Exploring a Possible Link Between Tinnitus and the Risk of Obstructive Sleep Apnea—A National Population-Based Cohort Study Using Propensity Score Matching Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. STOP-BANG Questionnaire (OSA Risk Evaluation)
2.3. Audiometric Evaluation
2.4. Tinnitus Evaluation
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. Study Population, Demographics, and Clinical Characteristics per OSA Risk
3.2. Hearing Levels per the OSA Risk
3.3. Tinnitus Levels per the OSA Risk
3.4. Factors Associated with Tinnitus
3.5. Effect of OSA Risk on Tinnitus After Controlling Other Factors
4. Discussion
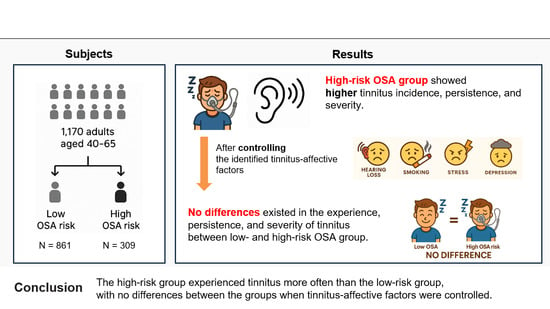
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.J.; Park, J.; Lee, S.Y.; Koo, J.W.; Vanneste, S.; De Ridder, D.; Lim, S.; Song, J.J. Triple network activation causes tinnitus in patients with sudden sensorineural hearing loss: A model-based volume-entropy analysis. Front. Neurosci. 2022, 16, 1028776. [Google Scholar] [CrossRef] [PubMed]
- Jarach, C.M.; Lugo, A.; Scala, M.; van den Brandt, P.A.; Cederroth, C.R.; Odone, A.; Garavello, W.; Schlee, W.; Langguth, B.; Gallus, S. Global Prevalence and Incidence of Tinnitus: A Systematic Review and Meta-analysis. JAMA Neurol. 2022, 79, 888–900. [Google Scholar] [CrossRef]
- Langguth, B.; Kreuzer, P.M.; Kleinjung, T.; De Ridder, D. Tinnitus: Causes and clinical management. Lancet Neurol. 2013, 12, 920–930. [Google Scholar] [CrossRef]
- Cho, Y.S.; Choi, S.H.; Park, K.H.; Park, H.J.; Kim, J.W.; Moon, I.J.; Rhee, C.S.; Kim, K.S.; Sun, D.I.; Lee, S.H.; et al. Prevalence of otolaryngologic diseases in South Korea: Data from the Korea national health and nutrition examination survey 2008. Clin. Exp. Otorhinolaryngol. 2010, 3, 183–193. [Google Scholar] [CrossRef]
- Choi, J.; Lee, C.H.; Kim, S.Y. Association of Tinnitus with Depression in a Normal Hearing Population. Medicina 2021, 57, 114. [Google Scholar] [CrossRef]
- De Ridder, D.; Vanneste, S.; Weisz, N.; Londero, A.; Schlee, W.; Elgoyhen, A.B.; Langguth, B. An integrative model of auditory phantom perception: Tinnitus as a unified percept of interacting separable subnetworks. Neurosci. Biobehav. Rev. 2014, 44, 16–32. [Google Scholar] [CrossRef]
- Biswas, R.; Genitsaridi, E.; Trpchevska, N.; Lugo, A.; Schlee, W.; Cederroth, C.R.; Gallus, S.; Hall, D.A. Low Evidence for Tinnitus Risk Factors: A Systematic Review and Meta-analysis. J. Assoc. Res. Otolaryngol. 2023, 24, 81–94. [Google Scholar] [CrossRef]
- Fioretti, A.B.; Fusetti, M.; Eibenstein, A. Association between sleep disorders, hyperacusis and tinnitus: Evaluation with tinnitus questionnaires. Noise Health 2013, 15, 91–95. [Google Scholar] [CrossRef]
- Franklin, K.A.; Lindberg, E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J. Thorac. Dis. 2015, 7, 1311–1322. [Google Scholar] [CrossRef]
- Hong, S.-E.; Kim, B.; Lee, B.-C.; Lee, M.-C.; Choi, I.J.; Ahn, J. Association Between Obstructive Sleep Apnea and Chronic Dizziness: Results of the Korean National Health and Nutrition Examination Survey 2019–2021. Korean J. Otorhinolaryngol.-Head Neck Surg. 2023, 66, 815–823. [Google Scholar] [CrossRef]
- Chung, F.; Yegneswaran, B.; Liao, P.; Chung, S.A.; Vairavanathan, S.; Islam, S.; Khajehdehi, A.; Shapiro, C.M. STOP questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology 2008, 108, 812–821. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Chen, P.Y.; Chuang, L.P.; Chen, N.H.; Tu, Y.K.; Hsieh, Y.J.; Wang, Y.C.; Guilleminault, C. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep. Med. Rev. 2017, 36, 57–70. [Google Scholar] [CrossRef]
- Kum, N.Y.; Alluşoğlu, S.; Çetin, M.A.; Kundi, F.C.S.; Yağmur, A.R.; Çolak, M.; İkincioğulları, A.; Özcan, K.M.; Dere, H.H. Is there a relation between sleep apnea, tinnitus, and hearing Loss? J. Contemp. Med. 2023, 13, 288–293. [Google Scholar] [CrossRef]
- Lu, C.T.; Lee, L.A.; Lee, G.S.; Li, H.Y. Obstructive Sleep Apnea and Auditory Dysfunction-Does Snoring Sound Play a Role? Diagnostics 2022, 12, 2374. [Google Scholar] [CrossRef]
- Lu, T.; Li, S.; Ma, Y.; Lai, D.; Zhong, J.; Li, G.; Zheng, Y. Positive Correlation between Tinnitus Severity and Poor Sleep Quality Prior to Tinnitus Onset: A Retrospective Study. Psychiatr. Q. 2020, 91, 379–388. [Google Scholar] [CrossRef]
- American Speech-Language-Hearing Association. Guidelines for manual pure-tone threshold audiometry. Asha 1978, 20, 297–301. [Google Scholar]
- Miller, J.N.; Kupzyk, K.A.; Zimmerman, L.; Pozehl, B.; Schulz, P.; Romberger, D.; Berger, A.M. Comparisons of measures used to screen for obstructive sleep apnea in patients referred to a sleep clinic. Sleep. Med. 2018, 51, 15–21. [Google Scholar] [CrossRef]
- Pereira, E.J.; Driver, H.S.; Stewart, S.C.; Fitzpatrick, M.F. Comparing a combination of validated questionnaires and level III portable monitor with polysomnography to diagnose and exclude sleep apnea. J. Clin. Sleep. Med. 2013, 9, 1259–1266. [Google Scholar] [CrossRef]
- Ha, S.C.; Lee, D.L.; Abdullah, V.J.; van Hasselt, C.A. Evaluation and validation of four translated Chinese questionnaires for obstructive sleep apnea patients in Hong Kong. Sleep. Breath. 2014, 18, 715–721. [Google Scholar] [CrossRef]
- Batts, S.; Stankovic, K.M. Tinnitus prevalence, associated characteristics, and related healthcare use in the United States: A population-level analysis. Lancet Reg. Health Am. 2024, 29, 100659. [Google Scholar] [CrossRef]
- McCormack, A.; Edmondson-Jones, M.; Somerset, S.; Hall, D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016, 337, 70–79. [Google Scholar] [CrossRef]
- Henry, J.A.; Dennis, K.C.; Schechter, M.A.; Zaugg, T.L. Tinnitus: An epidemiologic perspective. Otolaryngol. Clin. N. Am. 2020, 53, 481–499. [Google Scholar] [CrossRef]
- Ausland, J.H.; Engdahl, B.; Oftedal, B.; Steingrímsdóttir, Ó.A.; Nielsen, C.S.; Hopstock, L.A.; Johnsen, M.; Friborg, O.; Rosenvinge, J.H.; Eggen, A.E.; et al. Tinnitus and associations with chronic pain: The population-based Tromsø Study (2015–2016). PLoS ONE 2021, 16, e0247880. [Google Scholar] [CrossRef]
- Choo, O.S.; Kim, H.; Lee, S.J.; Kim, S.Y.; Lee, K.Y.; Lee, H.Y.; Moon, I.S.; Seo, J.H.; Rah, Y.C.; Song, J.J.; et al. Consensus Statements on the Definition, Classification, and Diagnostic Tests for Tinnitus: A Delphi Study Conducted by the Korean Tinnitus Study Group. J. Korean Med. Sci. 2024, 39, e49. [Google Scholar] [CrossRef]
- Aljuaid, S.M.; Mirza, A.A.; Habib, L.A.; AlHarthi, L.A.; Alansari, B.M.; AlQahtani, B.G.; Althobaiti, Y.A. Does Caffeine Intake Increase the Incidence of Tinnitus? A Systematic Review. Int. Arch. Otorhinolaryngol. 2021, 25, e628–e632. [Google Scholar] [CrossRef]
- Dixit, A.; Vaney, N.; Tandon, O.P. Effect of caffeine on central auditory pathways: An evoked potential study. Hear. Res. 2006, 220, 61–66. [Google Scholar] [CrossRef]
- Maitre, N.L.; Chan, J.; Stark, A.R.; Lambert, W.E.; Aschner, J.L.; Key, A.P. Effects of caffeine treatment for apnea of prematurity on cortical speech-sound differentiation in preterm infants. J. Child. Neurol. 2015, 30, 307–313. [Google Scholar] [CrossRef]
- Ledesma, A.L.L.; Leite Rodrigues, D.; Monteiro de Castro Silva, I.; Oliveira, C.A.; Bahmad, F., Jr. The effect of caffeine on tinnitus: Randomized triple-blind placebo-controlled clinical trial. PLoS ONE 2021, 16, e0256275. [Google Scholar] [CrossRef]
- Asokan, M.M.; Chen, Y.; Manohar, S.; Lalwani, A.K. Desensitizing nicotinic agents normalize tinnitus-related inhibitory synaptic activity in the dorsal cochlear nucleus. Front Neurosci. 2023, 17, 1161392. [Google Scholar]
- Delano, P.H.; Elgueda, D.; Hamame, C.M.; Robles, L. Nicotine enhances auditory processing in healthy non-smoking humans. Front Neurosci. 2020, 14, 49. [Google Scholar]
- Sheu, J.J.; Wu, C.S.; Lin, H.C. Association between obstructive sleep apnea and sudden sensorineural hearing loss: A population-based case-control study. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 55–59. [Google Scholar] [CrossRef]
- Lazarini, P.R.; Camargo, A.C. Idiopathic sudden sensorineural hearing loss: Etiopathogenic aspects. Braz. J. Otorhinolaryngol. 2006, 72, 554–561. [Google Scholar] [CrossRef]
- Dyken, M.E.; Im, K.B. Obstructive sleep apnea and stroke. Chest 2009, 136, 1668–1677. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, H.J. Is sleep apnea truly associated with hearing loss? A nationwide, population-based study with STOP-BANG questionnaire. Front. Public Health 2023, 11, 1170470. [Google Scholar] [CrossRef]
- Song, Z.; Wu, Y.; Tang, D.; Lu, X.; Qiao, L.; Wang, J.; Li, H. Tinnitus Is Associated With Extended High-frequency Hearing Loss and Hidden High-frequency Damage in Young Patients. Otol. Neurotol. 2021, 42, 377–383. [Google Scholar] [CrossRef]
- Weingarten, J.A.; Islam, A.; Dubrovsky, B.; Gharanei, M.; Coelho, D.H. The association of subjective and objective sleep measures with chronic tinnitus. J. Clin. Sleep. Med. 2024, 20, 399–405. [Google Scholar] [CrossRef]
| Low-Risk Group (%) | High-Risk Group (%) | p-Value | ||
|---|---|---|---|---|
| Number of total participants | 861 | 309 | 0.813 | |
| Participants according to each year | 2019 | 203 (23.6) | 70 (22.7) | |
| 2020 | 308 (35.8) | 131 (42.4) | ||
| 2021 | 350 (40.7) | 108 (35.0) | ||
| STOP-BANG score | 1.53 ± 0.63 | 5.14 ± 1.05 | <0.001 * | |
| Age | 50.62 ± 7.06 | 53.34 ± 6.84 | <0.001 * | |
| Sex (male:female) | 612:249 | 304:5 | <0.001 * | |
| Education level (=graduated) | Elementary school | 48 (5.6) | 23 (7.4) | 0.464 |
| Middle school | 66 (7.7) | 24 (7.8) | ||
| High school | 351 (40.8) | 113 (36.6) | ||
| College | 396 (46.0) | 149 (48.2) | ||
| Marriage status (not married) | 99 (11.5) | 18 (5.8) | 0.006 * | |
| Subjective health perception | Good | 284 (33.0) | 63 (20.4) | <0.001 * |
| Normal | 472 (54.8) | 178 (57.6) | ||
| Poor | 105 (12.2) | 68 (22.0) | ||
| BMI | 23.77 ± 2.84 | 27.58 ± 3.91 | <0.001 * | |
| HTN | Normal | 467 (54.2) | 43 (13.9) | <0.001 * |
| Pre-HTN | 304 (35.3) | 61 (19.7) | ||
| HTN | 90 (10.5) | 205 (66.3) | ||
| Hypercholesterolemia (=yes) | 205 (23.8) | 140 (45.3) | <0.001 * | |
| Hypertriglyceridemia (=yes) | 164 (19.0) | 93 (30.1) | <0.001 * | |
| Anemia (=yes) | 45 (5.2) | 7 (2.3) | 0.045 * | |
| Alcohol consumption | None | 16 (1.9) | 7 (2.3) | 0.085 |
| More than once per month | 511 (59.3) | 204 (66.0) | ||
| Less than once per month | 334 (38.8) | 98 (31.7) | ||
| Average sleep time | 7.29 ± 6.39 | 6.67 ± 4.94 | 0.079 | |
| Smoking | None | 59 (6.9) | 9 (2.9) | 0.006 * |
| Ex-smoker | 434 (50.4) | 182 (58.9) | ||
| Current smoker | 368 (42.7) | 118 (38.2) | ||
| Masticatory difficulties (=yes) | 188 (21.8) | 69 (22.3) | 0.92 | |
| Asthma (=yes) | 20 (2.3) | 11 (3.6) | 0.340 | |
| Thyroid disease (=yes) | 17 (2.0) | 7 (2.3) | 0.940 | |
| Depression (=yes) | 46 (5.3) | 8 (2.6) | 0.069 | |
| Otitis media (=yes) | 43 (5.0) | 20 (6.5) | 0.401 | |
| Renal disease (=yes) | 8 (0.9) | 3 (1.0) | 1.000 | |
| Hearing Threshold (dB) | Low-Risk Group | High-Risk Group | p-Value |
|---|---|---|---|
| Mean hearing threshold (better ear) | 13.58 ± 8.93 | 15.83 ± 9.85 | <0.001 * |
| Mean hearing threshold (worse ear) | 18.96 ± 11.43 | 22.01 ± 12.56 | <0.001 * |
| Mean high-frequency hearing threshold (better ear) | 19.99 ± 13.75 | 24.63 ± 14.82 | <0.001 * |
| Mean high-frequency hearing threshold (worse ear) | 28.00 ± 16.45 | 34.08 ± 17.95 | <0.001 * |
| Tinnitus | Low-risk Group (N, %) | High-risk Group (N, %) | p-value |
| Tinnitus experience (=yes) | 68 (7.9) | 36 (11.7) | 0.061 |
| Tinnitus persistence (more than 6 months) (=yes) | 59 (6.9) | 33 (10.7) | 0.043 * |
| Subjective distress level (mean NRS) | 0.29 ± 1.19 | 0.50 ± 1.58 | 0.036 * |
| NRS ≥ 7 | 10 (1.2) | 7 (2.3) | 0.171 |
| Variables | Number (%) | Experience of Tinnitus | Persistence of Tinnitus | Severity of Tinnitus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable ANALYSIS | Multivariable Analysis | Univariable Analysis | Multivariable Analysis | Univariable Analysis | Multivariable Analysis | |||||||||
| p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | |||
| Age | 1170 | <0.001 | 1.1 (1,1.1) | 0.12 | (1,1.1) | <0.001 | 1.1 (1,1.1) | 0.08 | 1 (1,1.1) | 0.02 | 1.1 (1,1.2) | 0.07 | 1.1 (1,1.3) | |
| Sex | Male | 916 (78.3) | ||||||||||||
| Female | 254 (21.7) | 0.04 | 0.5 (0.3,1) | 0.09 | 0.5 (0.3,1.1) | 0.04 | 0.5 (0.3,1) | 0.24 | 0.6 (0.3,1.4) | 0.33 | 0.5 (0.1,2.1) | |||
| Perceived stress | No | 832 (71.1) | ||||||||||||
| Yes | 338 (28.9) | 0.04 | 1.5 (1,2.3) | 0.02 * | 1.8 (1.1,2.9) | 0.29 | 1.3 (0.8,2) | 0.1 | 2.2 (0.8,5.8) | 0.21 | 2.7 (0.6,13.1) | |||
| Smoking | None | 68 (5.8) | ||||||||||||
| Ex-smoker | 616 (52.6) | 0.1 | 0.5 (0.3,1.1) | 0.01 * | 0.3 (0.1,0.7) | 0.06 | 0.5 (0.2,1) | 0 * | 0.3 (0.1,0.6) | 0.05 | 0.2 (0.1,1) | 0.06 | 0.1 (0,1.1) | |
| Current smoker | 486 (41.5) | 0.1 | 0.5 (0.3,1.1) | 0 * | 0.2 (0.1,0.6) | 0.03 | 0.4 (0.2,0.9) | <0.001 * | 0.2 (0.1,0.4) | 0.1 | 0.3 (0.1,1.3) | 0.04 * | 0.1 (0,0.9) | |
| Depression | No | 1116 (95.4) | ||||||||||||
| Yes | 54 (4.6) | 0.12 | 1.8 (0.8,4) | 0.04 * | 2.6 (1,6.7) | 0.37 | 1.5 (0.6,3.6) | 0 | 6.8 (2.1,21.6) | <0.001 * | 132.7 (12.5,1409.6) | |||
| Hearing level | Mean hearing level (better ear) | - | <0.001 | 1.1 (1,1.1) | <0.001 | 1.1 (1,1.1) | 0.55 | 1 (0.9,1) | <0.001 | 1.1 (1,1.1) | 0.54 | 1 (0.8,1.1) | ||
| Mean hearing level (worse ear) | - | <0.001 | 1 (1,1.1) | <0.001 | 1 (1,1.1) | 0.17 | 1 (0.9,1) | <0.001 | 1.1 (1,1.1) | 0.99 | 1 (0.9,1.1) | |||
| Mean hearing level (better ear, high frequency) | - | <0.001 | 1 (1,1.1) | <0.001 | 1 (1,1.1) | 0.8 | 1 (1,1.1) | <0.001 | 1.1 (1,1.1) | 0.34 | 1.1 (0.9,1.2) | |||
| Mean hearing level (worse ear, high frequency) | - | <0.001 | 1 (1,1.1) | <0.001 * | 1.1 (1,1.1) | <0.001 | 1 (1,1.1) | <0.001 * | 1.1 (1,1.1) | <0.001 | 1.1 (1,1.1) | 0.18 | 1.1 (1,1.2) | |
| Propensity Score Matching | Low-Risk Group (N = 861) | High-Risk Group (N = 309) | p-Value |
|---|---|---|---|
| 1. Experience of tinnitus | |||
| Model 1 (all combined) | 33 (10.7%) | 36 (11.7%) | 0.798 |
| Model 2 (perceived stress) | 24 (7.8%) | 36 (11.7%) | 0.135 |
| Model 3 (smoking) | 21 (6.8%) | 36 (11.7%) | 0.052 |
| Model 4 (depression) | 20 (6.5%) | 36 (11.7%) | 0.036 * |
| Model 5 (high-frequency HL on better side) | 36 (11.7%) | 36 (11.7%) | 1.000 |
| 2. Persistence of tinnitus | |||
| Model 1 (all combined) | 30 (9.7%) | 33 (10.7%) | 0.790 |
| Model 2 (smoking) | 19 (6.1%) | 33 (10.7%) | 0.060 |
| Model 3 (high-frequency HL on better side) | 34 (11.0%) | 33 (10.7%) | 1.000 |
| 3. Severity of tinnitus | |||
| Model 1 (all combined) | 0.29 ± 1.24 | 0.50 ± 1.58 | 0.071 |
| Model 2 (smoking) | 0.28 ± 1.23 | 0.50 ± 1.58 | 0.058 |
| Model 3 (depression) | 0.23 ± 1.00 | 0.50 ± 1.58 | 0.010 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.J.; Park, S.I.; Choi, I.S.; Lee, H.J.; Lee, J.M. Exploring a Possible Link Between Tinnitus and the Risk of Obstructive Sleep Apnea—A National Population-Based Cohort Study Using Propensity Score Matching Analysis. J. Clin. Med. 2025, 14, 7492. https://doi.org/10.3390/jcm14217492
Lee SJ, Park SI, Choi IS, Lee HJ, Lee JM. Exploring a Possible Link Between Tinnitus and the Risk of Obstructive Sleep Apnea—A National Population-Based Cohort Study Using Propensity Score Matching Analysis. Journal of Clinical Medicine. 2025; 14(21):7492. https://doi.org/10.3390/jcm14217492
Chicago/Turabian StyleLee, Seung Jae, Song I Park, Ick Soo Choi, Hyun Jin Lee, and Jeon Mi Lee. 2025. "Exploring a Possible Link Between Tinnitus and the Risk of Obstructive Sleep Apnea—A National Population-Based Cohort Study Using Propensity Score Matching Analysis" Journal of Clinical Medicine 14, no. 21: 7492. https://doi.org/10.3390/jcm14217492
APA StyleLee, S. J., Park, S. I., Choi, I. S., Lee, H. J., & Lee, J. M. (2025). Exploring a Possible Link Between Tinnitus and the Risk of Obstructive Sleep Apnea—A National Population-Based Cohort Study Using Propensity Score Matching Analysis. Journal of Clinical Medicine, 14(21), 7492. https://doi.org/10.3390/jcm14217492