Abstract
Background: Compared with isolated aortic valve replacement (AVR), echocardiographic hemodynamics after Wheat and Bentall procedures, both involving replacement of the proximal ascending aorta with a smaller-diameter graft, have been less thoroughly investigated. Methods: We analyzed 213 patients who received 21 mm or 23 mm aortic bioprostheses (AVR, n = 138; Wheat, n = 43; Bentall, n = 32). Transthoracic echocardiography was performed before and after surgery, and the proximal ascending aortic area (Aa) was assessed using contrast-enhanced computed tomography. Results: The maximal pressure gradient (PG max), derived from the simplified Bernoulli equation, was significantly lower in the Bentall group, whereas pressure recovery (PR), calculated using Voelker’s equation, was lower in the AVR group. A smaller Aa was associated with a higher PG max in the AVR group. The Bentall group exhibited significantly lower energy loss (EL). In propensity score-matched analyses to minimize potential confounding factors, the AVR group showed a significantly lower PR and higher EL than the Wheat group; a significantly higher PG max, lower PR, and higher EL than the Bentall group; and a significantly similar PR but lower EL in the Bentall group compared with the Wheat group. Conclusions: Although limited to bioprosthetic valves, caution is warranted when interpreting echocardiographic PG max after AVR in patients with a small ascending aorta. However, overestimation of PG max was not observed in either the Wheat or Bentall groups, even though both demonstrated higher PR and lower EL compared with the AVR group.
1. Introduction
The hemodynamic performance of aortic valve (AV) prostheses is typically evaluated by echocardiography, using the simplified Bernoulli equation to calculate transprosthetic maximal and mean pressure gradients (PGs) [1]. The simplified Bernoulli equation assumes that the increase in convective velocity across the AV is entirely due to pressure loss from AV stenosis, while neglecting local acceleration, viscous forces, and proximal left ventricular outflow tract velocity. In addition to this simplification, failure to account for pressure recovery (PR) is a well-described cause of the discrepancy between echocardiographic and catheterization-derived pressure PGs in AV prostheses [2,3].
PR is a phenomenon reflecting the dynamic interplay between kinetic and potential energy across the AV [1]. As blood flows across the AV, potential energy is converted into kinetic as flow velocity accelerates. Distal to the stenosis, some of this kinetic energy is reconverted into potential energy, resulting in a partial restoration of pressure detectable by catheterization—this is referred to as PR. Greater PR leads to an overestimation of PG by echocardiography compared to those measured by catheterization. PR is more pronounced in conditions such as high-flow states, small non-compliant aortas, and geometrical configurations that prevent the normal outward turbulent expansion of blood into the sinuses of Valsalva [4,5,6].
The flow characteristics of AV prostheses may differ following simple AV replacement (AVR), AVR with ascending aortic replacement (Wheat procedure), and the Bentall procedure, potentially affecting PR. In patients undergoing the Wheat and Bentall procedures, proximal aortic prosthetic grafts with smaller diameters and increased impedance may contribute to greater PR and an overestimation of the echocardiographic pressure gradient (PG) across the AV prosthesis. In the present study, we retrospectively compared the echocardiographic evaluation of AV prostheses following AVR, Wheat, and Bentall procedures.
2. Materials and Methods
2.1. Ethical Statement
This retrospective study to analyze single-center data was approved by the Fujita Health University Ethics Committee for clinical study and was conducted according to the ethical guidelines published by the Ministry of Health and the Helsinki Declaration. The approval number is HM24-607, 22 December 2024, including a waiver of informed consent.
2.2. Study Patients
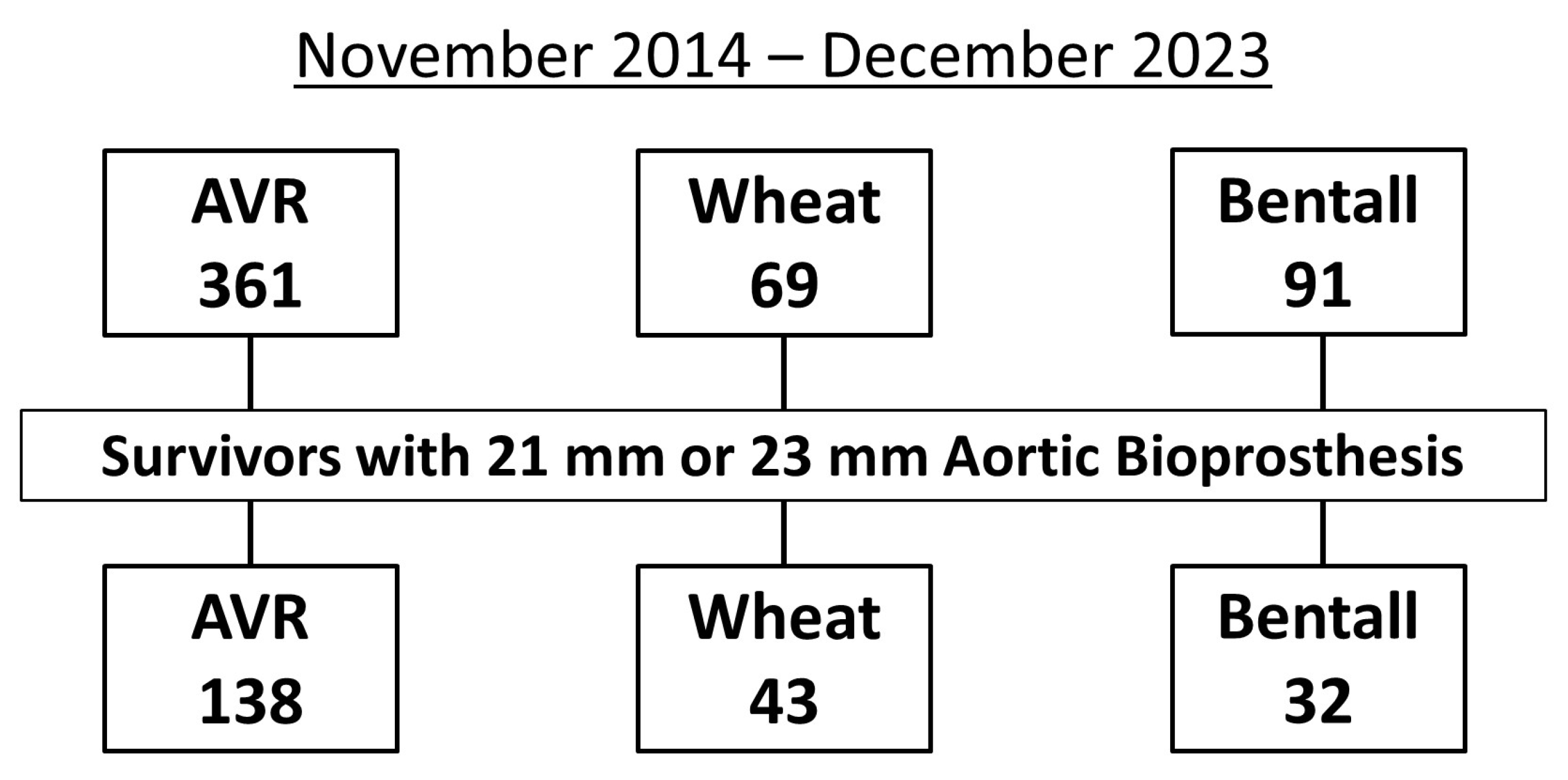
Between November 2014 and December 2023, 361 patients underwent AVR, 69 underwent the Wheat procedure, and 91 underwent the Bentall procedure. Of these, the study population (n = 213) consisted of 138 patients who underwent AVR, 43 who underwent the Wheat procedure, and 32 who underwent the Bentall procedure, all of whom received 21 mm or 23 mm aortic bioprostheses and were discharged alive after surgery (Figure 1).
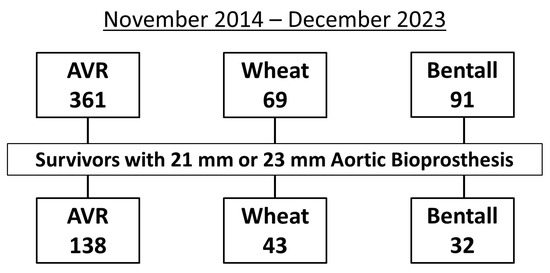
Figure 1.
Flowchart showing the inclusion criteria for study patients with 21 mm or 23 mm aortic bioprostheses after aortic valve replacement (AVR), Wheat procedure (AVR with ascending aortic replacement), and Bentall procedure.
The Bentall group underwent the aortic root replacement using a valve conduit according to the Svensson modification [7]. The distant left main coronary ostium was reattached to the ascending conduit via a 10 mm interposition prosthetic graft. The right coronary artery button was mobilized and conventionally reattached.
2.3. Echocardiography
Echocardiography was performed in the study patients at the outpatient clinic between 6 months and 1 year after surgery to remove the effects of the surgery. The following left ventricular (LV) systolic and diastolic parameters were assessed: LV end-diastolic dimension (LVDd), LV end-systolic dimension (LVDs), LV ejection fraction (LVEF), LV mass (LVM), and relative wall thickness (RWT). The LVEF was measured from the apical 4- and 2-chamber images using the biplane method of disks. LVM was calculated according to the following formula: LVM (g) = 0.8 {1.04 [([LVDd + interventricular septum thickness + LV posterior wall thickness]3 − LVEDd3)]} + 0.6. The LVM index was calculated as LVM/body surface area (BSA). RWT was calculated with the following formula: RWT = (2 × posterior wall thickness)/LVDd.
Maximal transvalvular pressure gradients (PG max) were calculated with the use of the simplified Bernoulli equation from the aortic velocity obtained by multiwindow continuous-wave Doppler interrogation. The effective orifice area (EOA) of the aortic bioprosthesis was calculated using the continuity equation as the stroke volume measured in the LV outflow tract (LVOT) divided by the aortic velocity–time integral. LVOT stroke volume was calculated as the product of the LVOT cross-sectional area and LVOT velocity–time integral measured by pulsed-wave Doppler. The pulsed-wave Doppler sample volume was located just apical to the prosthetic valve stent or sewing ring. The LVOT diameter was measured from the outer-to-outer border of the stent or sewing ring [8].
The following equations, using Doppler-derived parameters, whose problem was solved by developing the Voelker equation [9], were used to calculate PR, the PR index (PRI), energy loss (EL), and the energy loss coefficient (ELCo) referring to the corresponding “functional valve orifice area” [10,11]: PR (mmHg) = 4Vmax2 × 2EOA/Aa × (1 − EOA/Aa); PRI = 2(EOA/Aa − (EOA/Aa)2); EL (mmHg) = 4Vmax2 × (1−EOA/Aa)2; and ELCo (cm2/m2) = (EOA × Aa)/(Aa − EOA)/BSA, where Aa is the cross-sectional area of the aorta.
To analyze the Doppler data, the average of at least three cardiac cycles was used (10 cycles for patients with atrial fibrillation). Patients with heart rates below 40 or above 120 beats/min were excluded.
The proximal ascending aortic diameter (Ad) and area (Aa) were measured about 5 mm above the sinotubular junction on preoperative contrast CT scans in the patients undergoing AVR. This localization was previously recommended [3,4], because at this position, the blood flow should be laminar again, and thus the pressure recovery should be completed. In those undergoing Wheat and Bentall procedures, Ad and Aa, which were diameter and area of the prosthetic graft, were obtained on postoperative contrast-enhanced CT scans.
2.4. Statistical Analysis
All statistical analyses were executed using software (EZR, available online [12]). A p value less than 0.05 was deemed significant for all tests. Categorical variables were expressed as count and percentage and compared with the chi-square test. Continuous variables were expressed as means ± standard deviations and compared using analysis of variance (ANOVA) or the Kruskal–Wallis rank sum test with appropriate post hoc tests (Tukey HSD or Steel–Dwass, respectively), according to the Kolmogorov–Smirnov test to check for normal distribution.
To reduce potentially confounding factors, propensity score matching was performed to balance risk factors between the groups using 1:1 nearest-neighbor matching with caliper 0.1. To assess covariate balance (potential confounders), the standardized mean difference was used, and logistic regression was employed for propensity score matching.
3. Results
3.1. Baseline Characteristics
As shown in Table 1, the patients of the Bentall group were significantly younger than those of the AVR and Wheat groups. The AVR group had significantly higher prevalences of diabetes mellitus and atrial fibrillation on electrocardiography than the Wheat and Bentall groups.

Table 1.
Patient characteristics of the complete cohort.
3.2. Operative Data
As shown in Table 2, there was a significantly lower prevalence of AV stenosis as a primary disease of the AV in the Bentall group. In the AVR group, 36% of the patients underwent isolated AVR, while 64% underwent AVR with concomitant procedures, including mitral valve replacement/repair and coronary artery bypass grafting. The Bentall group included more patients undergoing surgery under circulatory arrest and therefore showed a significantly longer time for operation, cardiopulmonary bypass, and cardiac arrest. Also, the lowest body temperature during surgery was significantly lower in the Bentall group. As shown in Table 2, the kinds and sizes of the aortic bioprostheses and vascular grafts were not significantly different in the three groups. Triplex grafts (Terumo Corp, Tokyo, Japan), with sizes of 24, 26, and 28 mm, were mostly used in the Wheat and Bentall groups.

Table 2.
Operative data in the complete cohort.
3.3. Echocardiographic Data
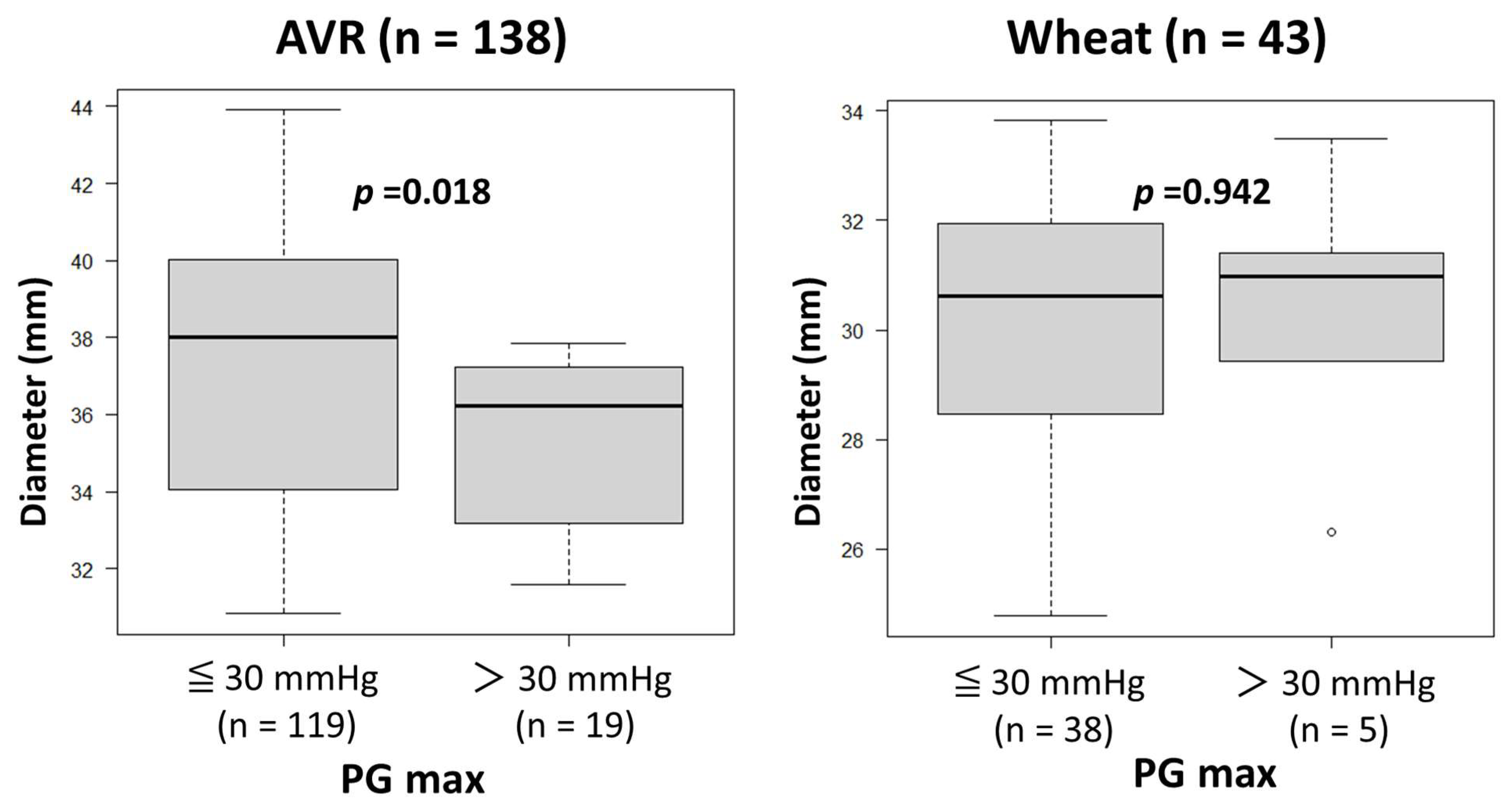
The heart rates during Doppler examination were 73 ± 16 beats/min (range, 45–112). No patients, including those with atrial fibrillation, were excluded from the study due to unsatisfactory Doppler data. As shown in Table 3, preoperative echocardiography showed similar variables other than LVEF, which was significantly lower in the Bentall group than in the AVR and Wheat groups. Postoperative echocardiography showed significantly lower PG max values in the Bentall group than in the AVR and Wheat groups, while EOAs were similar among the three groups. There were no patients with PG max > 30 mmHg in the Bentall group. A comparison of the patients with PG max > 30 mmHg and ≤30 mmHg revealed that the ascending aortic diameter was significantly smaller in those with PG max > 30 mmHg than in those with PG max ≤ 30 mmHg in the AVR group (Figure 2). However, in the Wheat group, there were no significant differences in aortic diameter between those with PG max > 30 mmHg and those with PG max ≤ 30 mmHg.

Table 3.
Echocardiographic data of the complete cohort.
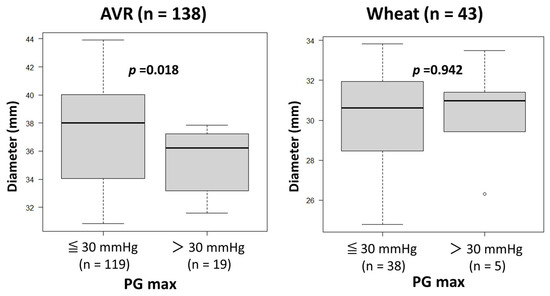
Figure 2.
Comparison of the ascending aortic diameters between the patients with maximal transvalvular pressure gradients (PG max) of ≤30 mmHg and those with a PG max of >30 mmHg after surgery in aortic valve replacement (AVR) and Wheat groups.
Since the aorta diameter and Aa measured on CT angiograms were significantly larger in the AVR group than in the Wheat and Bentall groups, PR and PRI were significantly lower in the AVR group than in the other two groups. The Bentall group demonstrated a significantly lower EL and higher ELCo than the other two groups (Table 3).
3.4. Propensity Score-Matched Comparisons
To compare the AVR and Wheat groups, propensity score matching was performed, controlling diabetes and atrial fibrillation to generate a balanced cohort made up of 38 pairs (Table 4). Both matched groups were similar across all demographic characteristics and preoperative echocardiographic data. Since the Aa was significantly larger in the AVR group, the AVR group showed significantly lower PR and higher EL values than the Wheat group.

Table 4.
Propensity score-matched comparisons between AVR and Wheat groups.
To compare the AVR and Bentall groups, propensity score matching was performed, controlling for diabetes, atrial fibrillation, and preoperative LVEF to generate a balanced cohort made up of 24 pairs (Table 5). Postoperative echocardiography showed a significantly higher PG max in the AVR group than in the Bentall group. Since the Aa was significantly larger in the AVR group, the AVR group showed significantly lower PR, lower PRI, and higher EL values than the Bentall group.

Table 5.
Propensity-matched comparisons between AVR and Bentall groups.
To compare the Wheat and Bentall groups, propensity score matching was performed, controlling for only preoperative LVEF to generate a balanced cohort made up of 21 pairs (Table 6). Since the Aa was similar in both groups, PR and PRI were also similar, but EL was significantly lower, and ELCo was significantly higher in the Bentall group than in the Wheat group.

Table 6.
Propensity-matched comparisons between Wheat and Bentall groups.
4. Discussion
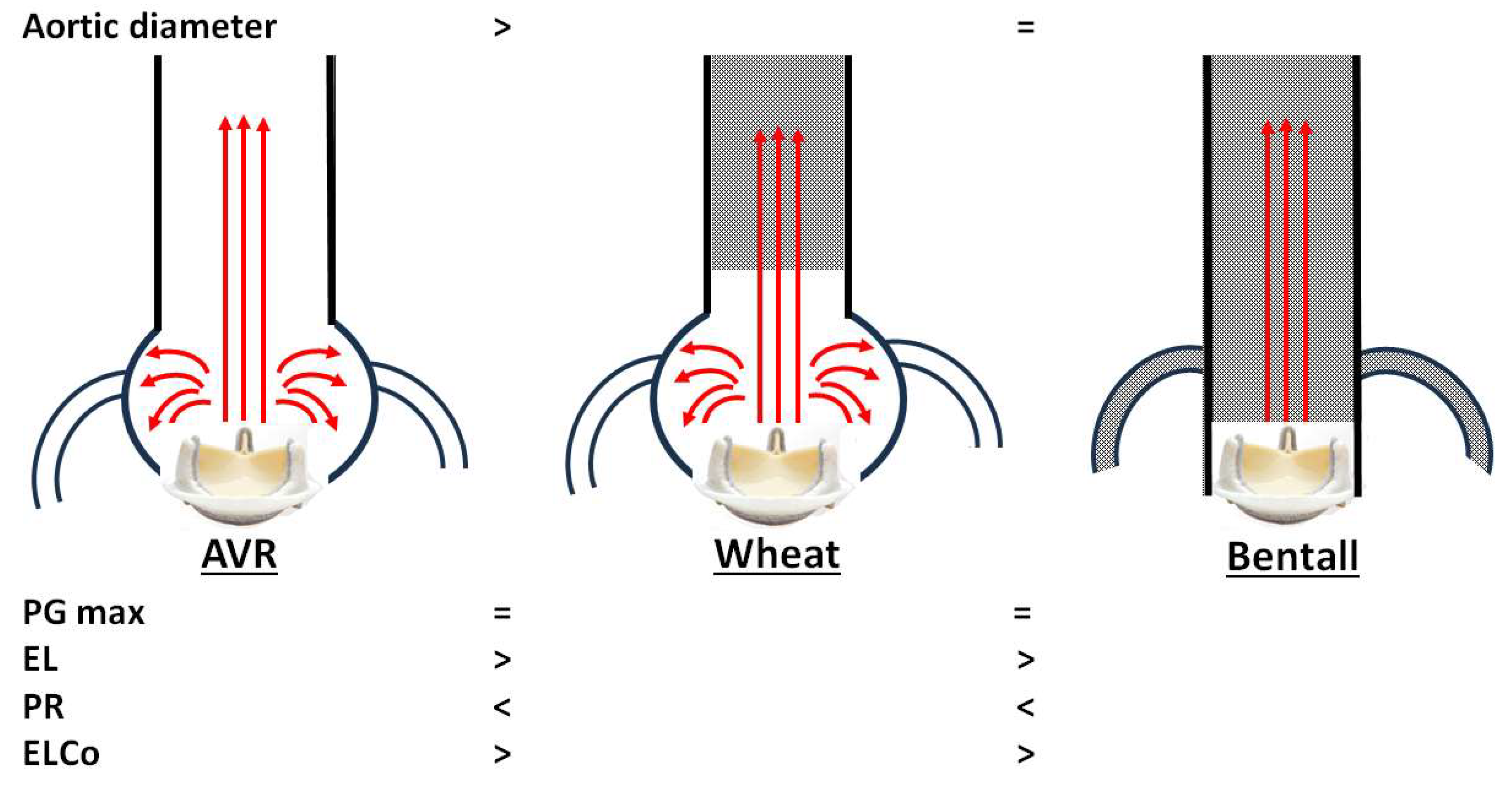
Our main echocardiographic findings comparing AV prostheses after AVR, Wheat, and Bentall procedures are summarized as follows, although these are limited to bioprosthetic valves (Figure 3):
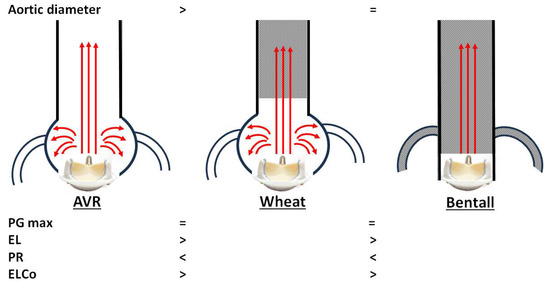
Figure 3.
Conceptual summary of our echocardiographic comparison of aortic valve prostheses after aortic valve replacement (AVR), Wheat, and Bentall procedures. PG max, maximal transvalvular pressure gradient; EL, energy loss; PR, pressure recovery; ELCo, energy loss coefficient.
- 1.
- Unexpectedly, overestimation of PG max was not observed in either the Wheat or Bentall groups, despite replacement of the ascending aorta with a smaller vascular graft.
- 2.
- In the AVR group, a smaller Ad was associated with a higher PG max after AVR. Patients with PG max > 30 mmHg had a significantly smaller Ad than those with PG max ≤ 30 mmHg.
- 3.
- Both the Wheat and Bentall groups demonstrated higher PR and lower EL values compared with the AVR group.
PR downstream of the AV is an important factor affecting the calculation of PG across the valve and thereby the estimation of the AV area [13]. PR represents the conversion of kinetic energy back into potential energy, which may lead to overestimation of echocardiographic PG. This phenomenon has been demonstrated experimentally [14], observed clinically [15], and precisely calculated using Doppler ultrasound based on Voelker’s equation [9]. The Aa and the presence of the sinus of Valsalva are critical determinants of PR. While the hemodynamic effects of surgical Aa reduction by graft replacement in Wheat and Bentall procedures have received little attention, valve performance and PR have been extensively studied in transcatheter aortic valve replacement (TAVR) [16,17,18]. The Edwards SAPIEN 3 (balloon-expandable valve) generates laminar flow, resulting in greater convective acceleration, increased PR, and higher echocardiographic PG. In contrast, the Medtronic Evolut R (self-expandable valve) produces slightly turbulent flow due to its stent structures that interact with the ascending aorta, resulting in less convective acceleration, lower PR values, and reduced echocardiographic PG.
One possible explanation for the absence of PG max overestimation in the Wheat and Bentall groups may be the unexpectedly high compliance of the Triplex graft used for ascending aortic replacement. The Triplex graft consists of three layers: an inner uncoated woven Dacron graft, an outer expanded polytetrafluoroethylene layer, and a middle non-biodegradable styrene elastomer resin layer [19]. Recent reports have described elongation of Triplex grafts, speculated to be due to reduced adhesion formation with surrounding tissues and insufficient stability and fixation of the prosthesis [20,21]. In addition, thermal crimping treatment is applied to induce a wavy textile configuration in the Dacron layer. This crimping helps preserve the tubular shape while providing bending flexibility and axial compliance [22].
As demonstrated in Figure 2, a smaller Aa increases PG max in the AVR group. Therefore, careful interpretation of echocardiographic PG max is warranted in AVR patients, especially with a smaller ascending aorta.
Our third finding is that the Bentall procedure may be associated with reduced LV work, as suggested by lower EL and higher ELCo values compared with the AVR and Wheat procedures. ELCo has been established as a useful parameter for stratifying patients with AV stenosis into high-, moderate-, or low-risk categories (with ELI < 0.6 cm2/m2 indicating severe aortic stenosis) [10]. A more accurate hemodynamic model considers not only velocity and pressure but also heat, as turbulent flow and frictional resistance across a valve result in energy loss as heat. Patients with a higher ELCo, reflecting less turbulence and shear stress, have been reported to show improved survival after TAVR [23]. The hemodynamic findings in the Bentall group, which lacks the sinus of Valsalva, indicate a valve–vessel interaction with more laminar flow. This led to significant PR gain and improved ELCo, essentially reflecting an increased functional AV orifice area.
The present study has several limitations. First, this was a single-center, retrospective observational study with a relatively small sample size, which may limit the generalizability of our findings. Second, although the Aa immediately distal to the sinotubular junction is critical for PR measurements, we did not account for the diameter or cross-sectional area of the sinus of Valsalva in the AVR and Wheat groups, which may also affect flow dynamics. Third, valve types were not uniform across the groups, and differences in valve material may have influenced the results. Fourth, the underlying aortic valve pathology differed among the groups: aortic stenosis was predominant in the AVR and Wheat groups, while aortic regurgitation was predominant in the Bentall group. Fifth, we did not take into consideration the viscosity of blood, which is also important for fluid dynamics through the AV prosthesis. Sixth, the AVR group included more patients with atrial fibrillation. Although propensity score matching was performed to minimize this potential confounding effect, it may still have influenced the Doppler measurements. Finally, the clinical implications of the present findings remain uncertain, and further investigation is required.
5. Conclusions
Although the present findings are limited to bioprosthetic valves, a smaller diameter of the ascending aorta was closely related to an increased PG max on echocardiography in the AVR group. In comparison to the AVR group, both the Wheat and Bentall groups showed a higher PR and lower EL. However, overestimation of PG max on echocardiography was not observed in both the Wheat and Bentall groups.
Author Contributions
Conceptualization, W.N., Y.T. (Yoshiyuki Takami), and Y.T. (Yasushi Takagi); methodology, W.N. and Y.T. (Yoshiyuki Takami); software, W.N. and Y.T. (Yoshiyuki Takami); validation, W.N. and Y.T. (Yasushi Takagi); formal analysis, W.N. and Y.T. (Yoshiyuki Takami); investigation, W.N. and Y.T. (Yoshiyuki Takami); resources, W.N., K.A. (Kiyotoshi Akita), A.M., K.Y., K.A. (Kentaro Amano), K.M., and T.A.; data curation, W.N. and Y.T. (Yoshiyuki Takami); writing—original draft preparation, W.N.; writing—review and editing, Y.T. (Yoshiyuki Takami); visualization, W.N. and Y.T. (Yoshiyuki Takami); supervision, Y.T. (Yasushi Takagi) and T.A.; project administration, W.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the medical ethics committee of Fujita Health University, Japan (HM24-607, 22 December 2024).
Informed Consent Statement
Patient consent was waived since no additional data were collected, and data assembled solely in the daily clinical routine were used.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abbas, A.E.; Mando, R.; Hanzel, G.; Goldstein, J.; Shannon, F.; Pibarot, P. Hemodynamic principles of prosthetic aortic valve evaluation in the transcatheter aortic valve replacement era. Echocardiography 2020, 37, 738–757. [Google Scholar] [CrossRef]
- Bach, D.S.; Schmitz, C.; Dohmen, G.; Aaronson, K.D.; Steinseifer, U.; Kleine, P. In vitro assessment of prosthesis type and pressure recovery characteristics: Doppler echocardiography overestimation of bileaflet mechanical and bioprosthetic aortic valve gradients. J. Thorac. Cardiovasc. Surg. 2012, 144, 453–458. [Google Scholar] [CrossRef]
- Baumgartner, H.; Schima, H.; Kühn, P. Discrepancies between Doppler and catheter gradients across bileaflet aortic valve prostheses. Am. J. Cardiol. 1993, 71, 1241–1243. [Google Scholar] [CrossRef]
- Garcia, D.; Dumesnil, J.G.; Durand, L.G.; Kadem, L.; Pibarot, P. Discrepancies between catheter and Doppler estimates of valve effective orifice area can be predicted from the pressure recovery phenomenon: Practical implications with regard to quantification of aortic stenosis severity. J. Am. Coll. Cardiol. 2003, 41, 435–442. [Google Scholar] [CrossRef]
- Midha, P.A.; Raghav, V.; Sharma, R.; Condado, J.F.; Okafor, I.U.; Rami, T.; Kumar, G.; Thourani, V.H.; Jilaihawi, H.; Babaliaros, V.; et al. The Fluid Mechanics of Transcatheter Heart Valve Leaflet Thrombosis in the Neosinus. Circulation 2017, 136, 1598–1609. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Miranda, W.R.; Nishimura, R.A. Measuring pressure gradients after transcatheter aortic valve implantation: Rethinking the Bernoulli principle. J. Am. Heart Assoc. 2021, 10, e022515. [Google Scholar] [CrossRef]
- Svensson, L.G. Approach for insertion of aortic composite valve grafts. Ann. Thorac. Surg. 1992, 54, 376–378. [Google Scholar] [CrossRef]
- Pibarot, P.; Salaun, E.; Dahou, A.; Avenatti, E.; Guzzetti, E.; Annabi, M.S.; Toubal, O.; Bernier, M.; Beaudoin, J.; Ong, G.; et al. PARTNER 3 Investigators. PARTNER 3 investigators. echocardiographic results of transcatheter versus surgical aortic valve replacement in low-risk patients: The PARTNER 3 Trial. Circulation 2020, 141, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Voelker, W.; Reul, H.; Stelzer, T.; Schmidt, A.; Karsch, K.R. Pressure recovery in aortic stenosis: An in vitro study in a pulsatile flow model. J. Am. Coll. Cardiol. 1992, 20, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Pibarot, P.; Dumesnil, J.G.; Sakr, F.; Durand, L.G. Assessment of aortic valve stenosis severity: A new index based on the energy loss concept. Circulation 2000, 101, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Reil, J.C.; Marquetand, C.; Busch-Tilge, C.; Ivannikova, M.; Rudolph, V.; Aboud, A.; Ensminger, S.; Schäfers, H.J.; Stierle, U.; Reil, G.H. Functional interaction of aortic valve and ascending aorta in patients after valve-sparing procedures. Sci. Rep. 2023, 13, 15340. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Bahlmann, E.; Cramariuc, D.; Gerdts, E.; Gohlke-Baerwolf, C.; Nienaber, C.A.; Eriksen, E.; Wachtell, K.; Chambers, J.; Kuck, K.H.; Ray, S. Impact of pressure recovery on echocardiographic assessment of asymptomatic aortic stenosis: A SEAS substudy. JACC Cardiovasc. Imaging 2010, 3, 555–562. [Google Scholar] [CrossRef]
- Heinrich, R.S.; Fontaine, A.A.; Grimes, R.Y.; Sidhaye, A.; Yang, S.; Moore, K.E.; Levine, R.A.; Yoganathan, A.P. Experimental analysis of fluid mechanical energy losses in aortic valve stenosis: Importance of pressure recovery. Ann. Biomed. Eng. 1996, 24, 685–694. [Google Scholar] [CrossRef]
- Singh, G.K.; Mowers, K.L.; Marino, C.; Balzer, D.; Rao, P.S. Effect of pressure recovery on pressure gradients in congenital stenotic outflow lesions in pediatric patients-clinical implications of lesion severity and geometry: A Simultaneous Doppler Echocardiography and Cardiac Catheter Correlative Study. J. Am. Soc. Echocardiogr. 2020, 33, 207–217. [Google Scholar] [CrossRef]
- Hatoum, H.; Hahn, R.T.; Lilly, S.; Dasi, L.P. Differences in pressure recovery between balloon expandable and self-expandable transcatheter aortic valves. Ann. Biomed. Eng. 2020, 48, 860–867. [Google Scholar] [CrossRef]
- Abbas, A.E.; Mando, R.; Kadri, A.; Khalili, H.; Hanzel, G.; Shannon, F.; Al-Azizi, K.; Waggoner, T.; Kassas, S.; Pilgrim, T.; et al. Comparison of transvalvular aortic mean gradients obtained by intraprocedural echocardiography and invasive measurement in balloon and self-expanding transcatheter valves. J. Am. Heart Assoc. 2021, 10, e021014. [Google Scholar] [CrossRef] [PubMed]
- Samaee, M.; Hatoum, H.; Biersmith, M.; Yeats, B.; Gooden, S.C.; Thourani, V.H.; Hahn, R.T.; Lilly, S.; Yoganathan, A.; Dasi, L.P. Gradient and pressure recovery of a self-expandable transcatheter aortic valve depends on ascending aorta size: In vitro study. JTCVS Open 2022, 9, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Morota, T.; Takamoto, S. Development and physical characteristics of novel zero-porosity vascular graft “triplex(®)”. Ann. Vasc. Dis. 2013, 6, 67–73. [Google Scholar] [CrossRef]
- Narita, M.; Shirasaka, T.; Ushioda, R.; Kamiya, H. Triplex vascular prostheses elongation in post-operative course. J. Surg. Case Rep. 2022, 6, rjac255. [Google Scholar] [CrossRef]
- Etz, C.D.; Homann, T.; Silovitz, D.; Bodian, C.A.; Luehr, M.; Di Luozzo, G.; Plestis, K.A.; Griepp, R.B. Vascular graft replacement of the ascending and descending aorta: Do Dacron grafts grow? Ann. Thorac. Surg. 2007, 84, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Nazer, R.I.; Alhothali, A.; Albarrati, A. In vitro modeling of crimped Dacron vascular grafts for aortic root replacement. Am. J. Cardiovasc. Dis. 2023, 13, 59–67. [Google Scholar] [PubMed]
- Johal, G.; Jonnala, V.; Pourafkari, L.; Sedghi, S.; Jafarsis, S.; Fernandez, S.; Iyer, V.; Nader, N.D. Energy loss index as a predictor of all-cause mortality after transcatheter aortic valve replacement: A long-term follow-up. Echocardiography 2023, 40, 327–334. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).