Long-Term Clinical Outcomes of Minimally Invasive Direct Coronary Artery Bypass Grafting
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- MIDCAB CR (CR-Complete revascularization) group (n = 260): 234 patients (90.0%) had isolated LAD disease and the rest (10.0%) had double vessel disease MIDCAB using either saphenous vein graft or radial artery connected to the LITA as a Y/T graft with LITA revascularizing the LAD.
- MIDCAB hybrid coronary revascularization (HCR) group (n = 142): these patients had multivessel disease with the heart team decision to perform percutaneous coronary intervention (PCI) and MIDCAB. 135 patients (95%) had isolated MIDCAB LITA to LAD and the rest (5%) had had double vessel disease MIDCAB using either saphenous vein graft or radial artery connected to the LITA as a Y/T graft with LITA revascularizing the LAD. First PCI strategy was performed in 61 patients (42.9%) and the rest 81 patients (57.1%) first MIDCAB strategy was performed.
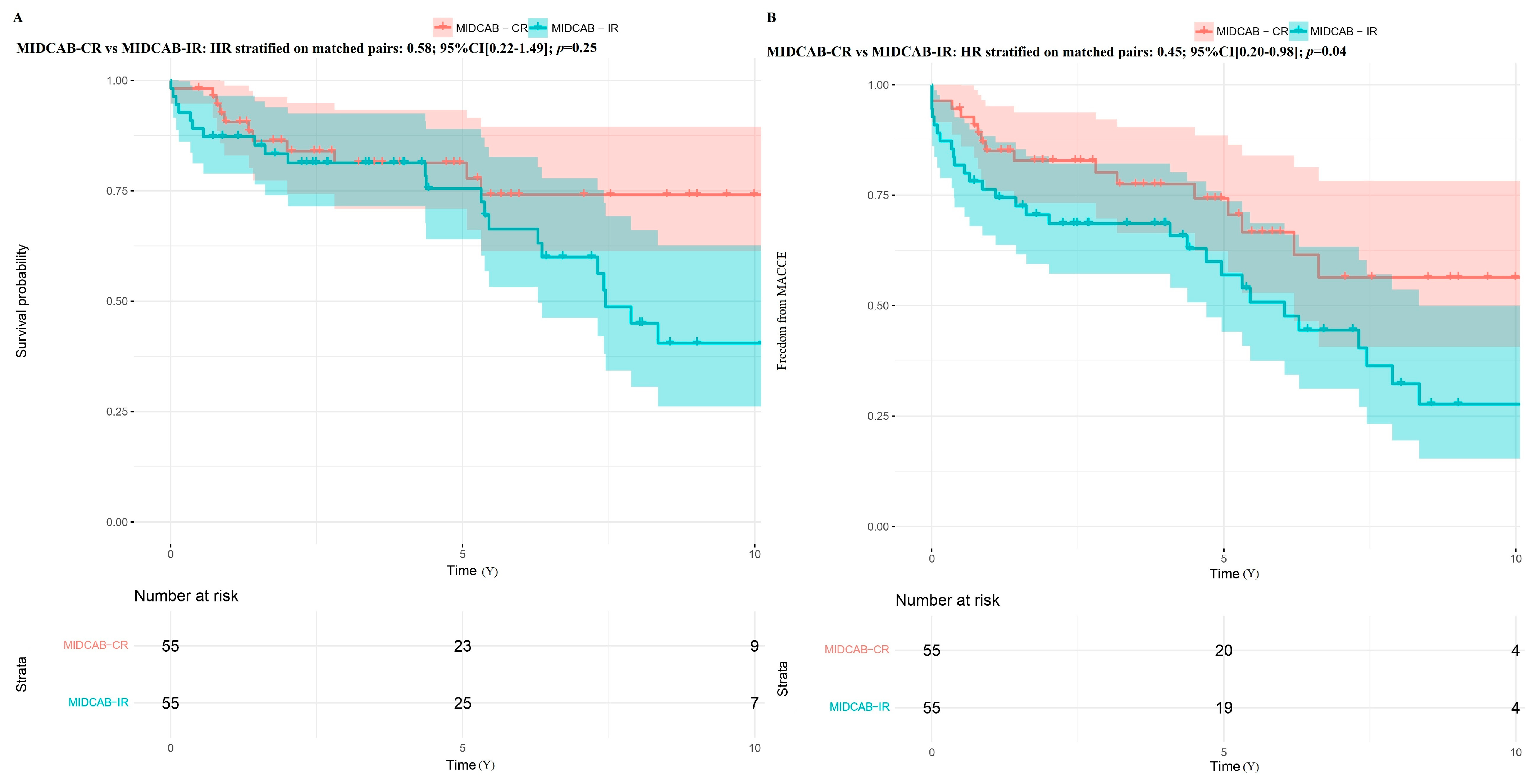
- MIDCAB IR (IR-Incomplete revascularization) group (n = 78): these patients had multivessel disease with the heart team decision to perform planned IR with only MIDCAB LITA-LAD due to high-risk profile of the patients and unfavorable PCI anatomy (IR was defined as an untreated significant stenosis of more than 70% of the vessel diameter).
2.2. Surgical Technique
2.3. Study Endpoints and Definitions
2.4. Statistical Analysis
3. Results
3.1. Early Outcomes
3.2. Long-Term Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CABG | Coronary artery bypass grafting |
| CI | Confidence interval |
| CR | Complete revascularization |
| DWI | Deep wound infection |
| HCR | Hybrid coronary revascularization |
| HRs | Hazard ratios |
| IABP | Intra-aortic balloon pump |
| IQR | Interquartile range |
| IR | Incomplete revascularization |
| LAD | Left anterior descending artery |
| LITA | Left internal thoracic artery |
| LOHS | Length of hospital stay |
| MACCE | Major adverse cardiac and cerebral events |
| MI | Myocardial infarction |
| MIDCAB | Minimally invasive direct coronary artery bypass grafting |
| PCI | Percutaneous coronary intervention |
| pRBC | Packed red blood cell transfusion |
| PS | Propensity score |
| RRT | Renal replacement therapy |
| SD | Standard deviation |
| SMD | Standardized mean difference |
| TTFM | Transit time flow measurement |
References
- Iribarne, A.; Zwischenberger, B.; Mehaffey, J.; Kaneko, T.; Wyler von Ballmoos, M.C.; Jacobs, J.P.; Krohn, C.; Habib, R.H.; Parsons, N.; Badhwar, V.; et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2024 update on national trends and outcomes. Ann. Thorac. Surg. 2025; in press. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Calafiore, A.M.; Giammarco, G.D.; Teodori, G.; Bosco, G.; D’Annunzio, E.; Barsotti, A.; Maddestra, N.; Paloscia, L.; Vitolla, G.; Sciarra, A.; et al. Left anterior descending coronary artery grafting via left anterior small thoracotomy without cardiopulmonary bypass. Ann. Thorac. Surg. 1996, 61, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Al-Ruzzeh, S.; Mazrani, W.; Wray, J.; Modine, T.; Nakamura, K.; George, S.; Ilsley, C.; Amrani, M. The clinical outcome and quality of life following minimally invasive direct coronary artery bypass surgery. J. Card. Surg. 2004, 19, 12–16. [Google Scholar] [CrossRef]
- Raja, S.G.; Benedetto, U.; Alkizwini, E.; Gupta, S.; Amrani, M. Propensity score adjusted comparison of MIDCAB versus full sternotomy left anterior descending artery revascularization. Innovations 2015, 10, 174–178. [Google Scholar] [CrossRef]
- Stanislawski, R.; Aboul-Hassan, S.S.; Marczak, J.; Stankowski, T.; Peksa, M.; Nawotka, M.; Cichon, R. Early and long-term clinical outcomes after minimally invasive direct coronary artery bypass grafting versus off-pump coronary surgery via sternotomy. J. Card. Surg. 2020, 35, 3412–3419. [Google Scholar] [CrossRef]
- Stanislawski, R.; Aboul-Hassan, S.S.; Pieszko, K.; Awad, A.K.; Stankowski, T.; Peksa, M.; Nawotka, M.; Moskal, L.; Marczak, J.; Torregrossa, G.; et al. Long-term outcomes of minimally invasive direct coronary artery bypass vs second generation drug eluting stent. Int. J. Cardiol. 2025, 422, 132935. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Ridderstolpe, L.; Gill, H.; Granfeldt, H.; Ahlfeldt, H.; Rutberg, H. Superficial and deep sternal wound complications: Incidence, risk factors and mortality. Eur. J. Cardiothorac. Surg. 2001, 20, 1168–1175. [Google Scholar] [CrossRef]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for prevention of surgical site infection, 1999. Am. J. Infect. Control 1999, 27, 97–132. [Google Scholar] [CrossRef]
- Lauer, M.S.; Blackstone, E.H.; Young, J.B.; Topol, E.J. Cause of death in clinical research: Time for a reassessment? J. Am. Coll. Cardiol. 1999, 34, 618–620. [Google Scholar] [CrossRef]
- Fitzgibbon, G.M.; Kafka, H.P.; Leach, A.J.; Keon, W.J.; Hooper, G.D.; Burton, J.R. Coronary bypass graft fate and patient outcome: Angiographic follow-up of 5065 grafts. J. Am. Coll. Cardiol. 1996, 28, 616–626. [Google Scholar] [CrossRef]
- Loop, F.D. Internal-thoracic-artery grafts: Biologically better coronary arteries. N. Engl. J. Med. 1996, 334, 263–265. [Google Scholar] [CrossRef]
- Tatoulis, J.; Buxton, B.F.; Fuller, J.A. Patencies of 2127 arterial to coronary conduits over 15 years. Ann. Thorac. Surg. 2004, 77, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Hannan, E.L.; Zhong, Y.; Cozzens, K.; Adams, D.H.; Girardi, L.; Chikwe, J.; Wechsler, A.; Sundt, T.M., 3rd; Smith, C.R.; Gold, J.P.; et al. Revascularization for isolated proximal left anterior descending artery disease. Ann. Thorac. Surg. 2021, 112, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Davierwala, P.M.; Verevkin, A.; Bergien, L.; von Aspern, K.; Deo, S.V.; Misfeld, M.; Holzhey, D.; Borger, M.A. Twenty-year outcomes of minimally invasive direct coronary artery bypass surgery: The Leipzig experience. J. Thorac. Cardiovasc. Surg. 2023, 165, 115–127. [Google Scholar] [CrossRef]
- Margari, V.; Squiccimarro, E.; Pascarella, C.; Carbone, C.; Paparella, D. Early and mid-term results of minimally invasive direct coronary artery bypass (MIDCAB) surgery. Vessel Plus 2024, 8, 38. [Google Scholar] [CrossRef]
- Akintoye, O.; Divya, A.; Farid, S.; Nashef, S.; De Silva, R. Sixteen-year outcomes of patients undergoing minimally invasive direct coronary artery bypass surgery: A single-center experience. Cardiothorac. Surg. 2024, 32, 16. [Google Scholar] [CrossRef]
- De Groote, S.; Marain, N.; Torregrossa, G.; Oosterlinck, W. Embracing industry in the development of robotic coronary bypass grafting—The sun rises in the East. Ann. Cardiothorac. Surg. 2024, 13, 417–424. [Google Scholar] [CrossRef]
- Raja, S.G. New clinical advances in minimally invasive coronary surgery. J. Clin. Med. 2025, 14, 3142. [Google Scholar] [CrossRef]
- Repossini, A.; Di Bacco, L.; Nicoli, F.; Passaretti, B.; Stara, A.; Jonida, B.; Muneretto, C. Minimally invasive coronary artery bypass: Twenty-year experience. J. Thorac. Cardiovasc. Surg. 2019, 158, 127–138.e1. [Google Scholar] [CrossRef]
- Weymann, A.; Amanov, L.; Beltsios, E.; Arjomandi Rad, A.; Szczechowicz, M.; Merzah, A.S.; Ali-Hasan-Al-Saegh, S.; Schmack, B.; Ismail, I.; Popov, A.-F.; et al. Minimally invasive direct coronary artery bypass grafting: Sixteen years of single-center experience. J. Clin. Med. 2024, 13, 3338. [Google Scholar] [CrossRef]
- Tajstra, M.; Hrapkowicz, T.; Hawranek, M.; Filipiak, K.; Gierlotka, M.; Zembala, M.; Gąsior, M.; Zembala, M.O. Hybrid coronary revascularization in selected patients with multivessel disease: 5-year clinical outcomes. JACC Cardiovasc. Interv. 2018, 11, 847–852. [Google Scholar] [CrossRef]
- Newman, J.S.; Jarral, O.A.; Kim, M.C.; Brinster, D.R.; Singh, V.P.; Scheinerman, S.J.; Patel, N.C. Ten-year outcomes of hybrid coronary revascularization at a single center. Ann. Cardiothorac. Surg. 2024, 13, 425–435. [Google Scholar] [CrossRef]
- Garcia, S.; Sandoval, Y.; Roukoz, H.; Adabag, S.; Canoniero, M.; Yannopoulos, D.; Brilakis, E.S. Outcomes after complete versus incomplete revascularization: A meta-analysis of 89,883 patients. J. Am. Coll. Cardiol. 2013, 62, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Hassan, S.S.; Awad, A.K.; Stankowski, T.; Perek, B.; Marczak, J.; Rodzki, M.; Jemielity, M.; Moskal, L.; Sá, M.P.; Torregrossa, G. Impact of incomplete revascularization on long-term survival based on revascularization strategy. Ann. Thorac. Surg. 2024, 118, 605–614. [Google Scholar] [CrossRef]
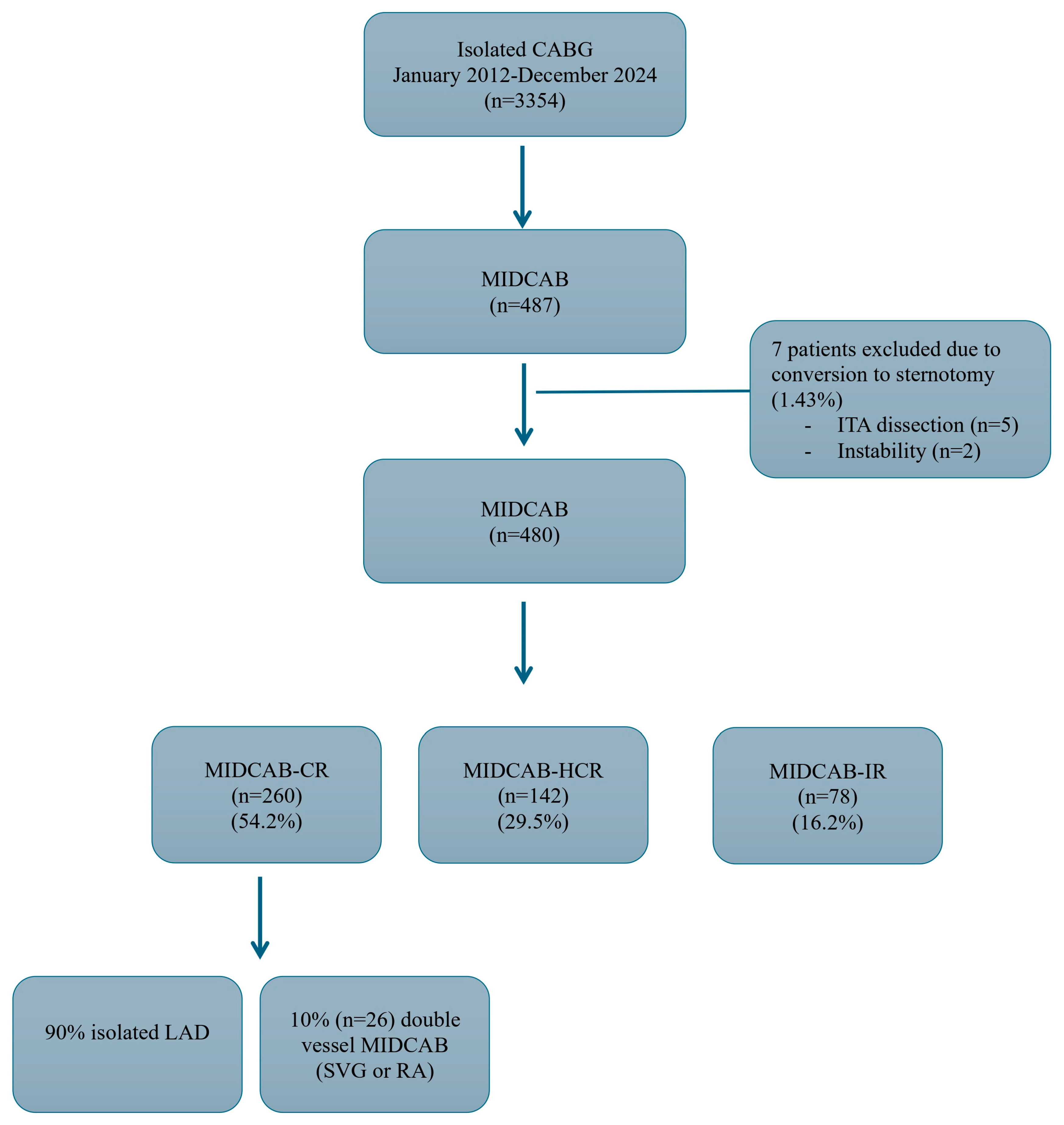

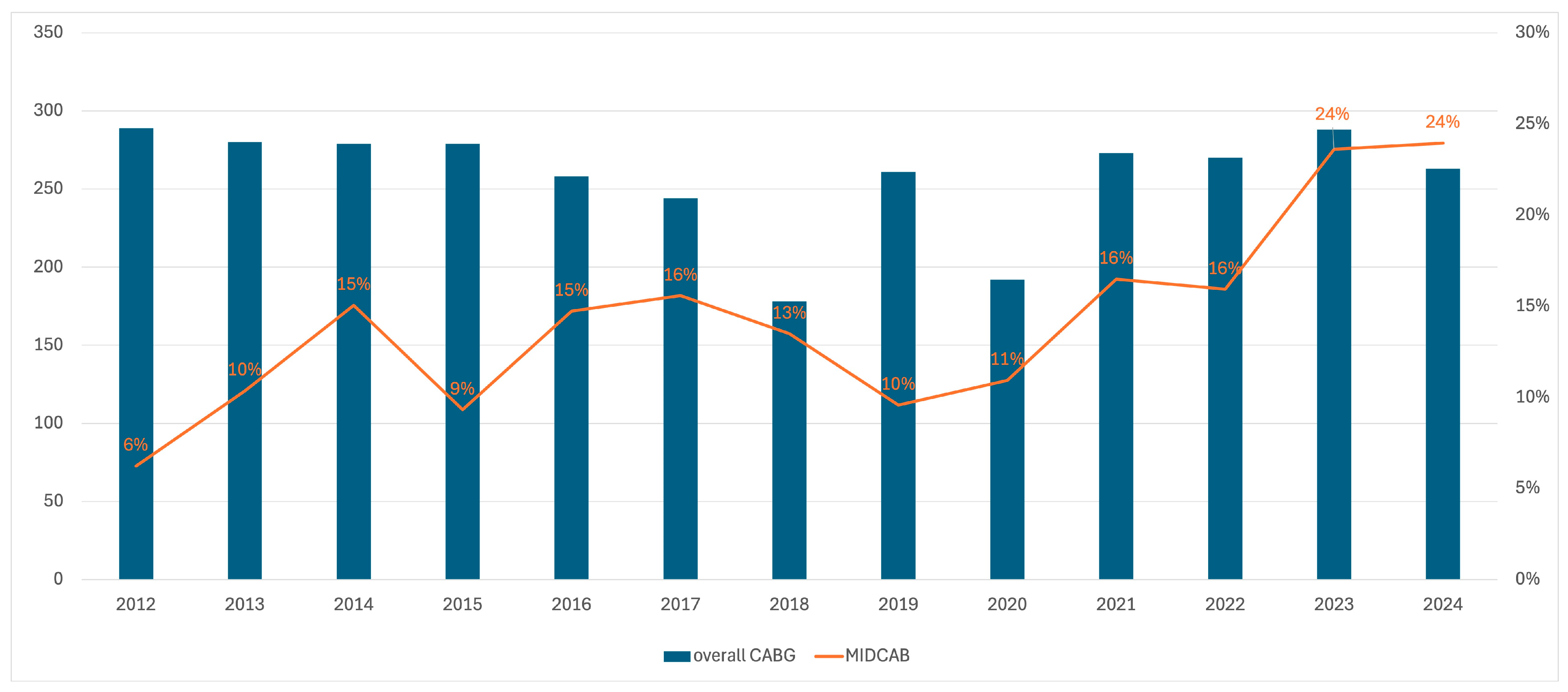
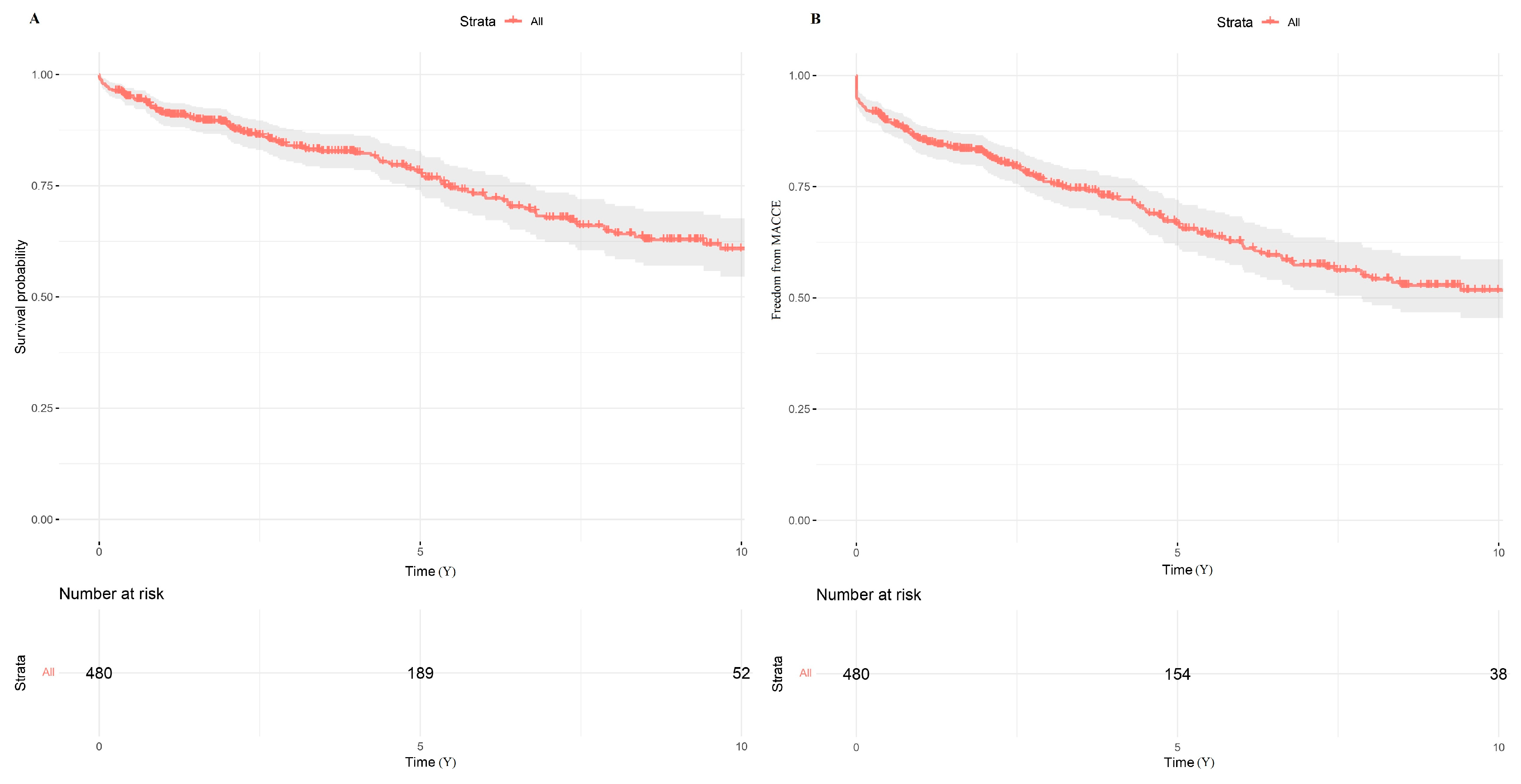
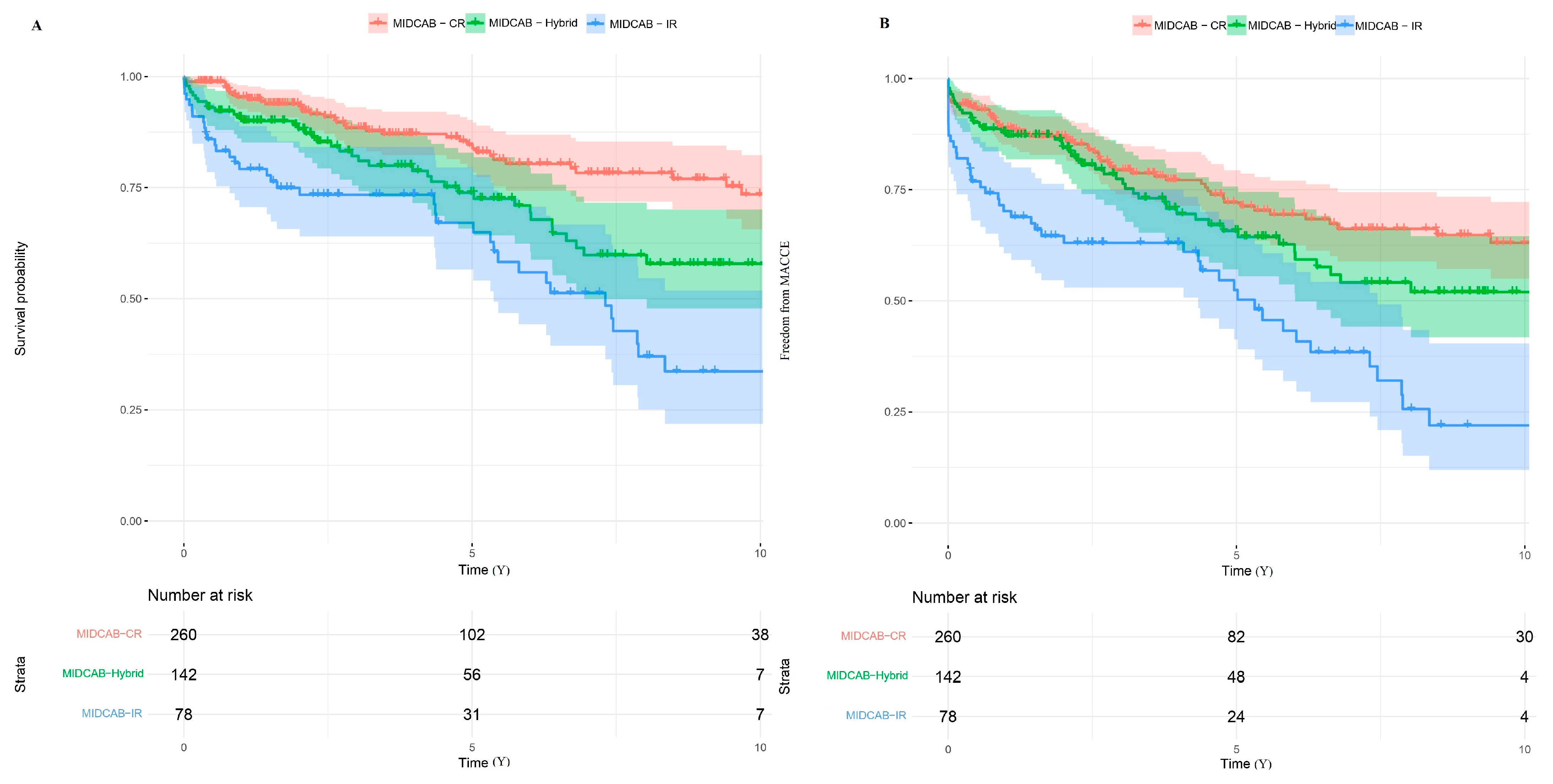


| Factor | MIDCAB | MIDCAB-CR | MIDCAB-HCR | MIDCAB-IR |
|---|---|---|---|---|
| n | n = 480 | n = 260 | n = 142 | n = 78 |
| Age (years) | 66.57 (9.33) | 65.77 (9.50) | 66.96 (9.24) | 68.58 (8.86) |
| Age above 75 (%) | 86 (19.9) | 43 (16.5) | 27 (19.0) | 16 (20.5) |
| Female gender (%) | 98 (20.4) | 58 (22.3) | 26 (18.3) | 14 (17.9) |
| BMI (Kg/m2) | 27.91 (4.49) | 27.81 (4.37) | 27.69 (4.81) | 28.70 (4.27) |
| Obesity (%) | (26.8) | 68 (26.2) | 34 (23.9) | 27 (34.6) |
| EF (%) | 51.47 (9.58) | 53.13 (8.43) | 50.18 (10.50) | 48.35 (10.43) |
| EF below 40 (%) | 90 (18.7) | 33 (12.7) | 35 (24.6) | 22 (28.2) |
| Diabetes (%) | 151 (31.4) | 71 (27.3) | 50 (35.2) | 30 (38.5) |
| Insulin (%) | 52 (10.8) | 19 (7.3) | 21 (14.8) | 12 (15.4) |
| Active smoker (%) | 86 (17.9) | 39 (15.0) | 31 (21.8) | 16 (20.5) |
| AF (%) | 49 (10.2) | 22 (8.5) | 15 (10.6) | 12 (15.4) |
| CLD (%) | 42 (8.7) | 15 (5.8) | 16 (11.3) | 11 (14.1) |
| Moderate RI (%) | 188 (39.1) | 104 (40.0) | 56 (39.4) | 28 (35.9) |
| Severe RI (%) | 49. (10.2) | 19 (7.3) | 14 (9.9) | 16 (20.5) |
| Dialysis (%) | 6 (1.2) | 5 (1.9) | 1 (0.7) | 0 (0.0) |
| History of CVAEs (%) | 8 (1.6) | 3 (1.2) | 3 (2.1) | 2 (2.6) |
| History of PCI (%) | 131 (27.2) | 60 (23.1) | 60 (42.3) | 11 (14.1) |
| LM (%) | 40 (8.3) | 0 (0.0) | 19 (13.4) | 21 (26.9) |
| NYHA III IV (%) | 32 (6.6) | 12 (4.6) | 15 (10.6) | 5 (6.4) |
| PAD (%) | 82 (17.0) | 30 (11.5) | 31 (21.8) | 21 (26.9) |
| Recent MI (%) | 124 (25.8) | 53 (20.4) | 49 (34.5) | 22 (28.2) |
| Urgent (%) | 171 (35.6) | 75 (28.8) | 66 (46.5) | 30 (38.5) |
| Euroscore II (%) | 1.99 (2.45) | 1.75 (2.57) | 2.10 (2.01) | 2.60 (2.70) |
| Factor | MIDCAB | MIDCAB-CR | MIDCAB-Hybrid | MIDCAB-IR | p | p | p |
|---|---|---|---|---|---|---|---|
| n | 480 | 260 | 142 | 78 | CR vs. HCR | CR vs. IR | HCR vs. IR |
| In hospital MACCE (%) | 27 (4.8) | 10 (3.8) | 7 (4.9) | 10 (12.8) | 0.60 | 0.005 | 0.04 |
| In hospital mortality (%) | 7 (1.4) | 1 (0.4) | 3 (2.1) | 3 (3.8) | 0.13 | 0.04 | 0.45 |
| Postoperative MI (%) | 20 (4.1) | 9 (3.5) | 4 (2.8) | 7 (9.0) | 0.72 | 0.05 | 0.05 |
| Postoperative Stroke (%) | 3 (0.6) | 0 (0.0) | 2 (1.4) | 1 (1.3) | 0.15 | 0.15 | 0.93 |
| MCS (%) | 2 (0.4) | 0 (0.0) | 0 (0.0) | 2 (2.6) | >0.99 | 0.06 | 0.15 |
| Any PRBC (%) | 107 (22.2) | 50 (19.2) | 37 (26.1) | 20 (25.6) | 0.11 | 0.22 | 0.94 |
| DWI (%) | 16 (3.3) | 6 (2.3) | 4 (2.8) | 6 (7.7) | 0.75 | 0.03 | 0.11 |
| New onset RRT (%) | 6 (1.2) | 0 (0.0) | 2 (1.4) | 4 (5.1) | 0.15 | 0.02 | 0.10 |
| Rethoracotomy (%) | 29 (6.0) | 16 (6.2) | 7 (4.9) | 6 (7.7) | 0.61 | 0.62 | 0.40 |
| LOHS | 5.90 (3.85) | 5.66 (2.20) | 6.03 (4.50) | 6.71 (6.39) | 0.18 | 0.03 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboul-Hassan, S.S.; Luszczyn, M.; Stanislawski, R.; Peksa, M.; Nawotka, M.; Amelchanka, S.; Moskal, L.; Stankowski, T.; Cichon, R. Long-Term Clinical Outcomes of Minimally Invasive Direct Coronary Artery Bypass Grafting. J. Clin. Med. 2025, 14, 7590. https://doi.org/10.3390/jcm14217590
Aboul-Hassan SS, Luszczyn M, Stanislawski R, Peksa M, Nawotka M, Amelchanka S, Moskal L, Stankowski T, Cichon R. Long-Term Clinical Outcomes of Minimally Invasive Direct Coronary Artery Bypass Grafting. Journal of Clinical Medicine. 2025; 14(21):7590. https://doi.org/10.3390/jcm14217590
Chicago/Turabian StyleAboul-Hassan, Sleiman Sebastian, Maria Luszczyn, Ryszard Stanislawski, Maciej Peksa, Marcin Nawotka, Siarhei Amelchanka, Lukasz Moskal, Tomasz Stankowski, and Romuald Cichon. 2025. "Long-Term Clinical Outcomes of Minimally Invasive Direct Coronary Artery Bypass Grafting" Journal of Clinical Medicine 14, no. 21: 7590. https://doi.org/10.3390/jcm14217590
APA StyleAboul-Hassan, S. S., Luszczyn, M., Stanislawski, R., Peksa, M., Nawotka, M., Amelchanka, S., Moskal, L., Stankowski, T., & Cichon, R. (2025). Long-Term Clinical Outcomes of Minimally Invasive Direct Coronary Artery Bypass Grafting. Journal of Clinical Medicine, 14(21), 7590. https://doi.org/10.3390/jcm14217590








