Effects of Personality Styles on Clinical Response to Intermittent Theta Burst Stimulation for Depression
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and rTMS-Protocol
2.2. Patients and Measures
2.3. Statistical Analysis
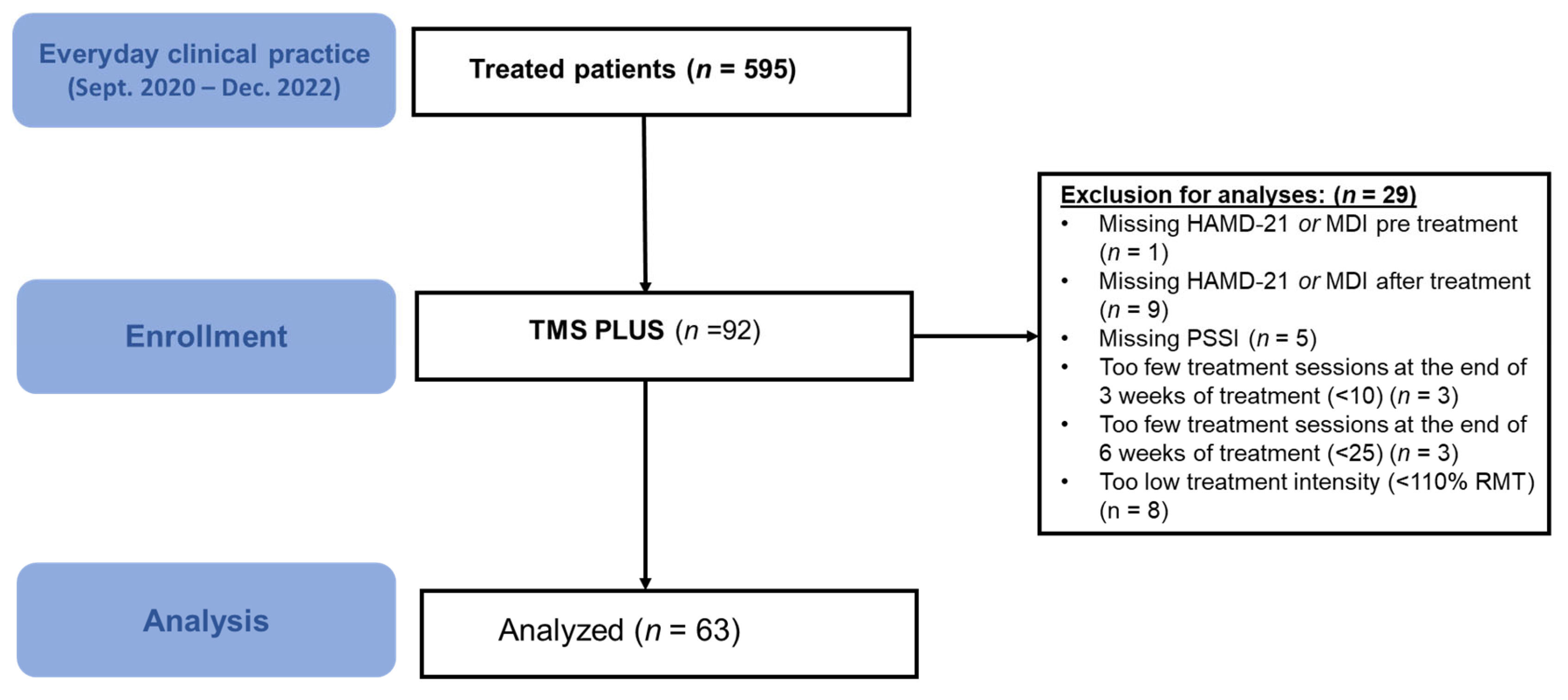
3. Results
4. Discussion
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kessler, R.C.; McGonagle, K.A.; Zhao, S.; Nelson, C.B.; Hughes, M.; Eshleman, S.; Wittchen, H.U.; Kendler, K.S. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch. Gen. Psychiatry 1994, 51, 8–19. [Google Scholar] [CrossRef]
- Sinyor, M.; Rezmovitz, J.; Zaretsky, A. Screen all for depression. BMJ 2016, 352, i1617. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.Y.; Tam, W.W.; Lu, Y.; Ho, C.S.; Zhang, M.W.; Ho, R.C. Prevalence of Depression in the Community from 30 Countries between 1994 and 2014. Sci. Rep. 2018, 8, 2861. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.F.; Ricci, J.A.; Chee, E.; Hahn, S.R.; Morganstein, D. Cost of lost productive work time among US workers with depression. JAMA 2003, 289, 3135–3144. [Google Scholar] [CrossRef] [PubMed]
- Daly, E.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Gaynes, B.N.; Warden, D.; Morris, D.W.; Luther, J.F.; Farabaugh, A.; Cook, I.; et al. Health-related quality of life in depression: A STAR*D report. Ann. Clin. Psychiatry 2010, 22, 43–55. [Google Scholar] [CrossRef]
- Large, M. Study on suicide risk assessment in mental illness underestimates inpatient suicide risk. BMJ 2016, 532, i267. [Google Scholar] [CrossRef]
- Cuijpers, P.; Smit, F. Excess mortality in depression: A meta-analysis of community studies. J. Affect. Disord. 2002, 72, 227–236. [Google Scholar] [CrossRef]
- Lépine, J.P.; Briley, M. The increasing burden of depression. Neuropsychiatr. Dis. Treat. 2011, 7, 3–7. [Google Scholar] [CrossRef]
- Fava, M. Diagnosis and definition of treatment-resistant depression. Biol. Psychiatry 2003, 53, 649–659. [Google Scholar] [CrossRef]
- Keller, M.B.; Lavori, P.W.; Mueller, T.I.; Endicott, J.; Coryell, W.; Hirschfeld, R.M.; Shea, T. Time to recovery, chronicity, and levels of psychopathology in major depression. A 5-year prospective follow-up of 431 subjects. Arch. Gen. Psychiatry 1992, 49, 809–816. [Google Scholar] [CrossRef]
- Sackeim, H.A. The definition and meaning of treatment-resistant depression. J. Clin. Psychiatry 2001, 62 (Suppl. S16), 10–17. [Google Scholar] [PubMed]
- Holtzheimer, P.E.; Nemeroff, C.B. Advances in the Treatment of Depression. NeuroRX 2006, 3, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P. Brain Stimulation Techniques for the Treatment of Depression and Other Psychiatric Disorders. Australas. Psychiatry 2008, 16, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; André-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.H.; Cantello, R.M.; Cincotta, M.; de Carvalho, M.; De Ridder, D.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef]
- George, M.S.; Taylor, J.J.; Short, E.B. The expanding evidence base for rTMS treatment of depression. Curr. Opin. Psychiatry 2013, 26, 13–18. [Google Scholar] [CrossRef]
- Cotovio, G.; Ventura, F.; Rodrigues da Silva, D.; Pereira, P.; Oliveira-Maia, A.J. Regulatory Clearance and Approval of Therapeutic Protocols of Transcranial Magnetic Stimulation for Psychiatric Disorders. Brain Sci. 2023, 13, 1029. [Google Scholar] [CrossRef]
- Hebel, T.; Grözinger, M.; Landgrebe, M.; Padberg, F.; Schecklmann, M.; Schlaepfer, T.; Schönfeldt-Lecuona, C.; Ullrich, H.; Zwanzger, P.; Langguth, B.; et al. Evidence and expert consensus based German guidelines for the use of repetitive transcranial magnetic stimulation in depression. World J. Biol. Psychiatry 2021, 23, 327–348. [Google Scholar] [CrossRef]
- NICE Guidance. Repetitive Transcranial Magnetic Stimulation for Depression. Available online: https://www.nice.org.uk/guidance/ipg542/chapter/1-recommendations (accessed on 20 June 2025).
- Levkovitz, Y.; Isserles, M.; Padberg, F.; Lisanby, S.H.; Bystritsky, A.; Xia, G.; Tendler, A.; Daskalakis, Z.J.; Winston, J.L.; Dannon, P.; et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: A prospective multicenter randomized controlled trial. World Psychiatry 2015, 14, 64–73. [Google Scholar] [CrossRef]
- O’Reardon, J.P.; Solvason, H.B.; Janicak, P.G.; Sampson, S.; Isenberg, K.E.; Nahas, Z.; McDonald, W.M.; Avery, D.; Fitzgerald, P.B.; Loo, C.; et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biol. Psychiatry 2007, 62, 1208–1216. [Google Scholar] [CrossRef]
- Gaynes, B.N.; Lloyd, S.W.; Lux, L.; Gartlehner, G.; Hansen, R.A.; Brode, S.; Jonas, D.E.; Swinson Evans, T.; Viswanathan, M.; Lohr, K.N. Repetitive transcranial magnetic stimulation for treatment-resistant depression: A systematic review and meta-analysis. J. Clin. Psychiatry 2014, 75, 477–489, quiz 489. [Google Scholar] [CrossRef]
- Voigt, J.; Carpenter, L.; Leuchter, A. A systematic literature review of the clinical efficacy of repetitive transcranial magnetic stimulation (rTMS) in non-treatment resistant patients with major depressive disorder. BMC Psychiatry 2019, 19, 13. [Google Scholar] [CrossRef]
- Brini, S.; Brudasca, N.I.; Hodkinson, A.; Kaluzinska, K.; Wach, A.; Storman, D.; Prokop-Dorner, A.; Jemioło, P.; Bala, M.M. Efficacy and safety of transcranial magnetic stimulation for treating major depressive disorder: An umbrella review and re-analysis of published meta-analyses of randomised controlled trials. Clin. Psychol. Rev. 2023, 100, 102236. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipovic, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Fregni, F.; Marcolin, M.A.; Myczkowski, M.; Amiaz, R.; Hasey, G.; Rumi, D.O.; Rosa, M.; Rigonatti, S.P.; Camprodon, J.; Walpoth, M.; et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int. J. Neuropsychopharmacol. 2006, 9, 641–654. [Google Scholar] [CrossRef]
- Lisanby, S.H.; Husain, M.M.; Rosenquist, P.B.; Maixner, D.; Gutierrez, R.; Krystal, A.; Gilmer, W.; Marangell, L.B.; Aaronson, S.; Daskalakis, Z.J.; et al. Daily Left Prefrontal Repetitive Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: Clinical Predictors of Outcome in a Multisite, Randomized Controlled Clinical Trial. Neuropsychopharmacology 2009, 34, 522–534. [Google Scholar] [CrossRef]
- Winninge, M.; Cernvall, M.; Persson, J.; Bodén, R. Early symptom improvement and other clinical predictors of response to repetitive transcranial magnetic stimulation for depression. J. Affect. Disord. 2024, 361, 383–389. [Google Scholar] [CrossRef]
- Rostami, R. Clinical Predictors of Response to rTMS in Patients with Depressive Disorder. J. Appl. Psychol. Res. 2017, 8, 181–200. [Google Scholar] [CrossRef]
- Rostami, R.; Kazemi, R.; Nitsche, M.A.; Gholipour, F.; Salehinejad, M.A. Clinical and demographic predictors of response to rTMS treatment in unipolar and bipolar depressive disorders. Clin. Neurophysiol. 2017, 128, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, A.; Calvet, B.; Laplace, B.; Lannaud, M.; Plansont, B.; Guignandon, S.; Balestrat, P.; Girard, M. Predictors of clinical response after rTMS treatment of patients suffering from drug-resistant depression. Transl. Psychiatry 2021, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Newton-Howes, G.; Tyrer, P.; Johnson, T. Personality disorder and the outcome of depression: Meta-analysis of published studies. Br. J. Psychiatry 2006, 188, 13–20. [Google Scholar] [CrossRef]
- Newton-Howes, G.; Tyrer, P.; Johnson, T.; Mulder, R.; Kool, S.; Dekker, J.; Schoevers, R. Influence of personality on the outcome of treatment in depression: Systematic review and meta-analysis. J. Personal. Disord. 2014, 28, 577–593. [Google Scholar] [CrossRef]
- Banyard, H.; Behn, A.J.; Delgadillo, J. Personality Disorders and Their Relation to Treatment Outcomes in Cognitive Behavioural Therapy for Depression: A Systematic Review and Meta-analysis. Cogn. Ther. Res. 2021, 45, 561–576. [Google Scholar] [CrossRef]
- Thaipisuttikul, P.; Ittasakul, P.; Waleeprakhon, P.; Wisajun, P.; Jullagate, S. Psychiatric comorbidities in patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2014, 10, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Skodol, A.E.; Grilo, C.M.; Keyes, K.M.; Geier, T.; Grant, B.F.; Hasin, D.S. Relationship of personality disorders to the course of major depressive disorder in a nationally representative sample. Am. J. Psychiatry 2011, 168, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Reich, J.H.; Green, A.I. Effect of personality disorders on outcome of treatment. J. Nerv. Ment. Dis. 1991, 179, 74–82. [Google Scholar] [CrossRef]
- Koppers, D.; Kool, M.; Van, H.; Driessen, E.; Peen, J.; Dekker, J. The effect of comorbid personality disorder on depression outcome after short-term psychotherapy in a randomised clinical trial. BJPsych Open 2019, 5, e61. [Google Scholar] [CrossRef]
- Bolton, J.M.; Pagura, J.; Enns, M.W.; Grant, B.; Sareen, J. A population-based longitudinal study of risk factors for suicide attempts in major depressive disorder. J. Psychiatr. Res. 2010, 44, 817–826. [Google Scholar] [CrossRef]
- Herwig, U.; Satrapi, P.; Schönfeldt-Lecuona, C. Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003, 16, 95–99. [Google Scholar] [CrossRef]
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985, 1, 1106–1107. [Google Scholar] [CrossRef]
- von Julius Kuhl, M.K. Das Persönlichkeits-Stil-und-Störungs-Inventar (PSSI): Manual; Hogrefe: Göttingen, Germany, 1997. [Google Scholar]
- Hamilton, M. Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 1967, 6, 278–296. [Google Scholar] [CrossRef]
- Bech, P.; Christensen, E.M.; Vinberg, M.; Østergaard, S.D.; Martiny, K.; Kessing, L.V. The performance of the revised Major Depression Inventory for self-reported severity of depression--implications for the DSM-5 and ICD-11. Psychother. Psychosom. 2013, 82, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Wongpakaran, N.; Wongpakaran, T.; Boonyanaruthee, V.; Pinyopornpanish, M.; Intaprasert, S. Comorbid personality disorders among patients with depression. Neuropsychiatr. Dis. Treat. 2015, 11, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Blumberger, D.M.; Vila-Rodriguez, F.; Thorpe, K.E.; Feffer, K.; Noda, Y.; Giacobbe, P.; Knyahnytska, Y.; Kennedy, S.H.; Lam, R.W.; Daskalakis, Z.J.; et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. Lancet 2018, 391, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Mulder, R.T. Personality pathology and treatment outcome in major depression: A review. Am. J. Psychiatry 2002, 159, 359–371. [Google Scholar] [CrossRef]
- Maciaszek, J.; Rymaszewska, J.; Wieczorek, T.; Piotrowski, P.; Szcześniak, D.; Beszłej, J.A.; Małecka, M.; Bogudzińska, B.; Senczyszyn, A.; Siwicki, D.; et al. Preliminary findings of a randomized controlled trial investigating the efficacy of transcranial magnetic stimulation in treatment-resistant depression: A post-hoc analysis on the role of co-occurring personality disorders. Front. Psychiatry 2024, 15, 1363984. [Google Scholar] [CrossRef]
- Krepel, N.; Rush, A.J.; Iseger, T.A.; Sack, A.T.; Arns, M. Can psychological features predict antidepressant response to rTMS? A Discovery–Replication approach. Psychol. Med. 2020, 50, 264–272. [Google Scholar] [CrossRef]
- Feffer, K.; Lee, H.H.; Wu, W.; Etkin, A.; Demchenko, I.; Cairo, T.; Mazza, F.; Fettes, P.; Mansouri, F.; Bhui, K.; et al. Dorsomedial prefrontal rTMS for depression in borderline personality disorder: A pilot randomized crossover trial. J. Affect. Disord. 2022, 301, 273–280. [Google Scholar] [CrossRef]
- Toth, C.; King Johnson, M.L.; Heinzerling, A.; Trapp, N. Response to TMS treatment for depression associated with higher levels of psychological well-being. J. Psychiatr. Res. 2022, 150, 142–146. [Google Scholar] [CrossRef]
- Baeken, C.; Desmyter, S.; Duprat, R.; De Raedt, R.; Van Denabbeele, D.; Tandt, H.; Lemmens, G.M.; Vervaet, M.; van Heeringen, K. Self-directedness: An indicator for clinical response to the HF-rTMS treatment in refractory melancholic depression. Psychiatry Res. 2014, 220, 269–274. [Google Scholar] [CrossRef]
- Berlim, M.T.; McGirr, A.; Beaulieu, M.M.; Van den Eynde, F.; Turecki, G. Are neuroticism and extraversion associated with the antidepressant effects of repetitive transcranial magnetic stimulation (rTMS)? An exploratory 4-week trial. Neurosci. Lett. 2013, 534, 306–310. [Google Scholar] [CrossRef]
- McGirr, A.; Van den Eynde, F.; Chachamovich, E.; Fleck, M.P.; Berlim, M.T. Personality dimensions and deep repetitive transcranial magnetic stimulation (DTMS) for treatment-resistant depression: A pilot trial on five-factor prediction of antidepressant response. Neurosci. Lett. 2014, 563, 144–148. [Google Scholar] [CrossRef]
- Siddiqi, S.H.; Chockalingam, R.; Cloninger, C.R.; Lenze, E.J.; Cristancho, P. Use of the Temperament and Character Inventory to Predict Response to Repetitive Transcranial Magnetic Stimulation for Major Depression. J. Psychiatr. Pract. 2016, 22, 193–202. [Google Scholar] [CrossRef][Green Version]
- Brown, L.L.; Acevedo, B.; Fisher, H.E. Neural correlates of four broad temperament dimensions: Testing predictions for a novel construct of personality. PLoS ONE 2013, 8, e78734. [Google Scholar] [CrossRef]
- Singh, A.; Erwin-Grabner, T.; Sutcliffe, G.; Antal, A.; Paulus, W.; Goya-Maldonado, R. Personalized repetitive transcranial magnetic stimulation temporarily alters default mode network in healthy subjects. Sci. Rep. 2019, 9, 5631. [Google Scholar] [CrossRef]
- Dinger, U.; Barrett, M.S.; Zimmermann, J.; Schauenburg, H.; Wright, A.G.; Renner, F.; Zilcha-Mano, S.; Barber, J.P. Interpersonal problems, dependency, and self-criticism in major depressive disorder. J. Clin. Psychol. 2015, 71, 93–104. [Google Scholar] [CrossRef]
- Torrico, T.J.; Madhanagopal, N. Schizoid Personality Disorder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Millon, T.; Grossman, S.; Millon, C.; Meagher, S.; Ramnath, R. Personality Disorders in Modern Life, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, US, 2004; p. xii, 610. [Google Scholar]

| Value (n = 63) | ||
|---|---|---|
| General variables | ||
| Sex: m/f | 27/36 | |
| Age: M (SD) | 39.24 (12.67) | |
| Age: range | 18–63 | |
| TMS variables | ||
| No. of sessions: M (SD) | 20.91 (1.20) | |
| Resting motor threshold (%): M (SD) | 39.98 (6.93) | |
| Intensity of treatment (%): M (SD) | 47.84 (7.74) | |
| Depression questionnaire scores: M (SD) | ||
| HAMD-21 | Baseline | 19.95 (5.14) |
| After treatment | 12.04 (5.80) | |
| MDI | Baseline | 32.27 (8.24) |
| After treatment | 21.16 (10.55) | |
| PSSI subscales | ||
| Self-willed–paranoid (PN) | 52.11 (12.86) | |
| Reserved–schizoid (SZ) | 57.10 (11.55) | |
| Suspicious–schizotypal (ST) | 44.29 (6.70) | |
| Spontaneous–borderline (BL) | 58.98 (8.00) | |
| Amiable–histrionic (HI) | 44.38 (9.80) | |
| Ambitious–narcissistic (NA) | 45.19 (8.74) | |
| Self-critical–self-insecure (SU) | 59.81 (8.49) | |
| Loyal–dependent (AB) | 54.44 (9.85) | |
| Meticulous–compulsive (ZW) | 54.70 (10.29) | |
| Critical–negativistic (NT) | 54.11 (10.90) | |
| Silent–depressive (DP) | 64.49 (9.27) | |
| Helpful–selfless (SL) | 55.67 (9.43) | |
| Optimistic–rhapsodic (RH) | 40.56 (8.69) | |
| Assertive–antisocial (AS) | 41.62 (9.19) | |
| Course of the Symptoms | PN | SZ | ST | BL | HI | NA | SU | AB | ZW | NT | DP | SL | RH | AS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAMD-21 | 0.028 | 0.129 | 0.102 | −0.016 | −0.050 | −0.065 | 0.191 | 0.102 | 0.063 | −0.228 | 0.047 | 0.067 | 0.010 | −0.094 |
| MDI | −0.068 | 0.141 | 0.173 | −0.066 | −0.151 | −0.155 | 0.054 | −0.030 | <0.001 | −0.288 * | −0.239 | 0.044 | −0.096 | −0.106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelnaim, M.A.; Hebel, T.; Kerkel, K.; Langguth, B.; Schecklmann, M.; Staudinger, S.; Reissmann, A. Effects of Personality Styles on Clinical Response to Intermittent Theta Burst Stimulation for Depression. J. Clin. Med. 2025, 14, 7612. https://doi.org/10.3390/jcm14217612
Abdelnaim MA, Hebel T, Kerkel K, Langguth B, Schecklmann M, Staudinger S, Reissmann A. Effects of Personality Styles on Clinical Response to Intermittent Theta Burst Stimulation for Depression. Journal of Clinical Medicine. 2025; 14(21):7612. https://doi.org/10.3390/jcm14217612
Chicago/Turabian StyleAbdelnaim, Mohamed A., Tobias Hebel, Katharina Kerkel, Berthold Langguth, Martin Schecklmann, Susanne Staudinger, and Andreas Reissmann. 2025. "Effects of Personality Styles on Clinical Response to Intermittent Theta Burst Stimulation for Depression" Journal of Clinical Medicine 14, no. 21: 7612. https://doi.org/10.3390/jcm14217612
APA StyleAbdelnaim, M. A., Hebel, T., Kerkel, K., Langguth, B., Schecklmann, M., Staudinger, S., & Reissmann, A. (2025). Effects of Personality Styles on Clinical Response to Intermittent Theta Burst Stimulation for Depression. Journal of Clinical Medicine, 14(21), 7612. https://doi.org/10.3390/jcm14217612






