RSV Monitoring in Germany: A Critical Overview of Available Surveillance Systems
Abstract
1. Introduction
2. Methods
- Geographic reach
- Included age groups and age stratification
- Information on manifestation of disease (pneumonia, bronchiolitis, bronchitis, lower respiratory tract infection, upper respiratory tract infection)
- Type of sampling (source, technique)
- Type of RSV diagnostic tools (e.g., bedside rapid antigen test, professional antigen test, PCR, serum antibodies)
- Information on health care sector: inpatient/outpatient
- Laboratory analysis of RSV Subtype
- Information on seasonality
- Time lag until data availability
- Frequency of updates (weekly reports/season reports/time lag until update in database)
- Risk of bias/limitations of the database
2.1. Data Evaluation
2.1.1. Robert Koch Institute (RKI)
2.1.2. Landesuntersuchungsanstalt (LUA) Saxony (Saxonian Health Institute)
2.1.3. German Society for Pediatric Infectious Diseases (DGPI)
2.1.4. GBE-Bund (National Health Reporting of Germany)
2.1.5. The German Institute for the Hospital Remuneration System (InEK)
2.1.6. RespVir/Clinical Virology Network
3. Surveillance Systems and Databases on RSV in Germany
3.1. Robert Koch Institute (RKI)
- (1)
- The RKI compiles and presents in its national surveillance database (SurvStat@RKI) data from mandatory reporting of RSVI, both from the outpatient and inpatient sector. Between 2002 and July 2023, Saxony was the only state with mandatory reporting of laboratory-confirmed RSV cases. In July 2023, mandatory reporting was extended across all German states. For the 2023/2024 season, a total of 57,541 cases and for the 2024/2025 season (as of 20 May 2025) 68,265 cases were reported based on the RKI’s official “Referenzdefinition” (reference case definition). In more than 60% of accumulated cases from both seasons, both clinical and laboratory criteria were met, meaning a clinical respiratory infection of a patient was confirmed as RSVI by a laboratory test. Another 34% of recorded cases arose from a positive laboratory test that could not be correlated to a specific sick patient (clinical criteria undetermined). Cases that relied only on clinical and epidemiological evidence in the absence of laboratory data or cases in which a positive lab test was not accompanied by clinical signs of RSVI accounted together for less than 6% of all reported cases.As a centralized reporting system for notifiable diseases, SurvStat provides near real-time monitoring and thus supports outbreak detection. However, the data lacks clinical detail. Additionally, data availability depends on reporting compliance, making it vulnerable to underreporting.
- (2)
- Physicians across Germany report current diagnoses of respiratory diseases in the outpatient sector to the online database called ARE Sentinel Surveillance. This data source consists of approximately 700 primary care practices as a representative sample of the population. The data collected include information on the severity and frequency of current RSVIs, specifically acute respiratory diseases, based on clinical symptoms. Additionally, virological surveillance is conducted in approximately 100 sentinel practices that submit patient samples of symptomatic patients to the National Reference Center to identify currently circulating respiratory viruses. The collected data is evaluated on a weekly basis and presented in the form of weekly reports [21]. Data on RSV consultation rates can be downloaded from an online repository [34] with an additional extraction step.The ARE Sentinel Surveillance offers broad insights into respiratory illness trends across age groups and regions in the outpatient sector by capturing syndromic as well as virological data. Although representative sampling is aimed for, the system is limited by its sample size (>1% coverage of primary care physicians in Germany) and regional differences in coverage.
- (3)
- Nationwide, “citizen scientists” can self-report cases of acute respiratory infections (ARIs) at the population level to the online database known as “GrippeWeb”. Individuals aged 16 years and above, residing primarily in Germany, can participate through the web portal in a population-based approach. They can self-report once a week whether they experienced a new respiratory illness in the preceding week. This process tracks the percentage of the entire population that has developed an acute respiratory infection on a weekly basis, including visits to their general practitioners (GPs) [35].A strength of GrippeWeb is the estimation of ARI incidence even independent of healthcare utilization by collecting self-reported data. However, the data may be biased or incomplete due to self-reporting, and it lacks clinical and virological specificity. The latter limitation is addressed by “GrippeWeb-Plus”.
- (4)
- Since 2020, the RKI has been conducting an additional virological surveillance program as part of GrippeWeb (influenza web), called “GrippeWeb-Plus.” In this program, a randomly selected sample of regularly reporting GrippeWeb participants receive swab materials. In the event of an acute respiratory infection, they take a sample from their frontal nasal area and subsequently send it to the RKI for testing for 24 different respiratory pathogens, including influenza viruses, SARS-CoV-2, and RSV. Currently, around 800 children and adults from approximately 480 different households participate in GrippeWeb-Plus. Since multiple individuals from one household participate in GrippeWeb-Plus, a household-adjusted positive rate is calculated [36].
- (5)
- The syndromic surveillance of severe acute respiratory infections (SARIs) in the inpatient environment involves the use of ICD-10 codes to monitor cases in sentinel hospitals (referred to as ICOSARI, [23]). This surveillance is conducted in approximately 70 selected hospitals, covering about 5–6% of all hospitalized patients in Germany, and is considered representative. As ICOSARI is dependent on ICD-10 codes, it may be affected by coding inaccuracies.
- (6)
- As of week 7, 2025, data on RSV detection in wastewater has been reported [37]. For the first report in February 2025, data from 25 wastewater treatment plants was analyzed and reported as viral load, stratified by RSV subgroup A and B [38]. Data provided by further wastewater treatment plants will be included subsequently. A key challenge lies in establishing standardized sampling frequencies and laboratory methodologies to ensure data comparability and reliability across surveillance sites.
| SurvStat | ARE Sentinel Surveillance | GrippeWeb | ICOSARI | ||
|---|---|---|---|---|---|
| Descriptor | Consultation | Virological Surveillance | |||
| Who reports | Physicians, laboratories, public health authorities | Physicians | Participating laboratories | Citizens | Physicians via ICD-10 coding |
| Geographic reach | Germany; stratification by state, territorial unit, and district | Germany | Reported for each German state, as well as Germany-wide | Germany | Germany |
| Age stratification | Fine-grained age stratification | 0–1 y, 2–4 y, 5–14 y, 15–34 y, 35–59 y, >59 y | 0–1 y, 2–4 y, 5–14 y, 15–34 y, 35–59 y, >59 y | 0–4 y, 5–14 y, 15–34 y, 35–59 y, >59 y | 0–4 y, 5–14 y, 15–34 y, 35–59 y, 60–79 y, >79 y |
| Information on manifestation of disease | No | Yes | No | Influenza-like illness (ILI) reported | Includes only severe acute respiratory infections |
| Type of sampling and test | Antigen test, PCR, or epidemiological confirmation | N/A | Usually nasopharyngeal swab; usually tested by RT—PCR [39] | N/A | N/A [22,23] |
| RS-virus subtyping | No | No | No | No | No |
| Information on seasonality | Yes | Yes | Yes | Yes, for ARE, not for RSV specifically | Yes, for ARE, not for RSV specifically |
| Frequency of updates | weekly | weekly | weekly | weekly | weekly |
| Limitations/Risk of bias | Includes only confirmed RSV cases. Testing frequency and quality varies substantially by patient age. | Syndromic reporting of patients with acute respiratory diseases; does not require testing. | Quality of sample material influences virus detection. | Convenient sampling, depending on voluntary reporting by citizens. Subjective self-reporting of symptoms. No testing for pathogens. | ICD-10 based surveillance; bias due to diagnosis- and coding-practice. Varying testing reported [22,23] |
| Descriptor | DGPI [40,41] | ClinicalVirology [33] | GBE-Bund/InEK [25] | ARE Surveillance by Federal States |
|---|---|---|---|---|
| Who reports | Participating hospitals | Participating laboratories | All hospitals billing according to the DRG reimbursement system | |
| Geographic reach | Germany | Germany (plus Austria and Switzerland) | Germany | 10 German Federal States (see Table 3) |
| Age stratification | 0–3 months, 4–11 months, 1–2 y, 3–4 y, 5–11 y, 12–18 y, ≥19 y | 0 < 6 y, 6 < 13 y, 13 < 19 y, 19 < 46 y, 46 < 65 y, ≥65 y | InEK: <28 days, 28 days—<1 y, 1–2 y, 3–5 y, 6–9 y, 10–15 y, 16–17 y, 18–29 y, 30–39 y, 40–49 y, 50–54 y, 55–59 y, 60–64 y, 64–74 y, 75–59 y, ≥80 y GBE-Bund: <1 y, 5 y increments | Depending on the state. E.g. Saxony-Anhalt: <2 y, 2–6 y, 7–17 y, 18–59 y, ≥ 80 y Often age stratified data not provided for RSV. |
| Information on manifestation of disease | All hospitalized cases. For 2021/22, 2022/23 and 2023/24 data for regular ward and ICU cases available. | No | Yes, by ICD-10 code | No |
| Type of sampling and test | Antigen test and/or PCR | Singleplex and Multiplex PCR | Not documented | For laboratory-based surveillance, respiratory samples collected by participating sentinel practices are most likely analyzed using PCR testing. |
| RS-virus subtyping | No | No | No | Usually not reported (exception Baden-Württemberg) |
| Information on seasonality | Yes, near real-time updates in the current season. | Yes, near real-time updates in the current season. | Yes (diagnoses provided by date, but with delay of several months) | Yes, near real-time updates in the current season. |
| Frequency of updates | Current case numbers provided on weekly basis. | Current case numbers and positivity rates provided on weekly basis. | The billing data in the InEK data browser is provided three times a year, on June 15th, October 15th, and January 15th. These data include discharges from January 1st to May 31st, January 1st to September 30th, and January 1st to December 31st of the current calendar year. | Weekly reports on current season. |
| Limitations/Risk of bias | Voluntary participation of hospitals | Voluntary participation of laboratories. In most settings, only patients with respiratory symptoms are tested. | Only ICD-10 coded diagnoses documented. | Usually preschool children as sentinels for virus activity. |
| Federal State | Independent ARE and RSV Surveillance | Notes |
|---|---|---|
| Baden-Württemberg | Yes https://www.gesundheitsamt-bw.de/aktuelles-und-service/newsletter-und-infodienste/are-bericht/ (last accessed on 14 October 2025). | Reports case numbers for RSV-A and -B separately |
| Bavaria | Yes https://www.lgl.bayern.de/gesundheit/infektionsschutz/molekulare_surveillance/bis_c/bisc_ergebnisse.htm (last accessed on 14 October 2025). | ARE surveillance, incl. RSV, established in 2009 (“Bayern Influenza Sentinel” (BIS)); wastewater monitoring for RSV introduced in 2025 |
| Berlin | Yes https://www.berlin.de/lageso/gesundheit/infektionskrankheiten/berichte-veroeffentlichungen/wochenberichte/ (last accessed on 14 October 2025). | RSV reported since season 2023/24 |
| Brandenburg | No | - |
| Bremen | No | - |
| Hamburg | No | - |
| Hesse | Yeshttps://hlfgp.hessen.de/gesundheitsschutz-gesundheitsdaten/gesundheitsdaten (last accessed on 14 October 2025). | RSV reporting established in September 2023 |
| Mecklenburg Western Pomerania | Yeshttps://www.lagus.mv-regierung.de/Gesundheit/InfektionsschutzPraevention/ARE/ (last accessed on 14 October 2025). | - |
| Lower Saxony | Yes https://www.nlga.niedersachsen.de/are/uebersicht-205132.html (last accessed on 14 October 2025). | ARE surveillance, incl. RSV, established in fall 2004 |
| Northrhine-Westphalia | Yes https://www.lzg.nrw.de/inf_schutz/meldewesen/infektionsberichte/wochen-infektionsberichte/index.html (last accessed on 14 October 2025). | ARE surveillance since season 2014/15; inclusion of RSV in season 2022/23 |
| Rhineland Palatinate | Yes, link https://lua.rlp.de/unsere-themen/humanmedizin/daten-zu-atemwegserkrankungen/wochenberichte#c79568 (last accessed on 14 October 2025). | ARE surveillance established in 2023 |
| Saarland | No | - |
| Saxony | Yes https://www.gesunde.sachsen.de/epidemiologische-berichte-4057.html (last accessed on 14 October 2025). | Mandatory reporting of laboratory-confirmed RSV cases since 2002 |
| Saxony-Anhalt | Yes https://verbraucherschutz.sachsen-anhalt.de/gesundheit/wasserhygiene/trinkwasser-1/surveillance-akuter-respiratorischer-erkrankungen-are (last accessed on 14 October 2025). | ARE surveillance established in 2007; Representative sample of children aged 3 to 6 years |
| Schleswig-Holstein | No | - |
| Thuringia | No | - |
3.2. Surveillance of ARE (“Akute Respiratorische Erkrankungen”, Acute Respiratory Illnesses) Surveillance by Federal States in Germany
3.3. German Institute for the Hospital Remuneration System (InEK)
3.4. German Society for Pediatric Infectious Diseases (DGPI)
3.5. RespVir, Clinical Virology Network
4. RSV Burden of Disease in Germany
4.1. RSV Epidemiology in Germany
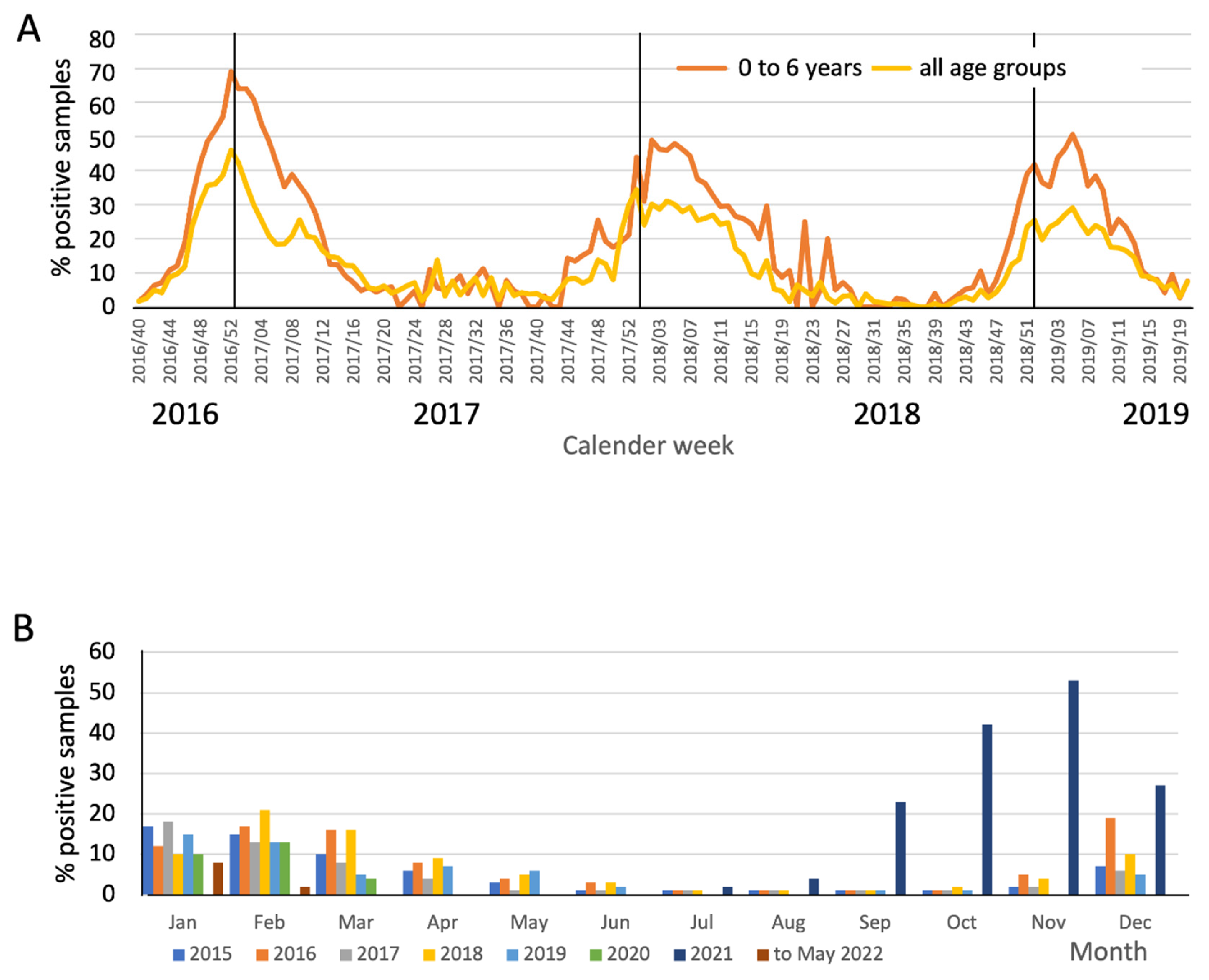
Seasonality of RSV
4.2. RSV Incidence
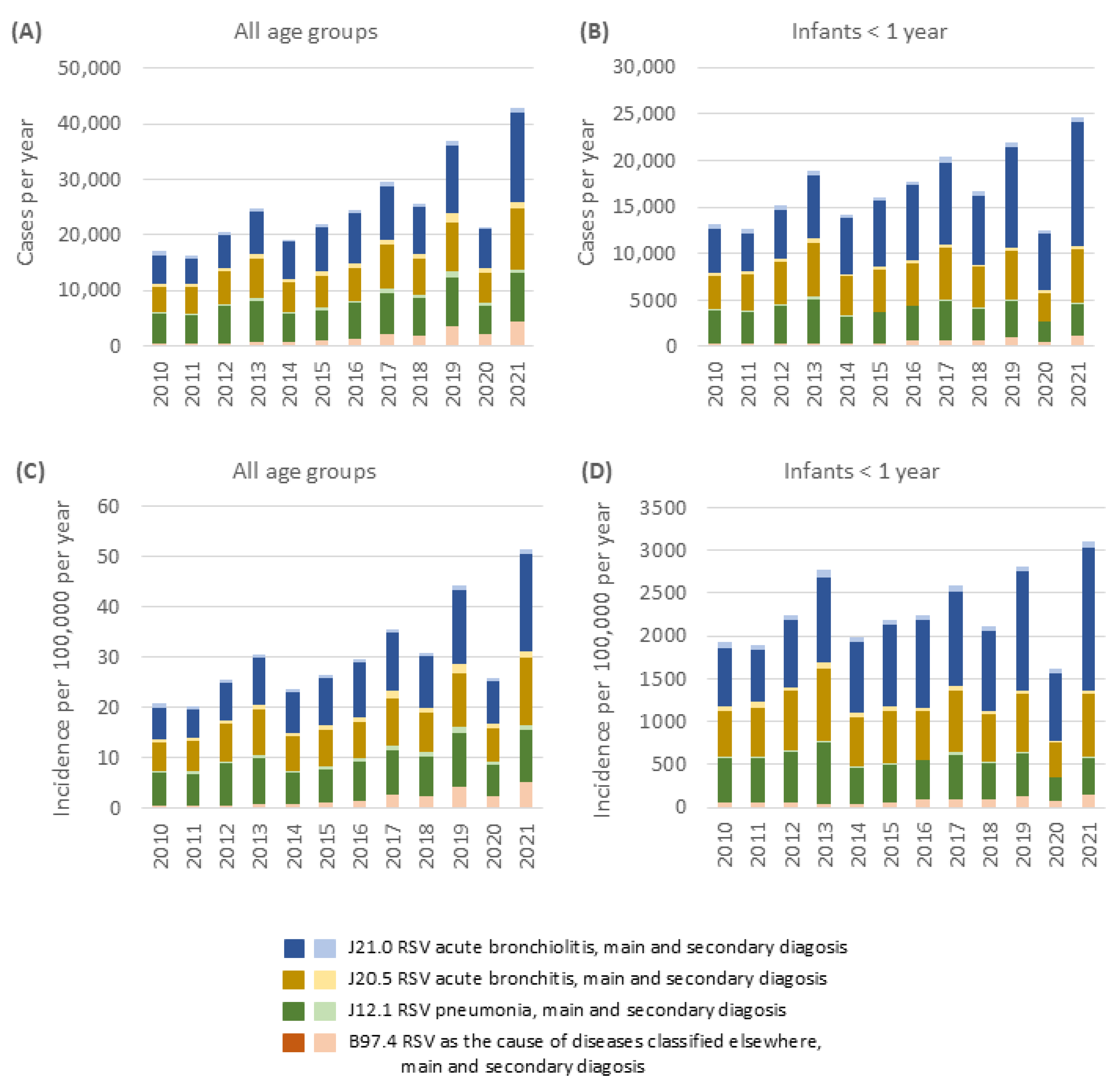
4.3. Severity and Hospitalization Burden of RSVI
5. Discussion
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AG | Arbeitsgruppe (working group) |
| ARE | Akute Respiratorische Erkrankung (acute respiratory illness) |
| ARI | Acute Respiratory Infection |
| BIS | Bayern Influenza Sentinel |
| CFR | Case Fatality Rate |
| CVN | Clinical Virology Network |
| DGPI | Deutsche Gesellschaft für Pädiatrische Infektiologie (German Society for Pediatric Infectious Diseases) |
| DRG | Diagnosis-Related Groups |
| GBE | Gesundheitsberichterstattung (health reporting system) |
| GfV | Gesellschaft für Virologie (German Society for Virology) |
| GP | General Practitioner |
| LRTI | Lower Respiratory Tract Illness |
| LUA | Landesuntersuchungsanstalt (State Institute for Investigation) |
| NPI | Non-Pharmaceutical Interventions |
| PCR | Polymerase Chain Reaction |
| RKI | Robert Koch Institut |
| SARI | Severe Acute Respiratory Infections |
| STIKO | Ständige Impfkommission (Standing Committee on Vaccination) |
References
- Shang, Z.; Tan, S.; Ma, D. Respiratory syncytial virus: From pathogenesis to potential therapeutic strategies. Int. J. Biol. Sci. 2021, 17, 4073–4091. [Google Scholar] [CrossRef]
- Ciarlitto, C.; Vittucci, A.C.; Antilici, L.; Concato, C.; Di Camillo, C.; Zangari, P.; Villani, A. Respiratory Syncityal Virus A and B: Three bronchiolitis seasons in a third level hospital in Italy. Ital. J. Pediatr. 2019, 45, 115. [Google Scholar] [CrossRef] [PubMed]
- Redlberger-Fritz, M.; Springer, D.N.; Aberle, S.W.; Camp, J.V.; Aberle, J.H. Respiratory syncytial virus surge in 2022 caused by lineages already present before the COVID-19 pandemic. J. Med. Virol. 2023, 95, e28830. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.T.; Cox, C.; Atkins, J.; Butler, I.J. Encephalopathy associated with respiratory syncytial virus bronchiolitis. J. Child Neurol. 2001, 16, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.P.; Busl, K.M. Neurologic Manifestations of Severe Respiratory Viral Contagions. Crit. Care Explor. 2020, 2, e0107. [Google Scholar] [CrossRef]
- Robert Koch Institut. RSV-Infektionen. Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Merkblaetter/Ratgeber_RSV.html (accessed on 11 January 2025).
- Rosas-Salazar, C.; Chirkova, T.; Gebretsadik, T.; Chappell, J.D.; Peebles, R.S., Jr.; Dupont, W.D.; Jadhao, S.J.; Gergen, P.J.; Anderson, L.J.; Hartert, T.V. Respiratory syncytial virus infection during infancy and asthma during childhood in the USA (INSPIRE): A population-based, prospective birth cohort study. Lancet 2023, 401, 1669–1680. [Google Scholar] [CrossRef]
- Eisenhut, M. Extrapulmonary manifestations of severe RSV bronchiolitis. Lancet 2006, 368, 988. [Google Scholar] [CrossRef]
- Ivey, K.S.; Edwards, K.M.; Talbot, H.K. Respiratory Syncytial Virus and Associations With Cardiovascular Disease in Adults. J. Am. Coll. Cardiol. 2018, 71, 1574–1583. [Google Scholar] [CrossRef]
- Kwong, J.C.; Schwartz, K.L.; Campitelli, M.A. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. N. Engl. J. Med. 2018, 378, 2540–2541. [Google Scholar] [CrossRef]
- Ramaswamy, M.; Groskreutz, D.J.; Look, D.C. Recognizing the importance of respiratory syncytial virus in chronic obstructive pulmonary disease. COPD J. Chronic Obstr. Pulm. Dis. 2009, 6, 64–75. [Google Scholar] [CrossRef]
- Falsey, A.R. Respiratory syncytial virus infection in adults. Semin. Respir. Crit. Care Med. 2007, 28, 171–181. [Google Scholar] [CrossRef]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Walsh, E.E. Respiratory syncytial virus infection in elderly adults. Drugs Aging 2005, 22, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Njue, A.; Nuabor, W.; Lyall, M.; Margulis, A.; Mauskopf, J.; Curcio, D.; Kurosky, S.; Gessner, B.D.; Begier, E. Systematic Literature Review of Risk Factors for Poor Outcomes Among Adults With Respiratory Syncytial Virus Infection in High-Income Countries. Open Forum Infect. Dis. 2023, 10, ofad513. [Google Scholar] [CrossRef] [PubMed]
- Niekler, P.; Goettler, D.; Liese, J.G.; Streng, A. Hospitalizations due to respiratory syncytial virus (RSV) infections in Germany: A nationwide clinical and direct cost data analysis (2010–2019). Infection 2023, 52, 1715–1724. [Google Scholar] [CrossRef]
- Polkowska-Kramek, A.; Begier, E.; Bruyndonckx, R.; Liang, C.; Beese, C.; Brestrich, G.; Tran, T.M.P.; Nuttens, C.; Casas, M.; Bayer, L.J.; et al. Estimated Incidence of Hospitalizations and Deaths Attributable to Respiratory Syncytial Virus Infections Among Adults in Germany Between 2015 and 2019. Infect. Dis. Ther. 2024, 13, 845–860. [Google Scholar] [CrossRef]
- Wick, M.; Poshtiban, A.; Kramer, R.; Bangert, M.; Lange, M.; Wetzke, M.; Damm, O. Inpatient burden of respiratory syncytial virus in children ≤2 years of age in Germany: A retrospective analysis of nationwide hospitalization data, 2019–2022. Influenza Other Respir. Viruses 2023, 17, e13211. [Google Scholar] [CrossRef]
- Ständige Impfkomission. Beschluss und wissenschaftliche Begründung zur Empfehlung der STIKO zur spezifischen Prophylaxe von RSV-Erkrankungen mit Nirsevimab bei Neugeborenen und Säuglingen in ihrer 1. RSV-Saison. Epidemiol. Bull. 2024, 26, 3–29. [Google Scholar]
- Ständige Impfkomission. Beschluss zur Empfehlung der STIKO für eine Standardimpfung gegen Erkrankungen durch Respiratorische Synzytial-Viren (RSV) für Personen ≥ 75 Jahre sowie zur Indikationsimpfung von Personen im Alter von 60 bis 74 Jahren mit Risikofaktoren. Epidemiol. Bull. 2024, 32, 3–26. [Google Scholar]
- Goerlitz, L.; Cai, W.; Tolksdorf, K.; Prahm, K.; Preuß, U.; Wolff, T.; Dürrwald, R.; Haas, W.; Buda, S. ICD-10-Code-basierte syndromische Surveillance akuter Atemwegserkrankungen mit COVID-19 im ambulanten Bereich. Epidemiol. Bull. 2021, 30, 3–10. [Google Scholar] [CrossRef]
- Cai, W.; Buda, S.; Schuler, E.; Hirve, S.; Zhang, W.; Haas, W. Risk factors for hospitalized respiratory syncytial virus disease and its severe outcomes. Influenza Other Respir. Viruses 2020, 14, 658–670. [Google Scholar] [CrossRef]
- Cai, W.; Tolksdorf, K.; Hirve, S.; Schuler, E.; Zhang, W.; Haas, W.; Buda, S. Evaluation of using ICD-10 code data for respiratory syncytial virus surveillance. Influenza Other Respir. Viruses 2020, 14, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Statistisches Bundesamt. Das Informationssystem der Gesundheitsberichterstattung des Bundes. Available online: https://www.gbe-bund.de/gbe/ (accessed on 11 January 2025).
- Institut für das Entgeltsystem im Krankenhaus (InEK). InEK DatenBrowser. Available online: https://datenbrowser.inek.org/ (accessed on 15 May 2024).
- Deutsche Gesellschaft für Pädiatrische Infektiologie. Ergebnisse der Adhoc-Erfassung der RSV-Assoziierten Krankheitslast bei Stationär Behandelten Kindern und Jugendlichen in Deutschland im Winter 2021–2022. Available online: https://dgpi.de/rsv-survey-update/ (accessed on 11 January 2025).
- Deutsche Gesellschaft für Pädiatrische Infektiologie. Ad hoc Atemwegsinfektions-Erfassung der Krankheitslast bei Stationär Behandelten Kindern und Jugendlichen in Deutschland—Ergebnisse der Ad hoc Atemwegsinfektions-Erfassung (13 September 2022–3 April 2023). Available online: https://dgpi.de/awi-erfassung-update/ (accessed on 11 January 2025).
- Deutsche Gesellschaft für Pädiatrische Infektiologie. Ad hoc Atemwegsinfektions-Erfassung der Krankheitslast bei Stationär Behandelten Kindern und Jugendlichen in Deutschland (Herbst/Winter 2023–2024). Available online: https://dgpi.de/awi-erfassung-update-2023-2024/ (accessed on 29 January 2025).
- Deutsche Gesellschaft für Pädiatrische Infektiologie. Ad hoc-Erfassung Aller stationären Aufnahmen Aufgrund Akuter Respiratorischer Infektionen (ARI) bei Kindern und Jugendlichen in Deutschland. Available online: https://dgpi.de/awi-erfassung-update-2024-2025/ (accessed on 19 May 2025).
- Clinical Virology Network. Clinical Virology Network. Available online: https://public.clinical-virology.net/ (accessed on 11 January 2025).
- Robert Koch Institut. SurvStat@RKI 2.0—Web-Basierte Abfrage der Meldedaten Gemäß Infektionsschutzgesetz (IfSG). Available online: https://survstat.rki.de/ (accessed on 11 January 2025).
- Landesuntersuchungsanstalt für das Gesundheits- und Veterinärwesen Sachsen. Epidemiologische Berichte. Available online: https://www.gesunde.sachsen.de/epidemiologische-berichte-4057.html (accessed on 11 January 2025).
- Clinical Virology Network. RespVir Dashboard. Available online: https://www.arcgis.com/apps/dashboards/2a29f2bebc524c67b6250b64beea12bf (accessed on 11 January 2025).
- Goerlitz, L.; Tolksdorf, K.; Prahm, K.; Preuß, U.; Krupka, S.; Wunderlich, J.; Gvaladze, T.; Buchholz, U.; Haas, W.; Buda, S. ARE-Konsultationsinzidenz; Robert Koch-Institut: Berlin, Germany, 2024. [Google Scholar] [CrossRef]
- Robert Koch Institut. GrippeWeb. Available online: https://www.rki.de/DE/Content/Infekt/Sentinel/Grippeweb/grippeweb_erlauterung_node.html (accessed on 11 January 2025).
- Buchholz, U.; Buda, S.; Eberle, C.; Hoffmeister, M.; Lehfeld, A.S.; Loenenbach, A.; Michel, J.; Prahm, K.; Preuß, U.; Haas, W.; et al. GrippeWeb-Wochenbericht KW 15/2024; GrippeWeb—Robert Koch-Institut: Berlin, Germany, 2024. [Google Scholar] [CrossRef]
- Robert Koch Institut. AMELAG: Abwasser Monitoring für die epi Demio Logische Lage Bewertung—Überwachung von SARS-CoV-2, Influenzaviren, RSV und Anderen Erregern im Abwasser. Available online: http://www.rki.de/abwassersurveillance (accessed on 11 January 2025).
- Robert Koch Institut. ARE-Wochenbericht KW 7/2025; Robert Koch Institut: Berlin, Germany, 2025. [Google Scholar]
- Robert Koch Institut. Diagramme Virologischer Surveillance—Deutschland (Gesamt) Saison 2024/2025; Robert Koch Institut: Berlin, Germany, 2025. [Google Scholar]
- Deutsche Gesellschaft für Pädiatrische Infektiologie. Ad hoc-Erfassung der Aufgrund von Atemwegsinfektionen Stationär Aufgenommenen und Intensivmedizinisch Behandelten Kinder und Jugendlichen in Deutschland; Klinik und Poliklinik für Kinder- und Jugendmedizin, Universitätsklinikum Carl Gustav Carus, TU Dresden: Dresden, Germany, 2023; Available online: https://dgpi.de/atemwegsinfektionen-survey/ (accessed on 4 March 2025).
- Deutsche Gesellschaft für Pädiatrische Infektiologie. FAQs: Häufig Gestellter Fragen zu den DGPI-Surveys; 2025. Available online: https://dgpi.de/faq-atemwegsinfektions-erfassung/ (accessed on 4 March 2025).
- Sächsisches Staatsministerium für Soziales und Verbraucherschutz. Verordnung des Sächsischen Staatsministeriums für Soziales und Verbraucherschutz über die Erweiterung der Meldepflicht für übertragbare Krankheiten und Krankheitserreger nach dem Infektionsschutzgesetz (IfSGMeldeVO); Sächsisches Staatsministerium: Dresden, Germany, 2002. [Google Scholar]
- Statistisches Bundesamt. Respiratory-Syncytial-Viren [RS-Viren]. Available online: https://www.gbe-bund.de/gbe/abrechnung.prc_abr_test_logon?p_uid=gast&p_aid=52372343&p_knoten=VR&p_sprache=D&p_suchstring=respiratory%20syncytial (accessed on 11 January 2025).
- Horemheb-Rubio, G.; Eggeling, R.; Schmeibetaer, N.; Pfeifer, N.; Lengauer, T.; Gartner, B.C.; Prifert, C.; Kochanek, M.; Scheid, C.; Adams, O.; et al. Respiratory viruses dynamics and interactions: Ten years of surveillance in central Europe. BMC Public Health 2022, 22, 1167. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Durrwald, R.; Biere, B.; Schweiger, B.; Haas, W.; Wolff, T.; Buda, S.; Reiche, J. Determination of respiratory syncytial virus epidemic seasons by using 95% confidence interval of positivity rates, 2011–2021, Germany. Influenza Other Respir. Viruses 2022, 16, 854–857. [Google Scholar] [CrossRef]
- Shaw, D.; Abad, R.; Amin-Chowdhury, Z.; Bautista, A.; Bennett, D.; Broughton, K.; Cao, B.; Casanova, C.; Choi, E.H.; Chu, Y.W.; et al. Trends in invasive bacterial diseases during the first 2 years of the COVID-19 pandemic: Analyses of prospective surveillance data from 30 countries and territories in the IRIS Consortium. Lancet Digit. Health 2023, 5, e582–e593. [Google Scholar] [CrossRef]
- Robert Koch Institut. Bericht zur Epidemiologie der Influenza in Deutschland, Saison 2018/19; Robert Koch-Institut: Berlin, Germany, 2019. [Google Scholar]
- Landesuntersuchungsanstalt für das Gesundheits- und Veterinärwesen (LUA). Jahresbericht 2019 der Landesuntersuchungsanstalt für das Gesundheits- und Veterinärwesen (LUA); Landesuntersuchungsanstalt für das Gesundheits- und Veterinärwesen Sachsen: Dresden, Germany, 2020. [Google Scholar]
- Sächsische Impfkommission (SIKO). 7. Positionspapier der SIKO—1. November 2023—RSV-Impfung; Sächsische Impfkommission; Sächsische Landesärztekammer: Dresden, Germany, 2023. [Google Scholar]
- Drews, S.J.; Branche, A.R.; Falsey, A.R.; Lee, N. What is the role of rapid molecular testing for seniors and other at-risk adults with respiratory syncytial virus infections? J. Clin. Virol. 2019, 117, 27–32. [Google Scholar] [CrossRef]
- Li, K.; Bont, L.J.; Weinberger, D.M.; Pitzer, V.E. Relating in vivo RSV infection kinetics to host infectiousness in different age groups. medRxiv 2024. [CrossRef]
- Onwuchekwa, C.; Moreo, L.M.; Menon, S.; Machado, B.; Curcio, D.; Kalina, W.; Atwell, J.E.; Gessner, B.D.; Siapka, M.; Agarwal, N.; et al. Underascertainment of Respiratory Syncytial Virus Infection in Adults Due to Diagnostic Testing Limitations: A Systematic Literature Review and Meta-analysis. J. Infect. Dis. 2023, 228, 173–184. [Google Scholar] [CrossRef]
- Garcia-Maurino, C.; Moore-Clingenpeel, M.; Thomas, J.; Mertz, S.; Cohen, D.M.; Ramilo, O.; Mejias, A. Viral Load Dynamics and Clinical Disease Severity in Infants With Respiratory Syncytial Virus Infection. J. Infect. Dis. 2019, 219, 1207–1215. [Google Scholar] [CrossRef]
- Onwuchekwa, C.; Atwell, J.; Moreo, L.M.; Menon, S.; Machado, B.; Siapka, M.; Agarwal, N.; Rubbrecht, M.; Aponte-Torres, Z.; Rozenbaum, M.; et al. Pediatric Respiratory Syncytial Virus Diagnostic Testing Performance: A Systematic Review and Meta-analysis. J. Infect. Dis. 2023, 228, 1516–1527. [Google Scholar] [CrossRef]
- Ramirez, J.; Carrico, R.; Wilde, A.; Junkins, A.; Furmanek, S.; Chandler, T.; Schulz, P.; Hubler, R.; Peyrani, P.; Liu, Q.; et al. Diagnosis of Respiratory Syncytial Virus in Adults Substantially Increases When Adding Sputum, Saliva, and Serology Testing to Nasopharyngeal Swab RT-PCR. Infect. Dis. Ther. 2023, 12, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Haeberer, M.; Bruyndonckx, R.; Polkowska-Kramek, A.; Torres, A.; Liang, C.; Nuttens, C.; Casas, M.; Lemme, F.; Ewnetu, W.B.; Tran, T.M.P.; et al. Estimated Respiratory Syncytial Virus-Related Hospitalizations and Deaths Among Children and Adults in Spain, 2016–2019. Infect. Dis. Ther. 2024, 13, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Pletz, M.; Ankert, J.; Schwarz, C.; Wang, L.; Hagel, S.; Gessner, B.; Theilacker, C. Primary International Classification of Diseases Codes Are Only Modestly Sensitive for Detection of Community-Acquired Pneumonia Hospitalization. In Proceedings of the 13th Meeting of the International Society of Pneumonia & Pneumococcal Diseases, Capetown, South Africa, 17–20 March 2024. [Google Scholar]
- Liang, C.; Begier, E.; Hagel, S.; Ankert, J.; Wang, L.; Schwarz, C.; Bayer, L.J.; von Eiff, C.; Liu, Q.; Southern, J.; et al. Incidence of RSV-related hospitalizations for ARIs, including CAP: Data from the German prospective ThEpiCAP study. J. Infect. 2025, 90, 106440. [Google Scholar] [CrossRef] [PubMed]
- Savic, M.; Penders, Y.; Shi, T.; Branche, A.; Pircon, J.Y. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: A systematic literature review and meta-analysis. Influenza Other Respir. Viruses 2023, 17, e13031. [Google Scholar] [CrossRef]
- Li, Y.; Kulkarni, D.; Begier, E.; Wahi-Singh, P.; Wahi-Singh, B.; Gessner, B.; Nair, H. Adjusting for Case Under-Ascertainment in Estimating RSV Hospitalisation Burden of Older Adults in High-Income Countries: A Systematic Review and Modelling Study. Infect. Dis. Ther. 2023, 12, 1137–1149. [Google Scholar] [CrossRef]
- Rozenbaum, M.H.; Begier, E.; Kurosky, S.K.; Whelan, J.; Bem, D.; Pouwels, K.B.; Postma, M.; Bont, L. Incidence of Respiratory Syncytial Virus Infection in Older Adults: Limitations of Current Data. Infect. Dis. Ther. 2023, 12, 1487–1504. [Google Scholar] [CrossRef]
- Rozenbaum, M.H.; Judy, J.; Tran, D.; Yacisin, K.; Kurosky, S.K.; Begier, E. Low Levels of RSV Testing Among Adults Hospitalized for Lower Respiratory Tract Infection in the United States. Infect. Dis. Ther. 2023, 12, 677–685. [Google Scholar] [CrossRef]
- Scholz, S.; Dobrindt, K.; Tufts, J.; Adams, S.; Ghaswalla, P.; Ultsch, B.; Gottlieb, J. The Burden of Respiratory Syncytial Virus (RSV) in Germany: A Comprehensive Data Analysis Suggests Underdetection of Hospitalisations and Deaths in Adults 60 Years and Older. Infect. Dis. Ther. 2024, 13, 1759–1770. [Google Scholar] [CrossRef]

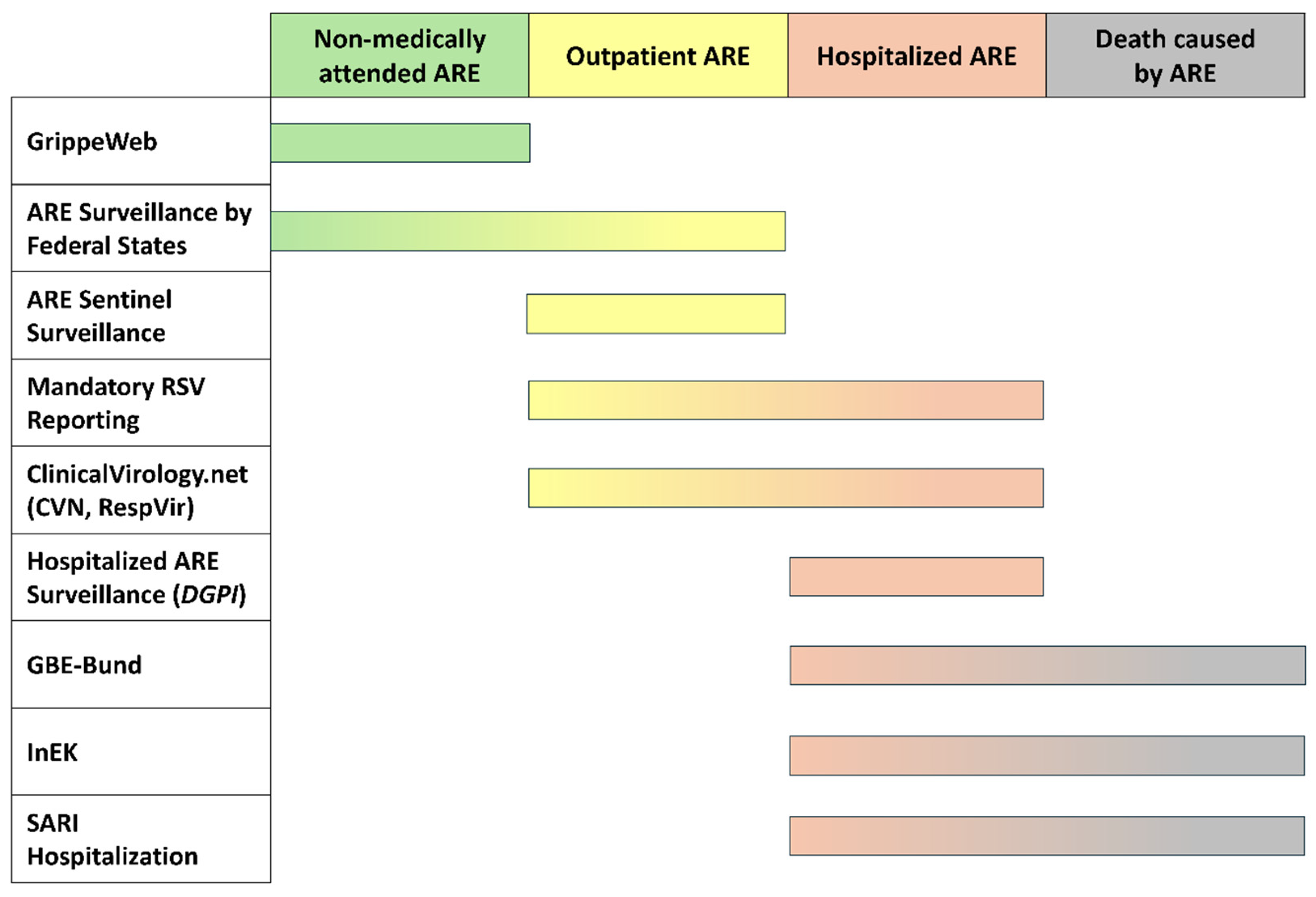
| Surveillance System/Database | Short Description | Access to Data | Access to Visualization | |
|---|---|---|---|---|
| RKI | Mandatory RSV reporting | Laboratory-confirmed RSV cases reported under the Infectious Disease Act; aggregated data published by RKI, publicly accessible. | SurvStat https://survstat.rki.de/Default.aspx (last accessed on 14 October 2025). | Infektionsradar https://infektionsradar.gesund.bund.de/de (last accessed on 14 October 2025). |
| GrippeWeb | Online self-reporting of acute respiratory symptoms; used to estimate incidence; aggregated data available on RKI website. | Weekly reports https://edoc.rki.de/handle/176904/39 GitHub https://github.com/robert-koch-institut/GrippeWeb_Daten_des_Wochenberichts (both accessed on 14 October 2025). | Dashboard GrippeWeb https://public.data.rki.de/t/public/views/ARE-Dashboard/BevoelkerungGrippeWeb-Inzidenzen (last accessed on 14 October 2025). | |
| ARE sentinel surveillance | Weekly reports from GPs and pediatricians on ARE and influenza-like illness; aggregated data published by RKI. | Weekly reports https://edoc.rki.de/handle/176904/39 GitHub https://github.com/robert-koch-institut/ARE-Konsultationsinzidenz (both accessed on 14 October 2025). | RKI Diagrams https://influenza.rki.de/Diagrams.aspx Dashboard surveillance https://public.data.rki.de/t/public/views/ARE-Dashboard/Arztpraxen (both accessed on 14 October 2025). | |
| SARI hospitalization | ICD-10-based hospital surveillance for severe respiratory infections; anonymized data from ~70 hospitals; aggregated results publicly available. | Weekly reports https://influenza.rki.de/Wochenberichte.aspx (last accessed on 14 October 2025). GitHub https://github.com/robert-koch-institut/ARE-Konsultationsinzidenz (last accessed on 14 October 2025). | Dashboard SARI in hospitals https://public.data.rki.de/t/public/views/ARE-Dashboard/Krankenhaeuser (last accessed on 14 October 2025). | |
| GBE-Bund | Federal health reporting system compiling official health data; reports and indicators publicly accessible. | ”Respiratory-Syncytial-Viren” „Diagnosedaten der Krankenhäuser nach Behandlungsort” https://www.gbe-bund.de/gbe/ (last accessed on 14 October 2025). | - | |
| InEK | Collects hospital performance and cost data for DRG reimbursement; aggregated data are publicly accessible via InEK data browser. | InEK Datenbrowser https://datenbrowser.inek.org/ (last accessed on 14 October 2025). | - | |
| Hospitalized ARE surveillance (DGPI) | Registry of hospitalized children with ARE (RSV, influenza, COVID-19); data visualization publicly available. | - | Surveillance | |
| • 2021/22 https://dgpi.de/rsv-survey-update/ • 2022/23 https://dgpi.de/awi-erfassung-update/ • 2023/24 https://dgpi.de/awi-erfassung-update-2023-2024/ • 2024/25 https://dgpi.de/awi-erfassung-update-2024-2025/ (all accessed on 14 October 2025). | ||||
| ClinicalVirology.net (CVN, RespVir) | Aggregates data on lab-confirmed respiratory pathogens (positive/negative, notifiable/non-notifiable) from ~60 labs in Germany, Austria, and Switzerland; trends are publicly available. | Upon request. | Dashboard https://www.arcgis.com/apps/dashboards/2a29f2bebc524c67b6250b64beea12bf (last accessed on 14 October 2025). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayer, L.J.; Brösamle, C.; Brestrich, G.; Najafi, B.; von Eiff, C.; Hösemann, C.; Stepan, H.; Gosch, G.; Wojcinski, M.; Abou-Dakn, M.; et al. RSV Monitoring in Germany: A Critical Overview of Available Surveillance Systems. J. Clin. Med. 2025, 14, 7487. https://doi.org/10.3390/jcm14217487
Bayer LJ, Brösamle C, Brestrich G, Najafi B, von Eiff C, Hösemann C, Stepan H, Gosch G, Wojcinski M, Abou-Dakn M, et al. RSV Monitoring in Germany: A Critical Overview of Available Surveillance Systems. Journal of Clinical Medicine. 2025; 14(21):7487. https://doi.org/10.3390/jcm14217487
Chicago/Turabian StyleBayer, Lea J., Christian Brösamle, Gordon Brestrich, Bahar Najafi, Christof von Eiff, Cornelia Hösemann, Holger Stepan, Gunther Gosch, Michael Wojcinski, Michael Abou-Dakn, and et al. 2025. "RSV Monitoring in Germany: A Critical Overview of Available Surveillance Systems" Journal of Clinical Medicine 14, no. 21: 7487. https://doi.org/10.3390/jcm14217487
APA StyleBayer, L. J., Brösamle, C., Brestrich, G., Najafi, B., von Eiff, C., Hösemann, C., Stepan, H., Gosch, G., Wojcinski, M., Abou-Dakn, M., Herting, E., Rose, M. A., Prelog, M., & Kaiser, R. (2025). RSV Monitoring in Germany: A Critical Overview of Available Surveillance Systems. Journal of Clinical Medicine, 14(21), 7487. https://doi.org/10.3390/jcm14217487







