Fit Hearts, Better Outcomes? A Systematic Review and Meta-Analysis of Exercise Intensity and Peak VO2 in Hypertrophic Cardiomyopathy
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Search Strategy and Data Extraction
2.3. Effect Measures
2.4. Quality Assessment
2.5. Sensitivity Analysis
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Included Studies and the Risk of Bias
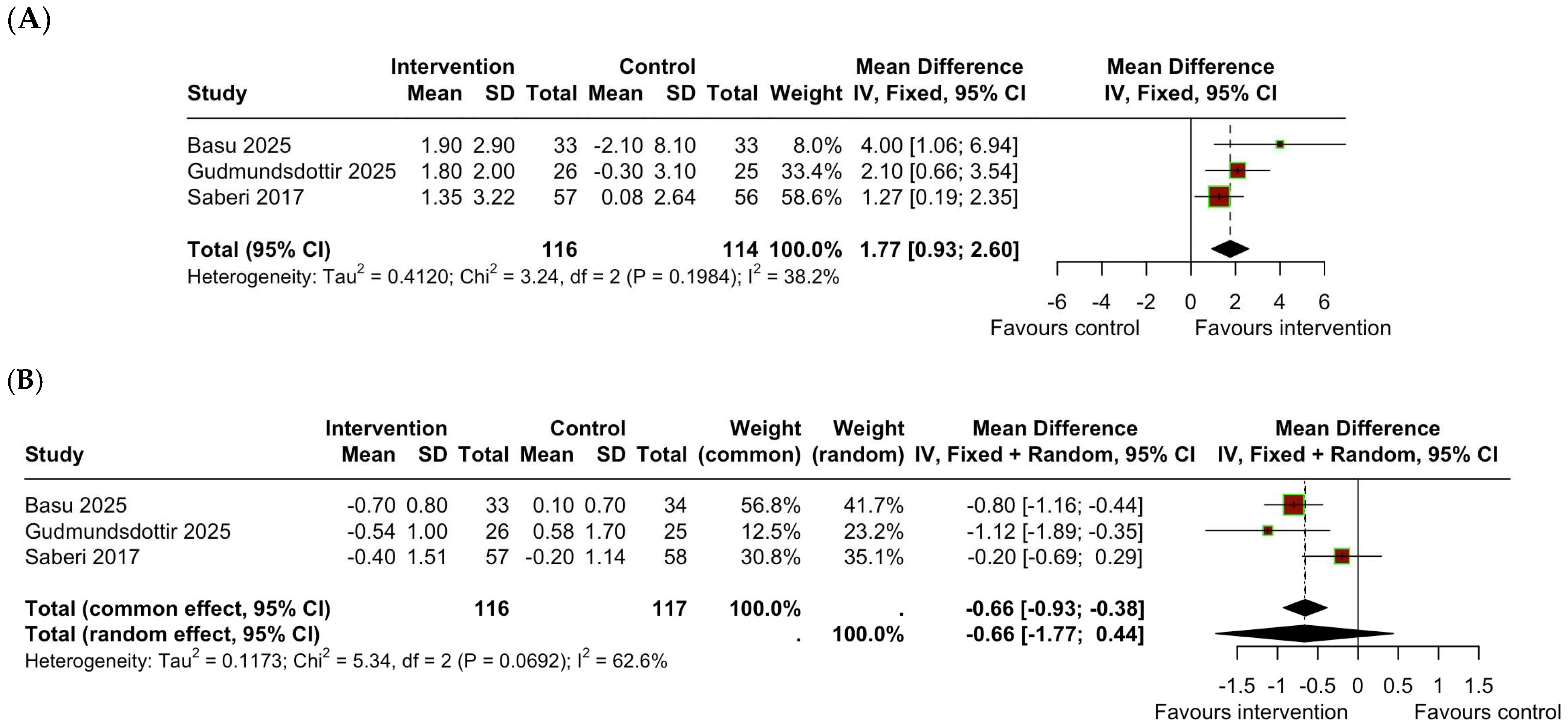
3.2. Change in VO2peak
3.3. Change in BMI
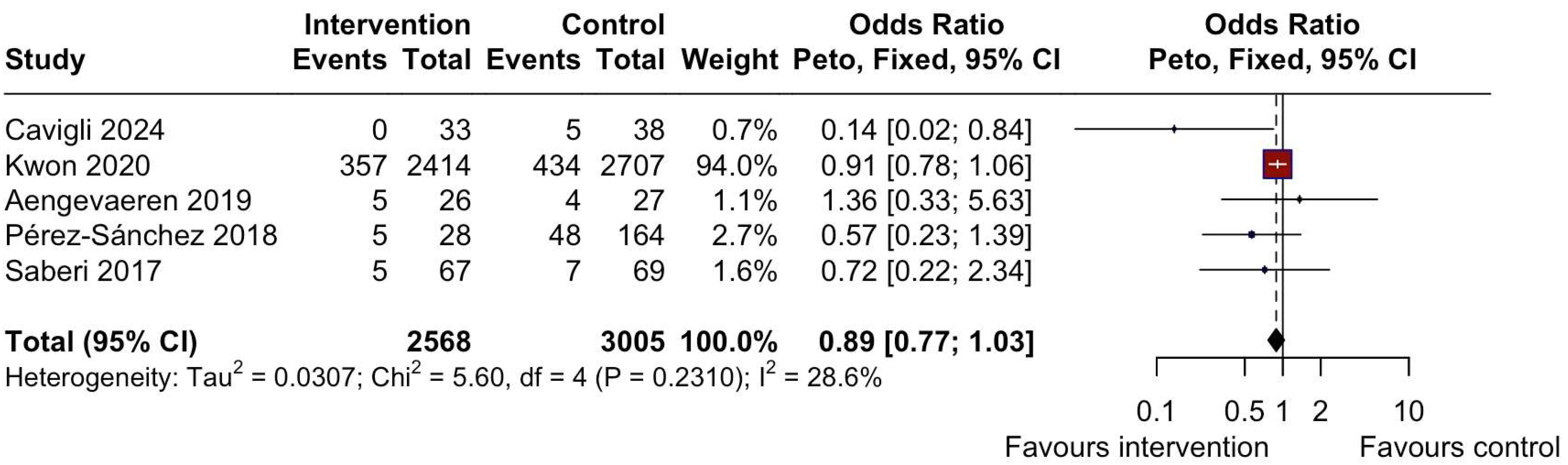
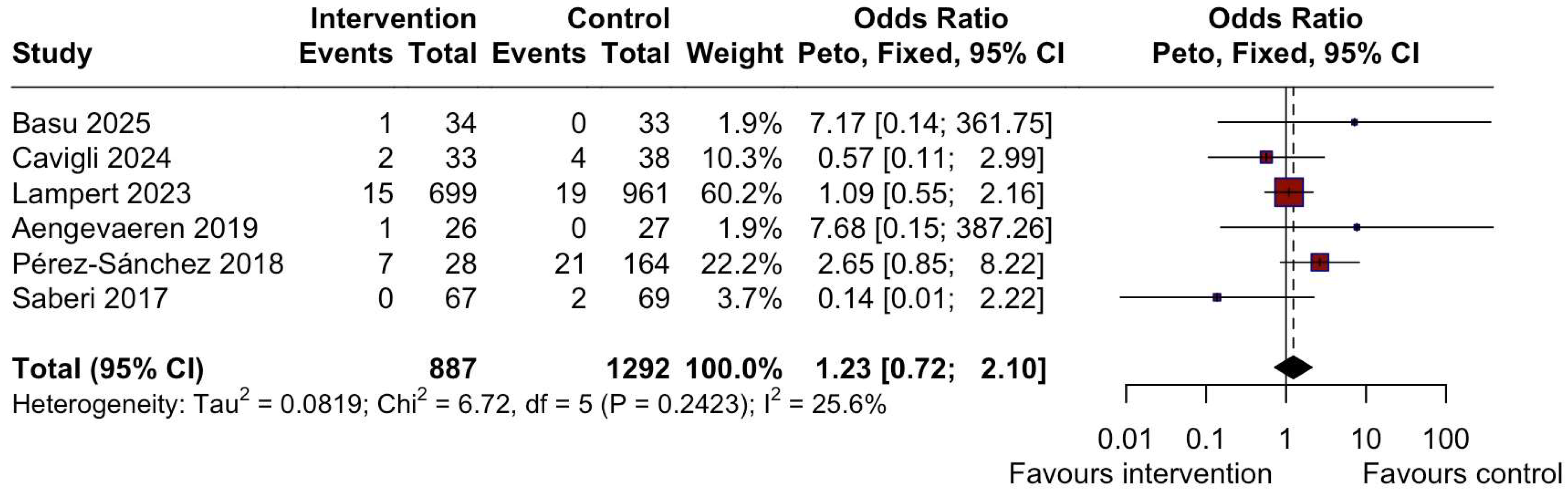
3.4. Adverse Outcomes
3.5. Leave-One-Out Analysis
4. Discussion
4.1. Exercise Interventions
4.2. Safety
4.3. Benefits of Exercise
4.4. Strengths
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Epps, K.C.; Evanovich, L.; Ferrari, V.A.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1239–e1311. [Google Scholar] [CrossRef]
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 372–389. [Google Scholar] [CrossRef]
- Lopes, L.R.; Ho, C.Y.; Elliott, P.M. Genetics of Hypertrophic Cardiomyopathy: Established and Emerging Implications for Clinical Practice. Eur. Heart J. 2024, 45, 2727–2734. [Google Scholar] [CrossRef]
- Marian, A.J.; Braunwald, E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ. Res. 2017, 121, 749–770. [Google Scholar] [CrossRef]
- Brinkley, D.M.; Wells, Q.S.; Stevenson, L.W. Avoiding Burnout From Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2020, 75, 3044–3047. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the Management of Cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Papadakis, M.; Tanzarella, G.; Dhutia, H.; Miles, C.; Tome, M.; Behr, E.R.; Sharma, S.; Sheppard, M.N. Sudden Death Can Be the First Manifestation of Hypertrophic Cardiomyopathy: Data from a United Kingdom Pathology Registry. JACC Clin. Electrophysiol. 2019, 5, 252–254. [Google Scholar] [CrossRef]
- Fumagalli, C.; Maurizi, N.; Day, S.M.; Ashley, E.A.; Michels, M.; Colan, S.D.; Jacoby, D.; Marchionni, N.; Vincent-Tompkins, J.; Ho, C.Y.; et al. Association of Obesity with Adverse Long-Term Outcomes in Hypertrophic Cardiomyopathy. JAMA Cardiol. 2020, 5, 65–72. [Google Scholar] [CrossRef]
- Semsarian, C.; Gray, B.; Haugaa, K.H.; Lampert, R.; Sharma, S.; Kovacic, J.C. Athletic Activity for Patients With Hypertrophic Cardiomyopathy and Other Inherited Cardiovascular Diseases: JACC Focus Seminar 3/4. J. Am. Coll. Cardiol. 2022, 80, 1268–1283. [Google Scholar] [CrossRef] [PubMed]
- World Obesity Federation. World Obesity Atlas 2025. Available online: https://data.worldobesity.org/publications/?cat=23 (accessed on 3 April 2025).
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, version 6.5; Wiley: Hoboken, NJ, USA, 2024; Available online: www.training.cochrane.org/handbook (accessed on 5 April 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan: A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Basu, J.; Nikoletou, D.; Miles, C.; MacLachlan, H.; Parry-Williams, G.; Tilby-Jones, F.; Bulleros, P.; Fanton, Z.; Baker, C.; Purcell, S.; et al. High-Intensity Exercise Programme in Patients with Hypertrophic Cardiomyopathy: A Randomized Trial. Eur. Heart J. 2025, 46, 1803–1815. [Google Scholar] [CrossRef]
- Gudmundsdottir, H.L.; Axelsson Raja, A.; Rossing, K.; Rasmusen, H.; Snoer, M.; Andersen, L.J.; Gottlieb, R.; Christensen, A.H.; Bundgaard, H.; Gustafsson, F.; et al. Exercise Training in Patients with Hypertrophic Cardiomyopathy Without Left Ventricular Outflow Tract Obstruction: A Randomized Clinical Trial. Circulation 2025, 151, 132–144. [Google Scholar] [CrossRef]
- Hassanzada, F.; Jansen, M.; van Lint, F.H.M.; Bosman, L.P.; Schmidt, A.F.; Dooijes, D.; van de Sande, D.; Miah, B.; van der Crabben, S.N.; Wilde, A.A.M.; et al. Recreational and Occupational Physical Activity and Risk of Adverse Events in Truncating MYBPC3 Founder Variant Carriers. Circ. Genom. Precis. Med. 2024, 17, e004561. [Google Scholar] [CrossRef]
- Cavigli, L.; Ragazzoni, G.L.; Vannuccini, F.; Targetti, M.; Mandoli, G.E.; Senesi, G.; Pastore, M.C.; Focardi, M.; Cameli, M.; Valente, S.; et al. Cardiopulmonary Fitness and Personalized Exercise Prescription in Patients with Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2024, 13, e036593. [Google Scholar] [CrossRef]
- Lampert, R.; Ackerman, M.J.; Marino, B.S.; Burg, M.; Ainsworth, B.; Salberg, L.; Tome Esteban, M.T.; Ho, C.Y.; Abraham, R.; Balaji, S.; et al. Vigorous Exercise in Patients with Hypertrophic Cardiomyopathy. JAMA Cardiol. 2023, 8, 595–605. [Google Scholar] [CrossRef]
- MacNamara, J.P.; Dias, K.A.; Hearon, C.M., Jr.; Ivey, E.; Delgado, V.A.; Saland, S.; Samels, M.; Hieda, M.; Turer, A.T.; Link, M.S.; et al. Randomized Controlled Trial of Moderate- and High-Intensity Exercise Training in Patients with Hypertrophic Cardiomyopathy: Effects on Fitness and Cardiovascular Response to Exercise. J. Am. Heart Assoc. 2023, 12, e031399. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, H.J.; Han, K.D.; Kim, D.H.; Lee, S.P.; Hwang, I.C.; Yoon, Y.; Park, J.B.; Lee, H.; Kwak, S.; et al. Association of Physical Activity With All-Cause and Cardiovascular Mortality in 7666 Adults with Hypertrophic Cardiomyopathy: More Physical Activity Is Better. Br. J. Sports Med. 2021, 55, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Aengevaeren, V.L.; Gommans, D.H.F.; Dieker, H.J.; Timmermans, J.; Verheugt, F.W.A.; Bakker, J.; Hopman, M.T.E.; DE Boer, M.-J.; Brouwer, M.A.; Thompson, P.D.; et al. Association Between Lifelong Physical Activity and Disease Characteristics in HCM. Med. Sci. Sports Exerc. 2019, 51, 1995–2002. [Google Scholar] [CrossRef]
- Wasserstrum, Y.; Barbarova, I.; Lotan, D.; Kuperstein, R.; Shechter, M.; Freimark, D.; Segal, G.; Klempfner, R.; Arad, M. Efficacy and Safety of Exercise Rehabilitation in Patients with Hypertrophic Cardiomyopathy. J. Cardiol. 2019, 74, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, J.; Ingles, J.; Ball, K.; Semsarian, C. A Control Theory-Based Pilot Intervention to Increase Physical Activity in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2018, 122, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, I.; Romero-Puche, A.J.; García-Molina Sáez, E.; Sabater-Molina, M.; López-Ayala, J.M.; Muñoz-Esparza, C.; López-Cuenca, D.; de la Morena, G.; Castro-García, F.J.; Gimeno-Blanes, J.R. Factors Influencing the Phenotypic Expression of Hypertrophic Cardiomyopathy in Genetic Carriers. Rev. Esp. Cardiol. (Engl. Ed.) 2018, 71, 146–154. [Google Scholar] [CrossRef]
- Dejgaard, L.A.; Haland, T.F.; Lie, O.H.; Ribe, M.; Bjune, T.; Leren, I.S.; Berge, K.E.; Edvardsen, T.; Haugaa, K.H. Vigorous Exercise in Patients With Hypertrophic Cardiomyopathy. Int. J. Cardiol. 2018, 250, 157–163. [Google Scholar] [CrossRef]
- Saberi, S.; Wheeler, M.; Bragg-Gresham, J.; Hornsby, W.; Agarwal, P.P.; Attili, A.; Concannon, M.; Dries, A.M.; Shmargad, Y.; Salisbury, H.; et al. Effect of Moderate-Intensity Exercise Training on Peak Oxygen Consumption in Patients with Hypertrophic Cardiomyopathy: A Randomized Clinical Trial. JAMA 2017, 317, 1349–1357. [Google Scholar] [CrossRef]
- Klempfner, R.; Kamerman, T.; Schwammenthal, E.; Nahshon, A.; Hay, I.; Goldenberg, I.; Freimark, D.; Arad, M. Efficacy of Exercise Training in Symptomatic Patients with Hypertrophic Cardiomyopathy: Results of a Structured Exercise Training Program in a Cardiac Rehabilitation Center. Eur. J. Prev. Cardiol. 2015, 22, 13–19. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on Sports Cardiology and Exercise in Patients with Cardiovascular Disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Abdelfattah, O.M.; Martinez, M.; Sayed, A.; ElRefaei, M.; Abushouk, A.I.; Hassan, A.; Masri, A.; Winters, S.L.; Kapadia, S.R.; Maron, B.J.; et al. Temporal and Global Trends of the Incidence of Sudden Cardiac Death in Hypertrophic Cardiomyopathy. JACC Clin. Electrophysiol. 2022, 8, 1417–1427. [Google Scholar] [CrossRef]
- Santoro, F.; Mango, F.; Mallardi, A.; D’Alessandro, D.; Casavecchia, G.; Gravina, M.; Correale, M.; Brunetti, N.D. Arrhythmic Risk Stratification among Patients with Hypertrophic Cardiomyopathy. J. Clin. Med. 2023, 12, 3397. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Mackey-Bojack, S.; Facile, E.; Duncanson, E.; Rowin, E.J.; Maron, M.S. Hypertrophic Cardiomyopathy and Sudden Death Initially Identified at Autopsy. Am. J. Cardiol. 2020, 127, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Link, M.S.; Bockstall, K.; Weinstock, J.; Alsheikh-Ali, A.A.; Semsarian, C.; Estes, N.A., 3rd; Spirito, P.; Haas, T.S.; Rowin, E.J.; Maron, M.S.; et al. Ventricular Tachyarrhythmias in Patients with Hypertrophic Cardiomyopathy and Defibrillators: Triggers, Treatment, and Implications. J. Cardiovasc. Electrophysiol. 2017, 28, 531–537. [Google Scholar] [CrossRef]
- Ackerman, M.; Atkins, D.L.; Triedman, J.K. Sudden Cardiac Death in the Young. Circulation 2016, 133, 1006–1026. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G.; Radaelli, D.; D’Errico, S.; Papadakis, M.; Behr, E.R.; Sharma, S.; Westaby, J.; Sheppard, M.N. Sudden Cardiac Death Among Adolescents in the United Kingdom. J. Am. Coll. Cardiol. 2023, 81, 1007–1017. [Google Scholar] [CrossRef]
- Bongini, C.; Ferrantini, C.; Girolami, F.; Coppini, R.; Arretini, A.; Targetti, M.; Bardi, S.; Castelli, G.; Torricelli, F.; Cecchi, F.; et al. Impact of Genotype on the Occurrence of Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2016, 117, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2024, 83, 109–279. [Google Scholar] [CrossRef]
- Guttmann, O.P.; Pavlou, M.; O’Mahony, C.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; Garcia-Pavia, P.; et al. Prediction of Thrombo-Embolic Risk in Patients with Hypertrophic Cardiomyopathy (HCM Risk-CVA). Eur. J. Heart Fail. 2015, 17, 837–845. [Google Scholar] [CrossRef]
- Franklin, B.A.; Thompson, P.D.; Al-Zaiti, S.S.; Albert, C.M.; Hivert, M.-F.; Levine, B.D.; Lobelo, F.; Madan, K.; Sharrief, A.Z.; Eijsvogels, T.M.H. Exercise-Related Acute Cardiovascular Events and Potential Deleterious Adaptations Following Long-Term Exercise Training: Placing the Risks Into Perspective—An Update: A Scientific Statement From the American Heart Association. Circulation 2020, 141, e705–e736. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Cameli, M.; Ciccone, M.M.; Maiello, M.; Modesti, P.A.; Mondillo, S.; Muiesan, M.L.; Scicchitano, P.; Novo, S.; Palmiero, P.; et al. The Controversial Relationship Between Exercise and Atrial Fibrillation: Clinical Studies and Pathophysiological Mechanisms. J. Cardiovasc. Med. 2015, 16, 802–810. [Google Scholar] [CrossRef]
- Garatachea, N.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Santos-Lozano, A.; Fiuza-Luces, C.; Morán, M.; Emanuele, E.; Joyner, M.J.; Lucia, A. Exercise Attenuates the Major Hallmarks of Aging. Rejuvenation Res. 2015, 18, 57–89. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, P.; Faselis, C.; Samuel, I.B.H.; Pittaras, A.; Doumas, M.; Murphy, R.; Heimall, M.S.; Sui, X.; Zhang, J.; Myers, J. Cardiorespiratory Fitness and Mortality Risk Across the Spectra of Age, Race, and Sex. J. Am. Coll. Cardiol. 2022, 80, 598–609. [Google Scholar] [CrossRef]
- Vazquez-Guajardo, M.; Rivas, D.; Duque, G. Exercise as a Therapeutic Tool in Age-Related Frailty and Cardiovascular Disease: Challenges and Strategies. Can. J. Cardiol. 2024, 40, 1458–1467. [Google Scholar] [CrossRef]
- Mandsager, K.; Harb, S.; Cremer, P.; Phelan, D.; Nissen, S.E.; Jaber, W. Association of Cardiorespiratory Fitness with Long-Term Mortality Among Adults Undergoing Exercise Treadmill Testing. JAMA Netw. Open 2018, 1, e183605. [Google Scholar] [CrossRef]
- Dias, K.A.; Link, M.S.; Levine, B.D. Exercise Training for Patients with Hypertrophic Cardiomyopathy: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.Y.; Bhonsale, A.; Patel, P.; Naji, P.; Smedira, N.G.; Thamilarasan, M.; Lytle, B.W.; Lever, H.M. Exercise Echocardiography in Asymptomatic HCM: Exercise Capacity, and Not LV Outflow Tract Gradient, Predicts Long-Term Outcomes. JACC Cardiovasc. Imaging 2014, 7, 26–36. [Google Scholar] [CrossRef]
- Mikic, L.; Ristic, A.; Markovic Nikolic, N.; Tesic, M.; Jakovljevic, D.G.; Arena, R.; Allison, T.G.; Popovic, D. The Role of Cardiopulmonary Exercise Testing in Hypertrophic Cardiomyopathy. Medicina 2023, 59, 1296. [Google Scholar] [CrossRef]
- Cui, H.; Schaff, H.V.; Olson, T.P.; Geske, J.B.; Dearani, J.A.; Nishimura, R.A.; Sun, D.; Ommen, S.R. Cardiopulmonary Exercise Test in Patients with Obstructive Hypertrophic Cardiomyopathy. J. Thorac. Cardiovasc. Surg. 2024, 167, 701–710.e3. [Google Scholar] [CrossRef]
- Larsen, C.M.; Ball, C.A.; Hebl, V.B.; Ong, K.C.; Siontis, K.C.; Olson, T.P.; Ackerman, M.J.; Ommen, S.R.; Allison, T.G.; Geske, J.B. Effect of Body Mass Index on Exercise Capacity in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2018, 121, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Sweatt, K.; Garvey, W.T.; Martins, C. Strengths and Limitations of BMI in the Diagnosis of Obesity: What Is the Path Forward? Curr. Obes. Rep. 2024, 13, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Rowe, S.; Ha, F.; Lim, H.; Playford, D.; Strange, G.; Ellims, A.; Semsarian, C.; La Gerche, A.; Paratz, E. Global and National Insights into the Decline of Hypertrophic Cardiomyopathy as a Cause of Sudden Death in the Young. Eur. Heart J. 2024, 45 (Suppl. S1), ehae666.888. [Google Scholar] [CrossRef]
- Fontana, L.; Fasano, A.; Chong, Y.S.; Vineis, P.; Willett, W.C. Transdisciplinary Research and Clinical Priorities for Better Health. PLoS Med. 2021, 18, e1003699. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Baggish, A.L.; Levine, B.D.; Ackerman, M.J.; Day, S.M.; Dineen, E.H.; Guseh, J.S., II; La Gerche, A.; Lampert, R.; Martinez, M.W.; et al. Clinical Considerations for Competitive Sports Participation for Athletes with Cardiovascular Abnormalities: A Scientific Statement from the American Heart Association and American College of Cardiology. J. Am. Coll. Cardiol. 2025, 85, 1059–1108. [Google Scholar] [CrossRef] [PubMed]


| Author and Publication Year | Design | Participant Number | Intervention | Control | Intervention Duration | Key Outcomes |
|---|---|---|---|---|---|---|
| Basu et al. 2025 [14], a | RCT | n = 80 | HI exercise program 3 h/week: −2 h/week supervised + 1 h/week home-based Aerobic + RT, 70 to 85% calculated HRR | Usual care | 12 weeks | After 12 weeks, participants with HCM who performed HI exercise vs. usual care, increased their VO2peak (+1.9 ± 2.9 mL/kg/min—exercise group and −2.1 ± 8.1 mL/kg/min -usual care group), decreased BMI (−0.7 ± 0.8 mL/kg/min—exercise group and +0.1 ± 0.7 mL/kg/min -usual care group), with no increase in arrhythmias, and one syncope episode in the exercise group. |
| Gudmundsdottir et al. 2025 [15], a | RCT | n = 59 | Supervised MI exercise programme 3 h/week −60% maximal work capacity −12–14 RPE—aerobic and RT | Usual activity | 12 weeks | In patients with HCM without LVOT obstruction, a 12-week supervised MI training intervention compared with usual activity increased VO2peak ((+1.8 ± 2.0 mL/kg/min—exercise group and −0.3 ± 3.1 mL/kg/min -usual care group) and decreased BMI (−0.54 ± 1.0 mL/kg/min—exercise group and +0.58 ± 1.7 mL/kg/min -usual care group). |
| Hassanzada et al. 2024 [16] | Cross-sectional | n = 133 | N/A | N/A | N/A Follow-up for 8.8 (4.3–16.5) y | In truncating MYBPC3 founder variant carriers, overall PA and high-static exercise are not associated with an increased risk of MCE and cardiomyopathy penetrance. Those who participated in the highest quartile of high-dynamic exercise had an increased risk of MVA. |
| Cavigli et al. 2024 [17], a | Cohort | n = 71 | Unsupervised-advice only -Personalized, tailored according to the CPET (aerobic MI, around VT1) −2 h/week and increased to 3–5 h/week -RT in non-obstructive patients, 40–70% 1RM. | N/A | N/A Reassessment in 6–12 months, followed up for max 3 years; 13 evaluated after 24 ± 12 months | Patients with HCM practicing regular MI aerobic exercise have a better functional capacity in the absence of relevant events vs. sedentary patients. A sedentary lifestyle led to a deterioration of cardiopulmonary functional capacity and fitness. |
| Lampert et al. 2023 [18], a | Cohort | n = 1660 | Self-reported PA in the past year (Minnesota Leisure Time Activity Questionnaire), classified according to the 2011 Compendium of Physical Activities | Sedentary | 36 months (outcome surveys every 6 months) | Among individuals with HCM or those who are genotype positive/phenotype negative and are treated in experienced centers, those exercising vigorously did not experience a higher rate of death or life-threatening arrhythmias than those exercising moderately or those who were sedentary (syncope episodes: 15 in the intervention group, 19 in the control group). |
| Mac Namara et al. 2023 [19], a | RCT | n = 22 | Randomized (LVOT 30 mmHg cutoff): 5 months MI (n = 9 completed) or 1 month MI + 4 months HI (n = 8 completed) -Intensity based on CPET. | MI | 5 months | In HCM patients, exercise training, both HI and MI, improved fitness without a clear superiority of either. Exercise training resulted in salutary peripheral and cardiac adaptations. No serious adverse events occurred (NSVT episodes: 2 in the intervention group, 2 in the control group). |
| Kwon et al. 2021 [20], a | Cross-sectional | n = 7666 | 7-day recall questionnaire | N/A | N/A | MI to vigorous- intensity PA, in a middle- aged population of patients with HCM, was associated with progressive reduction in all- cause and cardiovascular mortality. AF episodes: 357 in the intervention group, 434 in the control group. |
| Aengevaeren et al. 2019 [21], a | Cross-sectional | n = 102 | Questionnaire—lifelong PA per decade | N/A | N/A | Lifelong physical activity volumes are not associated with genotype-to-phenotype transition in HCM gene carriers. AF episodes: 5 in the intervention group, 4 in the control group; syncope episodes: 1 in the intervention group, 0 in the control group; For HI vs. MI, NSVT episodes: 8 in the intervention group, 7 in the control group. For MI vs. sedentary, NSVT episodes: 7 in the intervention group, 1 in the control group. |
| Wasserstrum et al. 2019 [22] | Pre-post | n = 45 | N/A (retrospective evaluation of the improvement in exercise capacity after cardiac rehabilitation) | Participants serving as their own controls | N/A | Exercise rehabilitation appears to be a suitable and safe option in HCM. It primarily benefits patients with significant functional limitations. No significant arrhythmias or adverse events were recorded during participation |
| Sweeting et al. 2018. [23] | Pre-post | n = 25 | Face-to-face motivational interview (based on principles of control theory) | Participants serving as their own controls | 12 w | A 12-week control theory-based intervention to increase physical activity in HCM patients led to significant improvement in physical quality of life and self-efficacy, and fewer barriers were identified. |
| Perez Sanchez et al. 2018 [24], a | Cohort | n = 272 | PA 2 years before the time of diagnosis in unaffected carriers or to the time of first evaluation in unaffected carriers. “Typical week” PA level is classified according to hours per week and type of activity, including physically demanding jobs | Sedentary | 5.5 ± 3.3 years follow-up | Men and athletes who are carriers of sarcomeric mutations are diagnosed earlier than women and sedentary individuals. Sex, hypertension, and the degree of PA were not significantly associated with the severity of LVH. AF episodes: 5 in the intervention group, 48 in the control group; syncope episodes: 7 in the intervention group, 21 in the control group; NSVT episodes: 7 in the intervention group, 42 in the control group). |
| Dejgaard et al. 2018 [25], a | Cross sectional | n = 187 | Lifelong PA (since the age of 6) | N/A | N/A | Increased lifetime vigorous exercise was associated with larger LV volumes in HCM, but correlated to LV mass only in Genotype+ LVH-. Vigorous exercise was associated with favorable diastolic function in HCM LVH+, and was not related to significant VA (NSVT episodes: 10 in the intervention group, 15 in the control group). |
| Saberi et al. 2017 [26], a | RCT | n = 136 | Unsupervised structured MI exercise programme according to the CPET: at least 3x/week and 20 min/session. -HR at 60% HRR -RPE 11–14 -Increasing gradually 5–10 min up to 60 min, 4–7x/week at 70% HRR. -Aerobic: cycling, walk-jog, elliptical. -No RT or burst-type activity | Usual activity | 16 weeks | After 16 weeks, MI exercise compared with usual activity resulted in an increase in VO2peak (+1.35 ± 3.22 mL/kg/min—exercise group and +0.8 ± 2.64 mL/kg/min -usual care group), slight reduction in BMI (−0.4 ± 1.51 mL/kg/min—exercise group and −0.2 ± 1.14 mL/kg/min -usual care group). There were two syncope episodes in the control group (0 in the exercise group), 5 AF episodes in the exercise group vs. 7 in the control group, 19 NSVT episodes in the exercise group vs. 15 in the control group, and no occurrences of sustained VA, SCA, appropriate defibrillator shock, or death in either group. |
| Klempfner et al. 2015 [27] | Pre-post | n = 20 | Supervised, aerobic, intensity according to the EST, gradually increased from 50% to 85% of the HRR (RPE 13–15), 2 h/week (ICD patients were limited to 20 bpm below therapy threshold) | Participants serving as their own controls | Not stated, patients completed an average of 41 ± 8 h of training | Patients with HCM who remain symptomatic despite medical therapy may achieve considerable functional improvement through a supervised ET program |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djuranovic, A.; Ristic, J.; Antic, M.; Rajovic, N.; Mirkovic, M.; Batinic, D.; Maletic, M.; Kizilkilic, S.E.; Zecchin Ferrara, V.; Prodanovic, V.; et al. Fit Hearts, Better Outcomes? A Systematic Review and Meta-Analysis of Exercise Intensity and Peak VO2 in Hypertrophic Cardiomyopathy. J. Clin. Med. 2025, 14, 7466. https://doi.org/10.3390/jcm14217466
Djuranovic A, Ristic J, Antic M, Rajovic N, Mirkovic M, Batinic D, Maletic M, Kizilkilic SE, Zecchin Ferrara V, Prodanovic V, et al. Fit Hearts, Better Outcomes? A Systematic Review and Meta-Analysis of Exercise Intensity and Peak VO2 in Hypertrophic Cardiomyopathy. Journal of Clinical Medicine. 2025; 14(21):7466. https://doi.org/10.3390/jcm14217466
Chicago/Turabian StyleDjuranovic, Andrija, Jovana Ristic, Milena Antic, Nina Rajovic, Mladen Mirkovic, Djordje Batinic, Milos Maletic, Sevda Ece Kizilkilic, Victoria Zecchin Ferrara, Verica Prodanovic, and et al. 2025. "Fit Hearts, Better Outcomes? A Systematic Review and Meta-Analysis of Exercise Intensity and Peak VO2 in Hypertrophic Cardiomyopathy" Journal of Clinical Medicine 14, no. 21: 7466. https://doi.org/10.3390/jcm14217466
APA StyleDjuranovic, A., Ristic, J., Antic, M., Rajovic, N., Mirkovic, M., Batinic, D., Maletic, M., Kizilkilic, S. E., Zecchin Ferrara, V., Prodanovic, V., Savic, S., Mazic, S., & Milic, N. (2025). Fit Hearts, Better Outcomes? A Systematic Review and Meta-Analysis of Exercise Intensity and Peak VO2 in Hypertrophic Cardiomyopathy. Journal of Clinical Medicine, 14(21), 7466. https://doi.org/10.3390/jcm14217466









