4.1. Key Findings
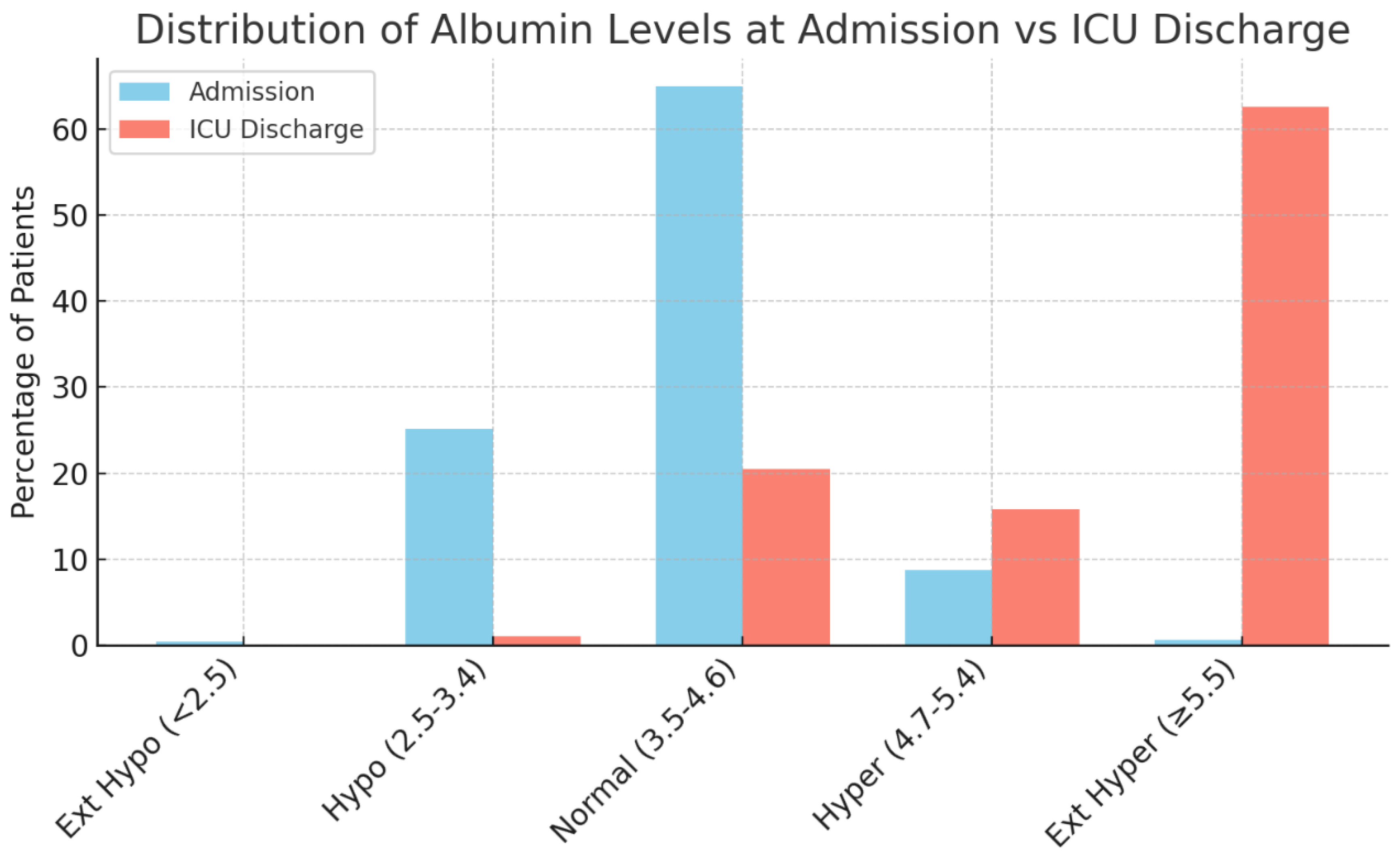
This single-center retrospective study of 925 severe TBI patients found that serum ALBs underwent a profound shift during hospitalization, yet showed no significant association with key clinical outcomes. On admission, about two-thirds of patients had normal albumin (3.5–4.6 g/dL), and roughly one-quarter presented with hypoalbuminemia (<3.5 g/dL). Extreme deviations were very uncommon initially (<1% for albumin <2.5 or ≥5.5 g/dL). By ICU discharge, however, ALBs had risen dramatically; nearly 80% of patients became hyperalbuminemic (>4.7 g/dL), with over 60% reaching extreme hyperalbuminemia (≥5.5 g/dL). This striking upward shift suggests that aggressive volume resuscitation and/or albumin supplementation in the ICU corrected initial deficits, virtually eliminating severe hypoalbuminemia by the time of transfer out of intensive care. While this therapeutic intervention could be considered an issue in evaluating ALB, these values are standardized through resuscitation and intervention protocols. Their corresponding clinical outcomes are compared not only against each other, but also against the findings of other literature done with different fluctuations in ALBs.
Despite these wide fluctuations in albumin, no statistically significant differences in outcomes were observed across albumin categories. In-hospital mortality was similar regardless of admission ALB: patients with hypoalbuminemia on arrival had a mortality of ~14.2% versus 12.2% in normoalbuminemic patients (p = 0.71). Even patients with extreme hyperalbuminemia at admission (n = 6) all survived, though this group was too small for meaningful comparison. By ICU discharge, survival rates remained comparable across ALBs (~13% mortality in each group), and the distribution of albumin did not significantly differ between survivors and non-survivors (χ2 = 3.47, p = 0.324). Similarly, other outcome measures showed no notable variation. Hospital length of stay (HLOS) was on the order of 7–8 days for each albumin group, with no significant differences by Kruskal–Wallis test (p > 0.5). ICU length of stay and days on mechanical ventilation were likewise equivalent across albumin strata. Patients presenting with low albumin did not experience longer hospitalizations or ICU stays than those with normal or high albumin, nor did high-albumin patients have shorter stays. Statistical analyses consistently confirmed the absence of strong associations; all p-values were well above significance thresholds for both categorical outcomes (mortality, discharge disposition) and continuous outcomes (durations of care).
Overall, the key finding is that serum ALB per se was not a clear prognostic predictor in this cohort of severe TBI patients. This contrasts with some other TBI-related laboratory parameters (e.g., dysnatremia or dyskalemia) that have shown prognostic significance. In our analysis, patients in all albumin categories had broadly similar clinical outcomes. The most notable observation was the dynamic albumin change over time: initial hypoalbuminemia was common, but after aggressive care in the ICU, most patients became hyperalbuminemic. Crucially, this rise in albumin did not translate into any outcome divergence between groups. In summary, while ALBs varied widely from admission to ICU discharge, these variations did not significantly influence mortality, length of stay, or ventilator days in our severe TBI population.
4.2. Comparison to Existing Literature
Our findings can be contextualized against a growing body of literature on albumin in critical illness and TBI. Hypoalbuminemia is widely recognized as a marker of illness severity and has been extremely common in trauma cohorts. For instance, a recent prospective study of 386 trauma patients reported early-onset hypoalbuminemia (albumin < 3.5 g/dL) in 53.1% of cases within the first week [
8]. Similarly, among severe TBI populations, roughly 30–45% of patients have been found to present with hypoalbuminemia on admission [
13,
14]. This prevalence aligns with our observation of ~25% hypoalbuminemia at admission (noting our cohort’s exclusion of moribund 24-h survivors might lower this rate). Historically, low albumin has often been associated with worse outcomes. Nayak et al. (2020), studying 80 severe head injury patients, found that those with admission albumin < 3.5 g/dL had significantly poorer 6-month neurological outcomes compared to normoalbuminemic patients (72.5% vs. 90% good recovery;
p = 0.01) [
15]. In their analysis, hypoalbuminemia emerged as an independent predictor of unfavorable outcome (adjusted odds ratio ~6.3 for poor Glasgow Outcome Score). This stands in contrast to our larger cohort, which did not confirm any outcome disadvantage for low-albumin patients in the acute hospital phase. One possible reason is that prior studies often examined longer-term functional outcomes (e.g., 3–6 month GOS) or were limited to smaller sample sizes, whereas our study focused on in-hospital metrics with a robust sample and aggressive albumin correction practices [
16,
17,
18]. It is conceivable that albumin’s impact might manifest more in long-term recovery or untreated states, whereas prompt critical care interventions (as in our cohort) can mitigate short-term risks associated with low albumin.
Recent research has also explored composite markers that integrate albumin with other indicators of physiologic stress. These studies generally reinforce the notion that while albumin alone has little prognostic value, it may be a stronger predictor when considered in combination with markers of inflammation or perfusion. For example, the C-reactive protein/albumin ratio (CAR), an index of systemic inflammation and nutritional status, has shown promise in TBI prognostication [
19]. Wang et al. (2023) reported that TBI patients who did not survive had a markedly higher admission CAR than survivors (median ~3.8 vs. 2.6,
p < 0.001) [
20]. In multivariate analysis, CAR was an independent risk factor for in-hospital mortality, outperforming some conventional predictors. This mirrors findings in other conditions that an elevated CAR portends worse outcomes, likely because it captures the dual hit of inflammation (high CRP) and poor protein reserves (low albumin). Similarly, the lactate-to-albumin ratio (LAR) has been investigated as a prognostic tool in neurotrauma. Lactate reflects tissue hypoperfusion and metabolic stress, while albumin reflects nutritional and inflammatory status; a high LAR thus indicates simultaneous shock and hypoalbuminemia. Lee et al. (2023) found that an elevated LAR on admission was associated with significantly higher 24-h mortality in TBI (odds ratio ~2.0 per unit increase in LAR) [
21]. Correspondingly, Wang et al. (2022) observed in 273 TBI patients that non-survivors had a mean LAR roughly double that of survivors (1.09 vs. 0.53) and that LAR was an independent predictor of in-hospital death (OR 1.70,
p = 0.022) [
22]. Notably, in that study, albumin by itself did have some prognostic signal—admission albumin under 3.5 g/dL was associated with worse outcomes—but the combined LAR provided better discrimination (area under ROC 0.78 for LAR vs. 0.74 for albumin alone). These findings suggest that albumin contributes meaningfully to outcome prediction when interpreted in context with concurrent physiologic disturbances. Our results, which showed no outcome difference across albumin categories alone, are thus consistent with the idea that albumin as a solitary measure may be an insufficient prognostic discriminator, even if extreme hypoalbuminemia indicates illness severity. Large critical-care studies have noted that while hypoalbuminemia independently correlates with higher mortality, its predictive power is limited. For example, an analysis of >18,000 ICU patients found that serum albumin < 3.0 g/dL was associated with ~1.5-fold higher odds of hospital death, but albumin had low sensitivity and specificity for mortality (many nonsurvivors had normal albumin and vice versa) [
23]. This aligns with our finding that albumin categories did not cleanly stratify outcomes; other factors in TBI likely dominate prognosis unless albumin is exceedingly low.
Inflammation-based albumin indices further underscore albumin’s contextual role. Aside from CAR and LAR, the neutrophil-to-albumin ratio (NAR) has recently been proposed as a superior composite biomarker in TBI [
24,
25]. In a recent study, they showed that NAR had stronger prognostic performance than traditional measures like neutrophil–lymphocyte ratio [
26]. Patients with poor 3-month neurological outcomes had significantly higher NAR on admission than those with good recoveries. Importantly, that study noted that hypoalbuminemia on its own was linked to elevated 30-day mortality rates in TBI patients, reinforcing the general association between low albumin and adverse outcome, but also that coupling albumin with an inflammatory marker like neutrophil count improved predictive accuracy. In summary, the literature suggests that albumin derangements after TBI are common and generally correlate with injury severity and outcome, but in isolation, albumin is a blunt prognostic instrument. Our findings add to this context by demonstrating in a large cohort that ALB, when aggressively managed, did not differentiate patient trajectories. This contrasts with smaller studies that reported significant outcome differences with hypoalbuminemia in non-traumatic care, as well as with composite indices where albumin plays a part [
27,
28]. One notable difference in our cohort is the routine correction of hypoalbuminemia; by ICU discharge, most patients were hyperalbuminemic, a scenario seldom reported elsewhere. Thus, our results support the notion from broader critical care research that while hypoalbuminemia is a red flag biologically, its prognostic impact can be blunted by prompt, effective supportive care. Indeed, the role of albumin in TBI is still debated on the therapeutic front: earlier trials (SAFE-TBI) suggested that infusing hypo-oncotic albumin (4% solution) in TBI resuscitation was associated with worse outcomes, whereas a more recent meta-analysis indicated that using hyperoncotic 20–25% albumin (as part of the “Lund concept” for intracranial pressure management) was associated with lower mortality (14.5% vs. 38.1% in controls,
p = 0.002) [
7]. These disparate findings reflect that albumin’s effects may depend on context, such as concentration, fluid shifts, and timing, and highlight the complexity in comparing across studies. In the context of prognostication, our study’s null results align with those larger patterns: albumin is a valuable clinical indicator of physiologic stress, but not a stand-alone predictor of outcome unless combined with other factors or left uncorrected.
4.3. Implications of Study Findings
From a clinical perspective, our findings suggest that routine albumin measurements in severe TBI may be more useful for guiding supportive therapy than for predicting patient outcomes. The dramatic rise in ALBs observed during ICU care indicates that modern critical care interventions (e.g., intravenous albumin administration, concentrated feeding, or hemoconcentration via diuresis) can effectively reverse initial hypoalbuminemia. Consequently, any potential prognostic disadvantage of low albumin on admission appears to have been mitigated in our cohort. In practical terms, this means that an admission albumin level below normal should prompt clinicians to ensure adequate volume resuscitation and nutritional support, but it should not be viewed as a definitive harbinger of poor outcome in isolation. Traditional teaching has linked hypoalbuminemia to higher morbidity and mortality, yet our data indicate that when hypoalbuminemia is promptly corrected, severe TBI patients can achieve outcomes comparable to those with normal albumin. This underscores the importance of addressing reversible factors (like protein depletion) early in the ICU course.
It is also notable that ALBs by ICU discharge were universally high without corresponding improvements in outcome. This implies there is no clear benefit (nor overt harm) in driving albumin to supranormal ranges with aggressive replacement. Therefore, management protocols might prioritize maintaining adequate albumin for colloid osmotic support and medication binding, but ultra-high ALBs should not be expected to confer additional outcome advantages. Our study reinforces that neurologic injury severity and systemic complications (rather than albumin per se) remain the dominant outcome determinants in severe TBI. Going forward, clinicians should continue to treat albumin derangements as an addressable marker of physiological stress, but not rely on albumin alone for prognostication. Instead, a more holistic approach, such as integrating albumin with other indicators (e.g., lactate, CRP, immune markers), may better inform risk, as suggested by emerging composite scores. In summary, our findings imply that maintaining albumin within a normal range is an important supportive goal in severe TBI care, but beyond that, ALB itself provides limited prognostic information, especially in the context of proactive critical care management.
4.4. Strengths, Limitations, and Future Perspectives
This investigation offers several strengths that add confidence to its principal finding, which is that serum albumin, when aggressively managed, does not independently predict acute outcomes in severe TBI. First, the cohort is large (n = 925) for a single-center neuro-trauma study and encompasses consecutive admissions over four years, reducing selection bias. Second, albumin was measured at multiple predefined junctures (admission, ICU admission, ICU discharge, hospital discharge, and death), permitting a rare view of its trajectory across the entire acute-care episode rather than the single time-point snapshots typical of earlier reports. Third, the institution’s standardized resuscitation, nutrition, and neurosurgical protocols minimize inter-patient treatment variability, allowing clearer attribution of observed albumin shifts to routine critical-care interventions. Finally, outcomes were captured comprehensively, including mortality, hospital and ICU length of stay, and ventilator days, providing a multidimensional assessment of clinical course.
Important limitations temper these strengths. The retrospective design precludes firm causal inference and is vulnerable to undocumented confounders. Exclusion of patients who died or were discharged within 24 h, although methodologically necessary to analyze ICU variables, omits the most fulminant presentations and may underestimate any association between extreme hypoalbuminemia and ultra-early mortality. Being a single-center study further constrains generalizability; other institutions may use different fluid or albumin-replacement strategies, potentially yielding divergent albumin trajectories and outcome patterns. Moreover, detailed data on nutritional intake, timing, and dose of albumin infusions, diuretic use, and fluid balance were not recorded. Without these variables, we cannot determine whether correction of hypoalbuminemia or iatrogenic hyperalbuminemia was uniformly beneficial, neutral, or occasionally harmful. We also lacked systematic recording of inflammatory markers (e.g., CRP), organ dysfunction scores, and chronic comorbidities, preventing multivariable adjustment that might have uncovered a subtler independent effect of albumin. Finally, follow-up ended at hospital discharge; potential links between albumin trends and longer-term neurological or functional recovery remain unexplored.
These gaps shape a clear research agenda. Prospective, multicenter studies should confirm whether albumin truly lacks prognostic value when contemporary critical-care practices are applied, and whether this holds in resource-limited settings where hypoalbuminemia might persist. Such studies should collect granular data on nutrition, albumin administration, fluid strategy, and inflammatory burden, enabling adjustment for key confounders and exploration of effect modification. A randomized trial focused on targeted nutritional or albumin supplementation in hypoalbuminemic TBI patients could clarify whether proactively raising albumin influences intracranial dynamics, complication rates, or longer-term outcomes. Composite biomarker models, like combining albumin with lactate, CRP, leukocyte profiles, or neuro-imaging severity indices, also warrant evaluation, as emerging evidence suggests albumin’s predictive power improves when contextualized within broader physiologic stress indicators. In parallel, mechanistic work should investigate how albumin kinetics interact with blood-brain-barrier permeability, cerebral edema, drug binding, and immunomodulation, thereby illuminating whether albumin is purely a marker or occasionally a mediator of secondary brain injury. Lastly, incorporating 6 or 12-month functional outcomes and quality-of-life metrics will be essential to determine if albumin trends have prognostic relevance beyond the acute hospitalization window.
In summary, our study strengthens the case that, under modern intensive care, albumin concentration alone is an unreliable short-term prognosticator in severe TBI. Future investigations—prospective, mechanistically informed, and enriched with long-term follow-up—are needed to ascertain the contexts in which albumin becomes clinically actionable and to integrate it intelligently into holistic risk-stratification and therapeutic frameworks.