Long-Term Outcome of Endothelial Keratoplasty Among Glaucoma Patients and the Risk of Prostaglandin Analogue (Latanoprost) Use on Graft Rejection
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patients
2.2. Patient Selection and Surgical Approach
2.3. Variables
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
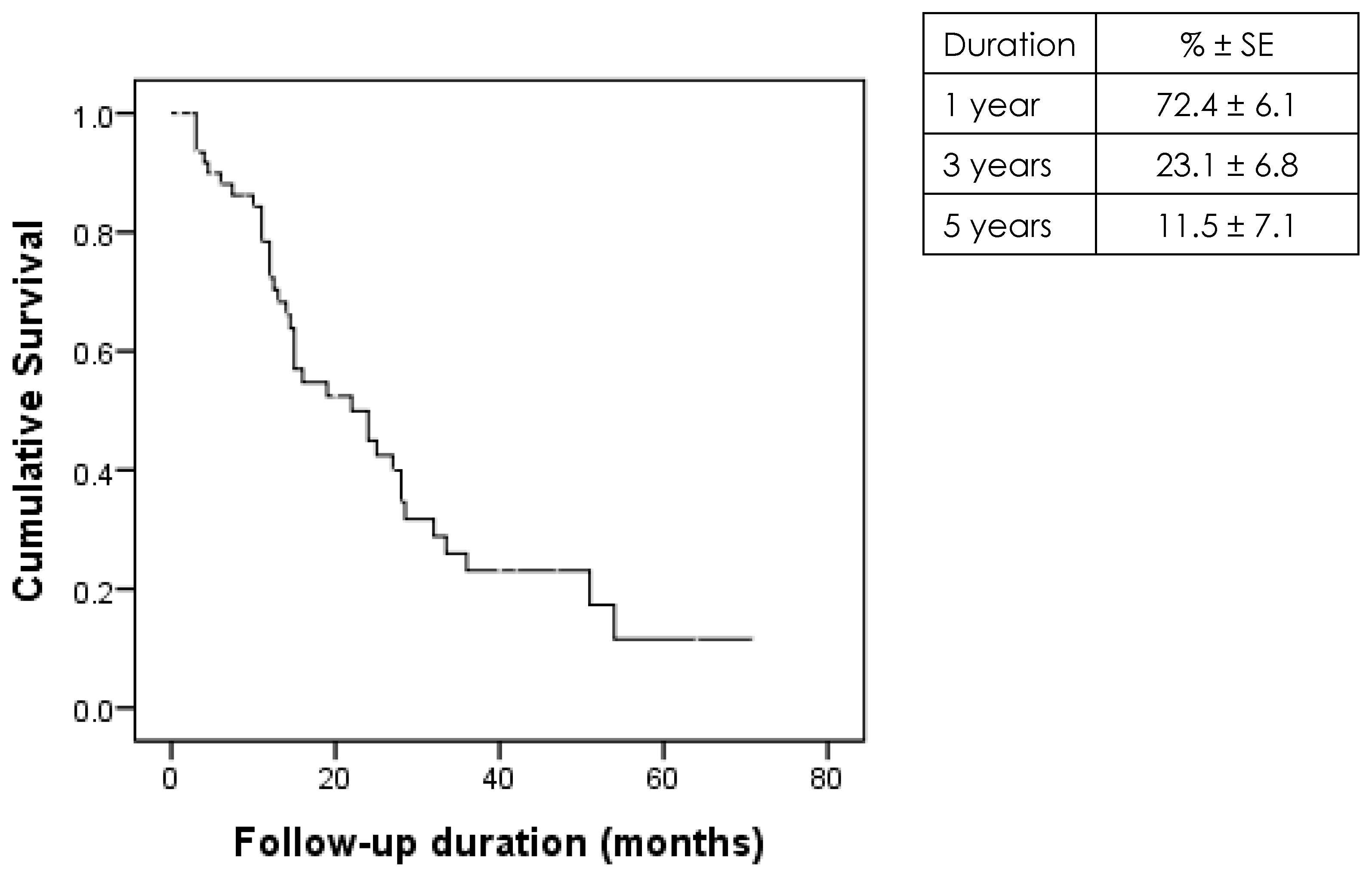
3.2. Graft Survival Rates
3.3. Antiglaucoma Drop Use and the Risk of Graft Failure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, D.A.; Kelley, M.; Price, F.W., Jr.; Price, M.O. Five-Year Graft Survival of Descemet Membrane Endothelial Keratoplasty (EK) versus Descemet Stripping EK and the Effect of Donor Sex Matching. Ophthalmology 2018, 125, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.A.; Aldave, A.J.; Szczotka-Flynn, L.B.; Liang, W.; Ayala, A.R.; Maguire, M.G.; Croasdale, C.; Daoud, Y.J.; Dunn, S.P.; Hoover, C.K.; et al. Donor, Recipient, and Operative Factors Associated with Graft Success in the Cornea Preservation Time Study. Ophthalmology 2018, 125, 1700–1709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wakimasu, K.; Kitazawa, K.; Kayukawa, K.; Yokota, I.; Inatomi, T.; Hieda, O.; Sotozono, C.; Kinoshita, S. Five-year follow-up outcomes after Descemet’s stripping automated endothelial keratoplasty: A retrospective study. BMJ Open Ophthalmol. 2020, 5, e000354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, L.; Hollick, E.J. Long-term Outcomes of Descemet Stripping Endothelial Keratoplasty: Ten-Year Graft Survival and Endothelial Cell Loss. Am. J. Ophthalmol. 2022, 234, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, T.I.; Gauché, L.; Böhringer, D.; Maier, P.; Heinzelmann, S.; Glegola, M.; Kammrath Betancor, P.; Reinhard, T. Ten-year outcomes after DMEK, DSAEK, and PK: Insights on graft survival, endothelial cell density loss, rejection and visual acuity. Sci. Rep. 2025, 15, 1249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wandling, G.R., Jr.; Rauen, M.P.; Goins, K.M.; Kitzmann, A.S.; Sutphin, J.E.; Kwon, Y.H.; Alward, W.L.; Wagoner, M.D. Glaucoma therapy escalation in eyes with pseudophakic corneal edema after penetrating keratoplasty and Descemet’s stripping automated endothelial keratoplasty. Int. Ophthalmol. 2012, 32, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Aldarrab, A.; Alsakran, W.; Al-Swailem, S.A.; Al-Shahwan, S.A. Comparison of Glaucoma Therapy Escalation After Penetrating Keratoplasty to Descemet Stripping Automated Endothelial Keratoplasty for the Treatment of Pseudophakic Bullous Keratopathy: A Cohort Study. Middle East Afr. J. Ophthalmol. 2023, 29, 72–79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takemori, H.; Higashide, T.; Kobayashi, A.; Yokogawa, H.; Sugiyama, K. Glaucoma-Related Risk Factors for Endothelial Cell Loss and Graft Failure After Descemet’s Stripping Automated Endothelial Keratoplasty. J. Glaucoma 2023, 32, e95–e102. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, I.B.; Ivarsen, A.; Hjortdal, J. Graft rejection and failure following endothelial keratoplasty (DSAEK) and penetrating keratoplasty for secondary endothelial failure. Acta Ophthalmol. 2015, 93, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Decroos, F.C.; Delmonte, D.W.; Chow, J.H.; Stinnett, S.S.; Kim, T.; Carlson, A.N.; Afshari, N.A. Increased Rates of Descemet’s Stripping Automated Endothelial Keratoplasty (DSAEK) Graft Failure and Dislocation in Glaucomatous Eyes with Aqueous Shunts. J. Ophthalmic Vis. Res. 2012, 7, 203–213. [Google Scholar] [PubMed] [PubMed Central]
- Aiello, F.; Matarazzo, F.; Phylactou, M.; Muthusamy, K.; Maurino, V. Endothelial Keratoplasty Following Glaucoma Filtration Surgery: A UK Tertiary Eye Care Referral Centre Experience. J. Clin. Med. 2024, 13, 6097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ward, M.S.; Goins, K.M.; Greiner, M.A.; Kitzmann, A.S.; Sutphin, J.E.; Alward, W.L.; Greenlee, E.C.; Kwon, Y.H.; Zimmerman, M.B.; Wagoner, M.D. Graft survival versus glaucoma treatment after penetrating or Descemet stripping automated endothelial keratoplasty. Cornea 2014, 33, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Quek, D.T.; Wong, T.; Tan, D.; Mehta, J.S. Corneal graft survival and intraocular pressure control after descemet stripping automated endothelial keratoplasty in eyes with pre-existing glaucoma. Am. J. Ophthalmol. 2011, 152, 48–54.e2. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Kaufmann, C.; Bachmann, L.M.; Tarantino-Scherrer, J.N.; Thiel, M.A.; Bochmann, F. Changes in intraocular pressure after descemet stripping automated endothelial keratoplasty: A retrospective analysis. Cornea 2015, 34, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Alshaker, S.; Mimouni, M.; Batawi, H.; Cohen, E.; Trinh, T.; Santaella, G.; Chan, C.C.; Slomovic, A.R.; Rootman, D.S.; Sorkin, N. Four-Year Survival Comparison of Endothelial Keratoplasty Techniques in Patients With Previous Glaucoma Surgery. Cornea 2021, 40, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.J.; Ritterband, D.C.; Lai, K.; Liebmann, J.M.; Seedor, J.A. Descemet Stripping Endothelial Keratoplasty in Eyes With Previous Glaucoma Surgery. Cornea 2016, 35, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Chiam, P.J.; Cheeseman, R.; Ho, V.W.; Romano, V.; Choudhary, A.; Batterbury, M.; Kaye, S.B.; Willoughby, C.E. Outcome of Descemet stripping automated endothelial keratoplasty in eyes with an Ahmed glaucoma valve. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Nahum, Y.; Mimouni, M.; Busin, M. Risk Factors Predicting the Need for Graft Exchange After Descemet Stripping Automated Endothelial Keratoplasty. Cornea 2015, 34, 876–879. [Google Scholar] [CrossRef] [PubMed]
- Fuest, M.; Ang, M.; Htoon, H.M.; Tan, D.; Mehta, J.S. Long-term Visual Outcomes Comparing Descemet Stripping Automated Endothelial Keratoplasty and Penetrating Keratoplasty. Am. J. Ophthalmol. 2017, 182, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Anshu, A.; Price, M.O.; Price, F.W. Descemet’s stripping endothelial keratoplasty: Long-term graft survival and risk factors for failure in eyes with pre-existing glaucoma. Ophthalmology 2012, 119, 1982–1987. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Khashabi, S.; Chopra, V.; Francis, B.; Heur, M.; Song, J.C.; Yiu, S.C. Descemet stripping with automated endothelial keratoplasty: A comparative study of outcome in patients with pre-existing glaucoma. Saudi J. Ophthalmol. 2013, 27, 73–78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schoenberg, E.D.; Levin, K.H.; Savetsky, M.J.; Mcintire, L.U.; Ayyala, R.S. Surgical Outcomes of DSAEK in Patients with Prior Ahmed Glaucoma Drainage Device Placement. Eur. J. Ophthalmol. 2013, 23, 807–813. [Google Scholar] [CrossRef]
- Aldave, A.J.; Chen, J.L.; Zaman, A.S.; Deng, S.X.; Yu, F. Outcomes After DSEK in 101 Eyes With Previous Trabeculectomy and Tube Shunt Implantation. Cornea 2014, 33, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Iverson, S.M.; Spierer, O.; Papachristou, G.C.; Feuer, W.J.; Shi, W.; Greenfield, D.S.; O’Brien, T.P. Comparison of primary graft survival following penetrating keratoplasty and Descemet’s stripping endothelial keratoplasty in eyes with prior trabeculectomy. Br. J. Ophthalmol. 2015, 99, 1477–1482. [Google Scholar] [CrossRef]
- Maurino, V.; Aiello, F. Glaucoma risks in advanced corneal surgery. Prog. Brain Res. 2015, 221, 271–295. [Google Scholar] [CrossRef]
- Kim, P.; Amiran, M.D.; Lichtinger, A.; Yeung, S.N.; Slomovic, A.R.; Rootman, D.S. Outcomes of Descemet stripping automated endothelial keratoplasty in patients with previous glaucoma drainage device insertion. Cornea 2012, 31, 172–175. [Google Scholar] [CrossRef]
- Wiaux, C.; Baghdasaryan, E.; Lee, O.L.; Bourges, J.L.; Deng, S.X.; Yu, F.; Aldave, A.J. Outcomes after Descemet stripping endothelial keratoplasty in glaucoma patients with previous trabeculectomy and tube shunt implantation. Cornea 2011, 30, 1304–1311. [Google Scholar] [CrossRef]
- Kaleem, M.; Ridha, F.; Shwani, Z.; Swenor, B.; Goshe, J.; Singh, A. Rates of Intraocular Pressure Elevation and Use of Topical Antihypertensive Medication After Descemet Stripping Automated Endothelial Keratoplasty. Cornea 2017, 36, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Jafari, K.; Ashena, Z.; Niestrata, M. A Review of Outcomes of Descemet Membrane Endothelial Keratoplasty and Descemet Stripping Automated Endothelial Keratoplasty Interventions in Patients with Pre-Existing Glaucoma. J. Clin. Med. 2025, 14, 3534. [Google Scholar] [CrossRef]
- Alm, A.; Stjernschantz, J. Effects on intraocular pressure and side effects of 0.005% latanoprost applied once daily, evening or morning. A comparison with timolol. Ophthalmology 1995, 102, 1743–1752. [Google Scholar] [CrossRef]
- Camras, C.B. Comparison of latanoprost and timolol in patients with ocular hypertension and glaucoma: A six-month masked, multicenter trial in the United States. The United States Latanoprost Study Group. Ophthalmology 1996, 103, 138–147. [Google Scholar] [CrossRef]
- Watson, P.; Stjernschantz, J. A six-month, randomized, double-masked study comparing latanoprost with timolol in open-angle glaucoma and ocular hypertension. The Latanoprost Study Group. Ophthalmology 1996, 103, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Maharana, P.K.; Mandal, S.; Kaweri, L.; Sahay, P.; Lata, S.; Asif, M.I.; Nagpal, R.; Sharma, N. Immunopathogenesis of corneal graft rejection. Indian J. Ophthalmol. 2023, 71, 1733–1738. [Google Scholar] [CrossRef]
- Nouri-Mahdavi, K.; Javadi, M.A.; Jafarinasab, M.R. Immunologic corneal graft rejection after administration of topical latanoprost: A report of two patients. J. Ophthalmic Vis. Res. 2011, 6, 127–130. [Google Scholar]
- Warwar, R.E.; Bullock, J.D.; Ballal, D. Cystoid macular edema and anterior uveitis associated with latanoprost use. Experience and incidence in a retrospective review of 94 patients. Ophthalmology 1998, 105, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Dvivedi, A.; Murthy, S.I.; Garudadri, C.; Sheba, E.; Sharma, S. Bilateral Severe Herpes Simplex Endotheliitis with a Possible Association with Latanoprost. Ocul. Immunol. Inflamm. 2023, 31, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- Miyake, K.; Ota, I.; Maekubo, K.; Ichihashi, S.; Miyake, S. Latanoprost accelerates disruption of the blood-aqueous barrier and the incidence of angiographic cystoid macular edema in early postoperative pseudophakias. Arch. Ophthalmol. 1999, 117, 34–40. [Google Scholar] [CrossRef]
- Arcieri, E.S.; Santana, A.; Rocha, F.N.; Guapo, G.L.; Costa, V.P. Blood-aqueous barrier changes after the use of prostaglandin analogues in patients with pseudophakia and aphakia: A 6-month randomized trial. Arch. Ophthalmol. 2005, 123, 186–192. [Google Scholar] [CrossRef]
| Characteristic | ALL (n = 61) n (%) |
|---|---|
| Age in years, median (IQR) | 68 (60–76) |
| Gender | |
| Male | 32 (52.5) |
| Female | 29 (47.5) |
| Nationality | |
| Saudi | 55 (90.2) |
| Non-Saudi | 6 (9.8) |
| All | Group I Glaucoma Pre-Operative | Group II Glaucoma Postoperative | p Value | |
|---|---|---|---|---|
| Number | 65 | 53 | 12 | ---- |
| Medically treated | 48 (73.8) | 39 (73.6) | 9 (75.0) | 0.998 |
| Brimonidine (Alphagan) | 22 (45.8) | 18 (46.2) | 4 (44.4) | 0.998 |
| Timolol | 8 (16.7) | 5 (12.8) | 3 (33.3) | 0.159 |
| Cosopt | 23 (47.9) | 18 (46.2) | 5 (55.6) | 0.719 |
| Latanoprost (Xalatan) | 14 (29.2) | 13 (33.3) | 1 (11.1) | 0.250 |
| Surgically treated | 44 (67.7) | 41 (77.4) | 3 (25.0) | 0.001 * |
| Trabeculectomy MMC | 20 (45.5) | 20 (48.8) | 0 (0.0) | 0.239 |
| Ahmed Valve | 11 (25.0) | 10 (24.4) | 1 (33.3) | 0.998 |
| PI | 9 (20.5) | 7 (17.1) | 2 (66.7) | 0.101 |
| Both—Medical and surgical | 30 (46.2) | 29 (54.7) | 1 (8.3) | 0.004 * |
| Risk Factor | Success (n = 37) n (%) | Failure (n = 28) n (%) | p Value |
|---|---|---|---|
| Glaucoma | |||
| Pre-operative (n = 53) | 30 (56.6) | 23 (43.4) | 0.913 |
| Post-Operative (n = 12) | 7 (58.3) | 5 (41.7) | |
| Treated medically | |||
| No (n = 17) | 10 (58.8) | 7 (41.2) | 0.854 |
| Yes (n = 48) | 27 (56.3) | 21 (43.8) | |
| Brimonidine (Alphagan) (n = 22) | 11 (50.0) | 11 (50.0) | 0.422 |
| Timolol (n = 8) | 4 (50.0) | 4 (50.0) | 0.715 |
| Cosopt (n = 23) | 12 (52.2) | 11 (47.8) | 0.585 |
| Latanoprost (Xalatan) (n = 14) | 4 (28.6) | 10 (71.4) | 0.024 * |
| Treated surgically | |||
| No (n = 21) | 12 (57.1) | 9 (42.9) | 0.980 |
| Yes (n = 44) | 25 (56.8) | 19 (43.2) |
| Total (n = 14) n (%) | Success (n = 4) n (%) | Failure (n = 10) n (%) | p Value | |
|---|---|---|---|---|
| Primary graft failure | 2 (14.3) | 0 (0.0) | 2 (20.0) | 0.998 |
| Secondary graft failure | 8 (57.1) | 0 (0.0) | 8 (80.0) | 0.015 * |
| Endothelial rejection | 5 (35.7) | 0 (0.0) | 5 (50.0) | 0.221 |
| Latanoprost (Xalatan) (n = 14) n (%) | Others (n = 34) n (%) | p Value | |
|---|---|---|---|
| Age in years, median (IQR) | 69 (65–81) | 68 (61–78.5) | 0.633 |
| Gender | |||
| Female (n = 23) | 7 (50.0) | 16 (47.1) | 0.998 |
| Male (n = 25) | 7 (50.0) | 18 (52.9) | |
| Treated surgically | |||
| No (n = 30) | 9 (64.3) | 21 (61.8) | 0.870 |
| Yes (n = 18) | 5 (35.7) | 13 (38.2) | |
| Developed glaucoma | |||
| No (n = 39) | 13 (92.9) | 26 (76.5) | 0.250 |
| Yes (n = 9) | 1 (7.1) | 8 (23.5) | |
| Lens status | |||
| Pseudophakic (n = 47) | 14 (100) | 33 (97.1) | 0.998 |
| Aphakic (n = 1) | 0 (0.0) | 1 (2.9) | |
| PBK diagnosis | |||
| No (n = 16) | 4 (28.6) | 12 (35.3) | 0.746 |
| Yes (n = 32) | 10 (71.4) | 22 (64.7) | |
| Vascularization | |||
| No (n = 46) | 13 (92.9) | 33 (97.1) | 0.503 |
| Yes (n = 2) | 1 (7.1) | 1 (2.9) | |
| Combined surgery | |||
| No (n = 44) | 13 (92.9) | 31 (91.2) | 0.998 |
| Yes (n = 4) | 1 (7.1) | 3 (8.8) | |
| Post-operative Complications | |||
| No (n = 20) | 4 (28.6) | 16 (47.1) | 0.338 |
| Yes (n = 28) | 10 (71.4) | 18 (52.9) | |
| Lenticule detachment | |||
| No (n = 42) | 13 (92.9) | 29 (85.3) | 0.656 |
| Yes (n = 6) | 1 (7.1) | 5 (14.7) | |
| Rebubbling (n = 5) | |||
| No (n = 1) | 1 (100) | 0 (0.0) | 0.200 |
| Yes (n = 4) | 0 (0.0) | 4 (100) | |
| Endothelial rejection | |||
| No (n = 39) | 9 (64.3) | 30 (88.2) | 0.099 |
| Yes (n = 9) | 5 (35.7) | 4 (11.8) | |
| High IOP | |||
| No (n = 41) | 12 (85.7) | 29 (85.3) | 0.998 |
| Yes (n = 7) | 2 (14.3) | 5 (14.7) | |
| Primary failure | |||
| No (n = 44) | 12 (85.7) | 32 (94.1) | 0.569 |
| Yes (n = 4) | 2 (14.3) | 2 (5.9) | |
| Secondary failure | |||
| No (n = 31) | 6 (42.9) | 25 (73.5) | 0.043 * |
| Yes (n = 17) | 8 (57.1) | 9 (26.5) | |
| Previous grafts | |||
| First (n = 36) | 11 (78.6) | 25 (73.5) | 0.998 |
| Repeated (n = 12) | 3 (21.4) | 9 (26.5) | |
| Surgical technique | |||
| Lens glide technique (n = 12) | 3 (21.4) | 9 (26.5) | 0.998 |
| Busin glide technique (n = 36) | 11 (78.6) | 25 (73.5) | |
| Physicians’ workload | |||
| ≥15 grafts (n = 45) | 13 (92.9) | 32 (94.1) | 0.998 |
| <15 grafts (n = 3) | 1 (7.1) | 2 (5.9) | |
| Histopathology for corneal dystrophies | |||
| Fuch’s dystrophy (n = 6) | 3 (21.4) | 3 (8.8) | 0.339 |
| No dystrophy and others (n = 42) | 11 (78.6) | 31 (91.2) | |
| Graft size for donor, Median (IQR) | 7.6 (7.5–7.8) | 7.8 (7.5–8.0) | 0.901 |
| Graft size for host, Median (IQR) | 8.0 (7.3–8.0) | 7.8 (7.5–8.0) | 0.622 |
| Duration of FU, months, Median (IQR) | 27.5 (10.8–44.8) | 15.0 (9.0–27.3) | 0.196 |
| Presence of Diabetes (n = 44) | |||
| No (n = 22) | 5 (45.5) | 17 (51.5) | 0.728 |
| Yes (n = 22) | 6 (54.5) | 16 (48.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkharashi, M.S.; Abusayf, M.M.; Aldofyan, M.Z. Long-Term Outcome of Endothelial Keratoplasty Among Glaucoma Patients and the Risk of Prostaglandin Analogue (Latanoprost) Use on Graft Rejection. J. Clin. Med. 2025, 14, 7650. https://doi.org/10.3390/jcm14217650
Alkharashi MS, Abusayf MM, Aldofyan MZ. Long-Term Outcome of Endothelial Keratoplasty Among Glaucoma Patients and the Risk of Prostaglandin Analogue (Latanoprost) Use on Graft Rejection. Journal of Clinical Medicine. 2025; 14(21):7650. https://doi.org/10.3390/jcm14217650
Chicago/Turabian StyleAlkharashi, Majed S., Mohammed M. Abusayf, and Munirah Z. Aldofyan. 2025. "Long-Term Outcome of Endothelial Keratoplasty Among Glaucoma Patients and the Risk of Prostaglandin Analogue (Latanoprost) Use on Graft Rejection" Journal of Clinical Medicine 14, no. 21: 7650. https://doi.org/10.3390/jcm14217650
APA StyleAlkharashi, M. S., Abusayf, M. M., & Aldofyan, M. Z. (2025). Long-Term Outcome of Endothelial Keratoplasty Among Glaucoma Patients and the Risk of Prostaglandin Analogue (Latanoprost) Use on Graft Rejection. Journal of Clinical Medicine, 14(21), 7650. https://doi.org/10.3390/jcm14217650





