D.A.; Pollak, A.W.; Phillips, S.D.; Smith, T.L.; Svatikova, A.; Hayes, S.N.; Mulvagh, S.L.; Norris, C.; Roger, V.L.; Noseworthy, P.A.; et al. Cardiovascular disease screening in women: Leveraging artificial intelligence and digital tools. Circ. Res. 2022, 130, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Erdemoglu, E.; Serel, T.A.; Karacan, E.; Köksal, O.K.; Turan, İ.; Öztürk, V.; Bozkurt, K.K. Artificial intelligence for prediction of endometrial intraepithelial neoplasia and endometrial cancer risks in pre-and postmenopausal women. AJOG Glob. Rep. 2023, 3, 100154. [Google Scholar] [CrossRef]
- Yoldemir, T. Artificial intelligence and women’s health. Climacteric 2020, 23, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Pan, W.; Jin, L.; Li, Y.; Geng, Y.; Gao, C.; Chen, G.; Wang, H.; Ma, D.; Liao, S. Artificial intelligence in reproductive medicine. Reproduction 2019, 158, R139–R154. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, W.; Lin, X.; Li, F. AI algorithms for accurate prediction of osteoporotic fractures in patients with diabetes: An up-to-date review. J. Orthop. Surg. Res. 2023, 18, 956. [Google Scholar] [CrossRef]
- Whittier, D.E.; Samelson, E.J.; Hannan, M.T.; Burt, L.A.; Hanley, D.A.; Biver, E.; Szulc, P.; Sornay-Rendu, E.; Merle, B.; Chapurlat, R.; et al. Bone microarchitecture phenotypes identified in older adults are associated with different levels of osteoporotic fracture risk. J. Bone Miner. Res. 2020, 37, 428–439. [Google Scholar] [CrossRef]
- Garg, R.; Munshi, A. Revolutionizing Menopause Management: Harnessing the Potential of Artificial Intelligence. J. Mid-Life Health 2024, 15, 53–54. [Google Scholar] [CrossRef]
- Vargas-Hernandez, V.M. Artificial Intelligence in Menopause Management. Mathews J. Gynecol. Obstet. 2025, 9, 1–4. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G.; Boutsinas, B. Digital and AI-Enhanced Cognitive Behavioral Therapy for Insomnia: Neurocognitive Mechanisms and Clinical Outcomes. J. Clin. Med. 2025, 14, 2265. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Lin, F.; Hu, Q.; Tang, Z.; Jin, C. AI-enabled diagnosis of spontaneous rupture of ovarian endometriomas: A PSO enhanced random forest approach. IEEE Access 2020, 8, 132253–132264. [Google Scholar] [CrossRef]
- Diaz, Z.M.R.; Muka, T.; Franco, O.H. Personalized solutions for menopause through artificial intelligence: Are we there yet? Maturitas 2019, 129, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Schwalbe, N.; Wahl, B. Artificial intelligence and the future of global health. Lancet 2020, 395, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Wahl, B.; Cossy-Gantner, A.; Germann, S.; Schwalbe, N.R. Artificial intelligence (AI) and global health: How can AI contribute to health in resource-poor settings? BMJ Glob. Health 2018, 3, e000798. [Google Scholar] [CrossRef] [PubMed]
- Nan, T.; Zheng, S.; Qiao, S.; Quan, H.; Gao, X.; Niu, J.; Zheng, B.; Guo, C.; Zhang, Y.; Wang, X.; et al. Deep learning quantifies pathologists’ visual patterns for whole-slide image classification. Nat. Biomed. Eng. 2025, 16, 5493. [Google Scholar] [PubMed]
- Hu, D.; Liang, K.; Dong, Z.; Wang, J.; Zhao, Y.; He, K. Effective multi-modal clustering method via skip aggregation network for parallel scRNA-seq and scATAC-seq data. Brief. Bioinform. 2024, 25, bbae102. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Liu, J.; Chen, S. High-order topology for deep single-cell multiview fuzzy clustering. IEEE Trans. Fuzzy Syst. 2024, 32, 4448–4459. [Google Scholar] [CrossRef]
- Hadley, T.D.; Pettit, R.W.; Malik, T.; Khoei, A.A.; Salihu, H.M. Artificial intelligence in global health—A framework and strategy for adoption and sustainability. Int. J. Matern. Child Health AIDS 2020, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Woods, D.; Jordan, N.; Starren, J. The role of artificial intelligence for the application of integrating electronic health records and patient-generated data in clinical decision support. AMIA Summits Transl. Sci. Proc. 2024, 2024, 459–467. [Google Scholar]
- Jones, C.; Neal, D.; Hillman, T. Artificial intelligence and clinical decision support. BMJ Health Care Inform. 2023, 30, e100810. [Google Scholar] [PubMed Central]
- Perez, K.; Wisniewski, D.; Ari, A.; Lee, K.; Lieneck, C.; Ramamonjiarivelo, Z. Investigation into application of AI and telemedicine in rural communities: A systematic literature review. Healthcare 2025, 13, 324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Butzner, M.; Cuffee, Y. Telehealth interventions and outcomes across rural communities in the United States: Narrative review. J. Med. Internet Res. 2021, 23, e29575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, K.E.; Davis, S.E. Gaps in artificial intelligence research for rural healthcare in the United States: A scoping review. medRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Elhaddad, M.; Hamam, S. AI-driven clinical decision support systems: An ongoing pursuit of potential. Cureus 2024, 16, e57609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ouanes, K.; Farhah, N. Effectiveness of AI-driven clinical decision support systems on patient and clinical outcomes: Systematic review. Digit Health 2024, 10, 205520762412. [Google Scholar] [PubMed]
- Glasgow, R.E.; Harden, S.M.; Gaglio, B.; Rabin, B.; Smith, M.L.; Porter, G.C.; Ory, M.G.; Estabrooks, P.A. RE-AIM planning and evaluation framework: Adapting to new science and practice with a 20-year review. Front Public Health 2019, 7, 64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hong, N.; Cho, S.W.; Shin, S.; Lee, S.; AJang, S.; Roh, S.; Lee, Y.H.; Rhee, Y.; Cummings, S.R.; Kim, H.; et al. Deep-learning-based detection of vertebral fracture and osteoporosis using lateral spine X-ray radiography. J. Bone Miner. Res. 2020, 38, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Baker, F.C.; Lampio, L.; Saaresranta, T.; Polo-Kantola, P. Sleep and sleep disorders in the menopausal transition. Sleep Med. Clin. 2018, 13, 443–456. [Google Scholar] [CrossRef] [PubMed]
| Year | Author/Study | Technique | Results and Limitations |
|---|---|---|---|
| 2023 | Ahn et al. [83] | Review of AI in breast cancer diagnosis and personalized treatment | AI shows promise in screening, staging, and treatment prediction, especially in imaging and pathology. Limitations include clinical validation needs, model generalizability, and integration challenges. |
| 2025 | Carlson & Nguyen [86] | Educational Review on GSM | Discusses hormonal changes and symptoms during menopause and outlines treatment options. Limitations include underdiagnosis and hesitancy among women to seek care. |
| 2024 | Hu et al. [82] | Unsupervised ML clustering using EMR data (K-means, PCA) | Achieved predictive accuracy > 85% in identifying CVD cases. Limitations include a lack of longitudinal design and a need for further refinement for clinical use. |
| 2024 | Khatiwada et al. [87] | Systematic review using PRISMA and Rayyan on PGHD | Identified key challenges in PGHD, including privacy, security, and stakeholder understanding. Limitations include fragmented standards and a lack of regulatory clarity. |
| 2023 | Ong et al. [90] | Systematic review of AI classification of osteoporosis via CT | AI achieved accuracy between 61.8 and 99.4% using CT scans. Limitations involve variability in methods, need for validation, and comparison with the DEXA gold standard. |
| 2023 | Davis et al. [91] | Scientific review on menopause biology and treatment | MHT and non-hormonal treatments are effective; personalized care is emphasized. Limitations include a lack of data on perimenopausal women and treatment safety. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panjwani, G.A.R.; Maddukuri, S.; Ansari, R.A.; Jain, S.; Chavan, M.; Gogula, N.S.A.R.; Yerrapragada, G.; Elangovan, P.; Shariff, M.N.; Natarajan, T.; et al. Artificial Intelligence in Postmenopausal Health: From Risk Prediction to Holistic Care. J. Clin. Med. 2025, 14, 7651. https://doi.org/10.3390/jcm14217651
Panjwani GAR, Maddukuri S, Ansari RA, Jain S, Chavan M, Gogula NSAR, Yerrapragada G, Elangovan P, Shariff MN, Natarajan T, et al. Artificial Intelligence in Postmenopausal Health: From Risk Prediction to Holistic Care. Journal of Clinical Medicine. 2025; 14(21):7651. https://doi.org/10.3390/jcm14217651
Chicago/Turabian StylePanjwani, Gianeshwaree Alias Rachna, Srivarshini Maddukuri, Rabiah Aslam Ansari, Samiksha Jain, Manisha Chavan, Naga Sai Akhil Reddy Gogula, Gayathri Yerrapragada, Poonguzhali Elangovan, Mohammed Naveed Shariff, Thangeswaran Natarajan, and et al. 2025. "Artificial Intelligence in Postmenopausal Health: From Risk Prediction to Holistic Care" Journal of Clinical Medicine 14, no. 21: 7651. https://doi.org/10.3390/jcm14217651
APA StylePanjwani, G. A. R., Maddukuri, S., Ansari, R. A., Jain, S., Chavan, M., Gogula, N. S. A. R., Yerrapragada, G., Elangovan, P., Shariff, M. N., Natarajan, T., Janarthanan, J., Karrupiah, S. S., Gopalakrishnan, K., Sood, D., & Arunachalam, S. P. (2025). Artificial Intelligence in Postmenopausal Health: From Risk Prediction to Holistic Care. Journal of Clinical Medicine, 14(21), 7651. https://doi.org/10.3390/jcm14217651






