Superior Vena Cava as an Arrhythmogenic Substrate in Atrial Fibrillation: Anatomical, Electrophysiological and Clinical Perspectives
Abstract
1. Introduction
2. Information Sources and Search Strategy
3. Superior Vena Cava Serving as the AF Arrhythmogenic Source
4. Induction Protocols Used for Non-PV Trigger Detection
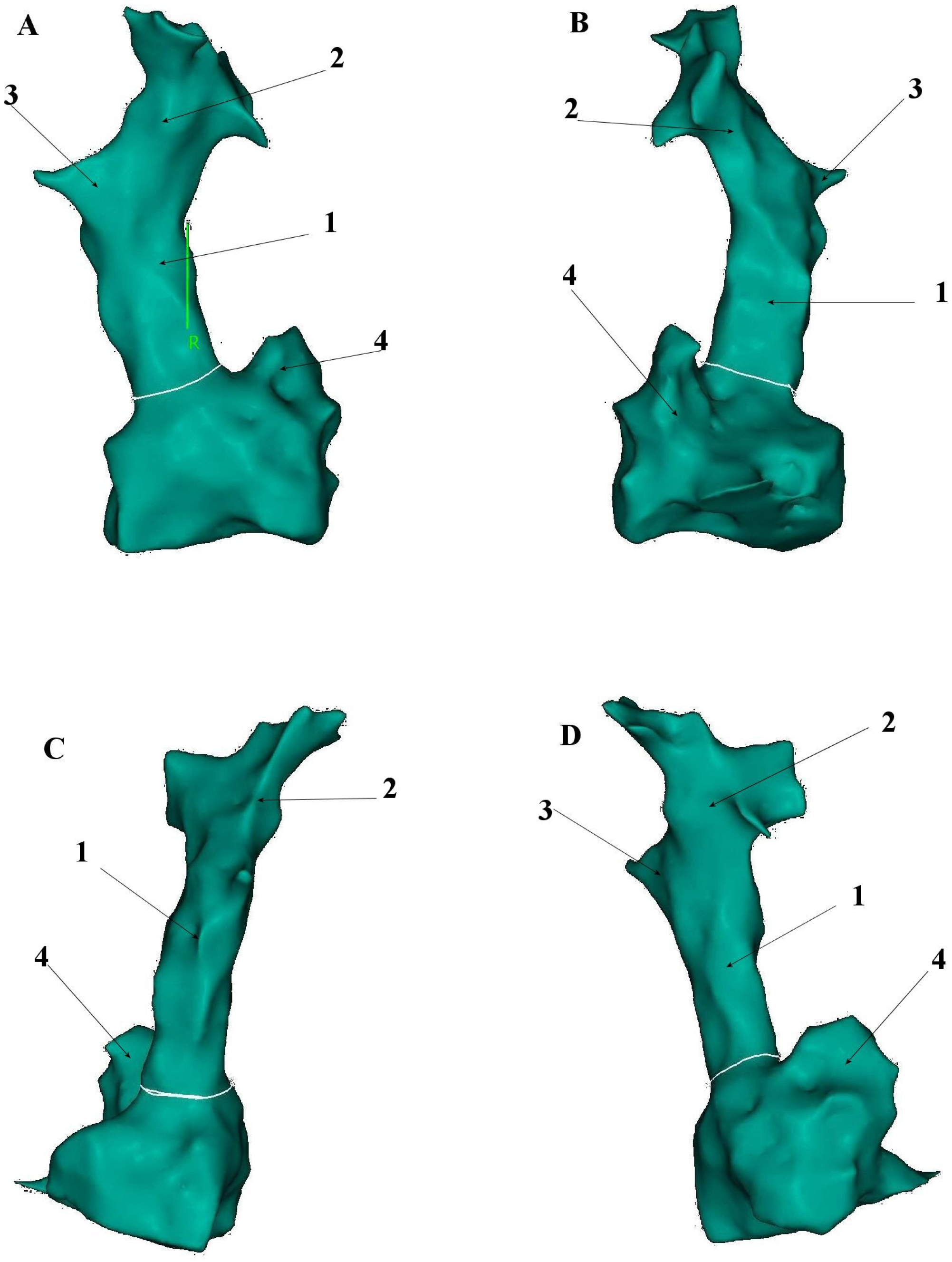
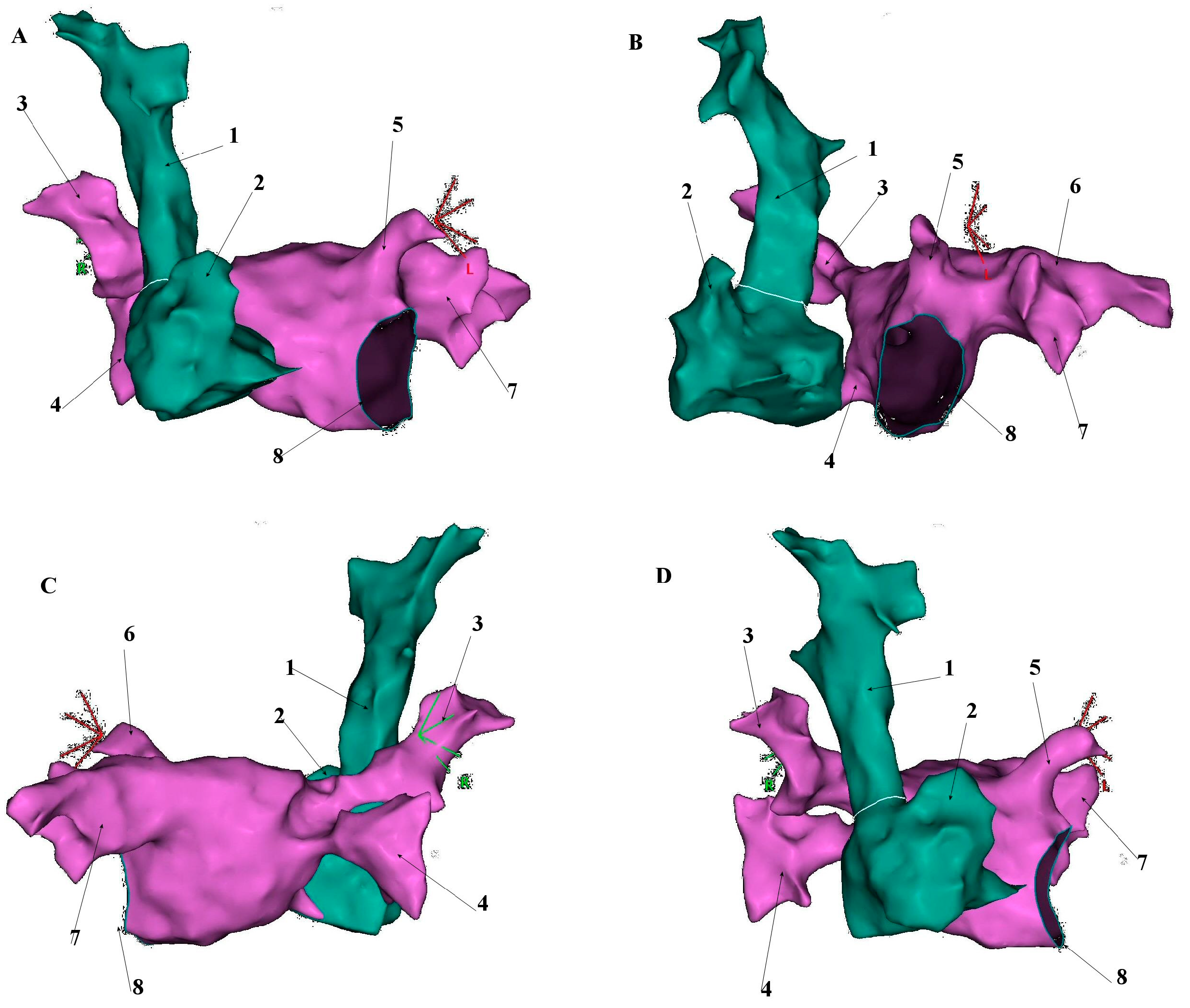
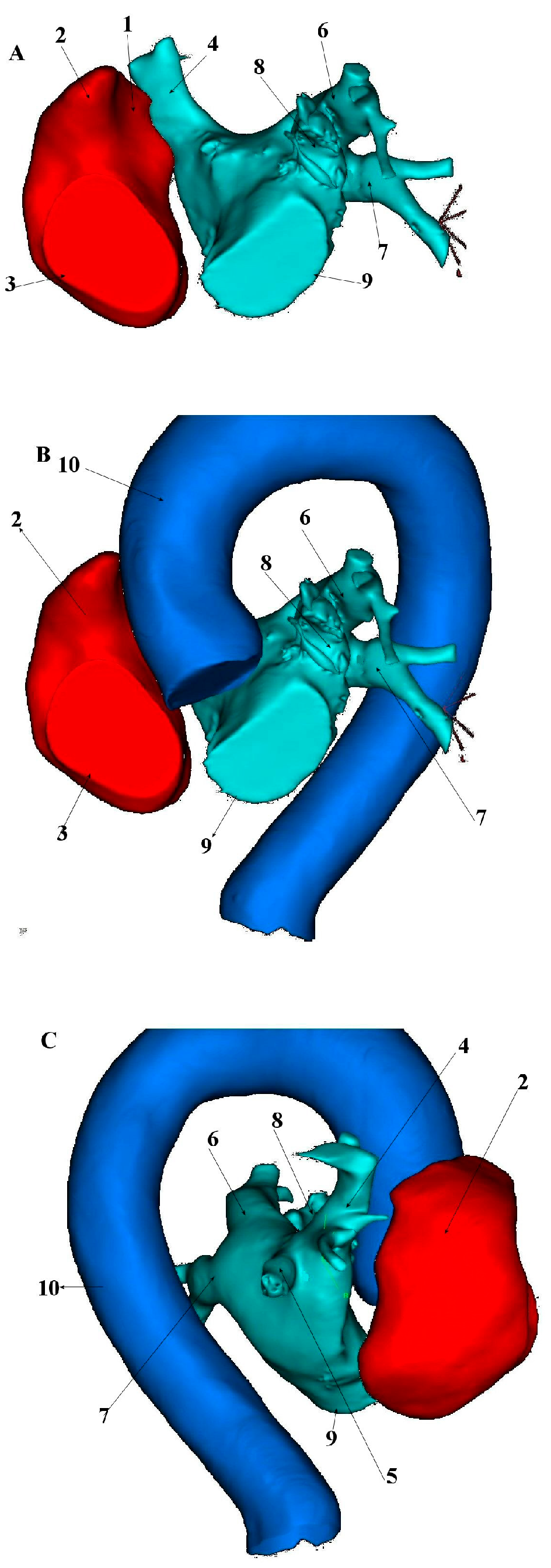
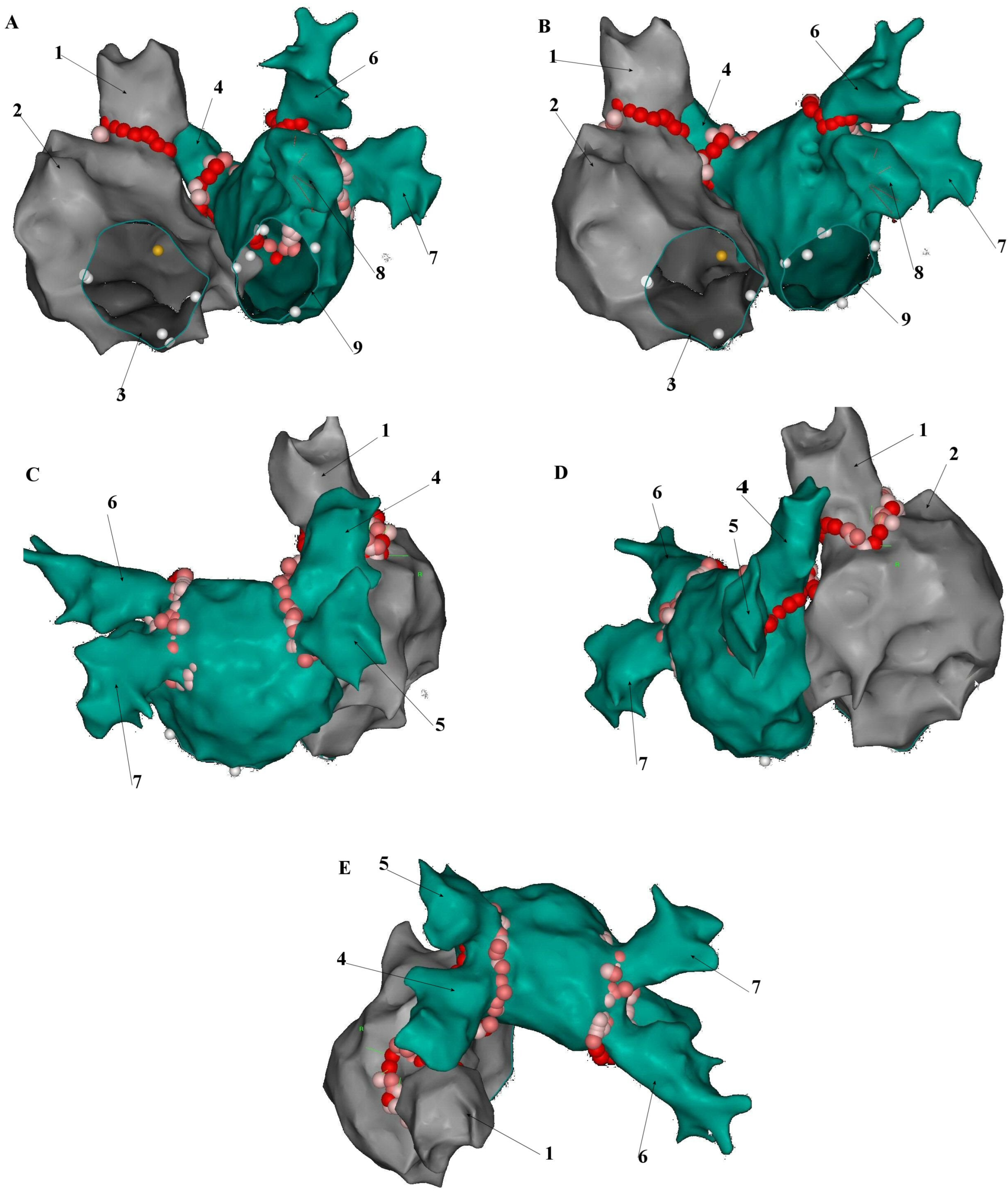
5. Anatomical and Histological Properties of the SVC
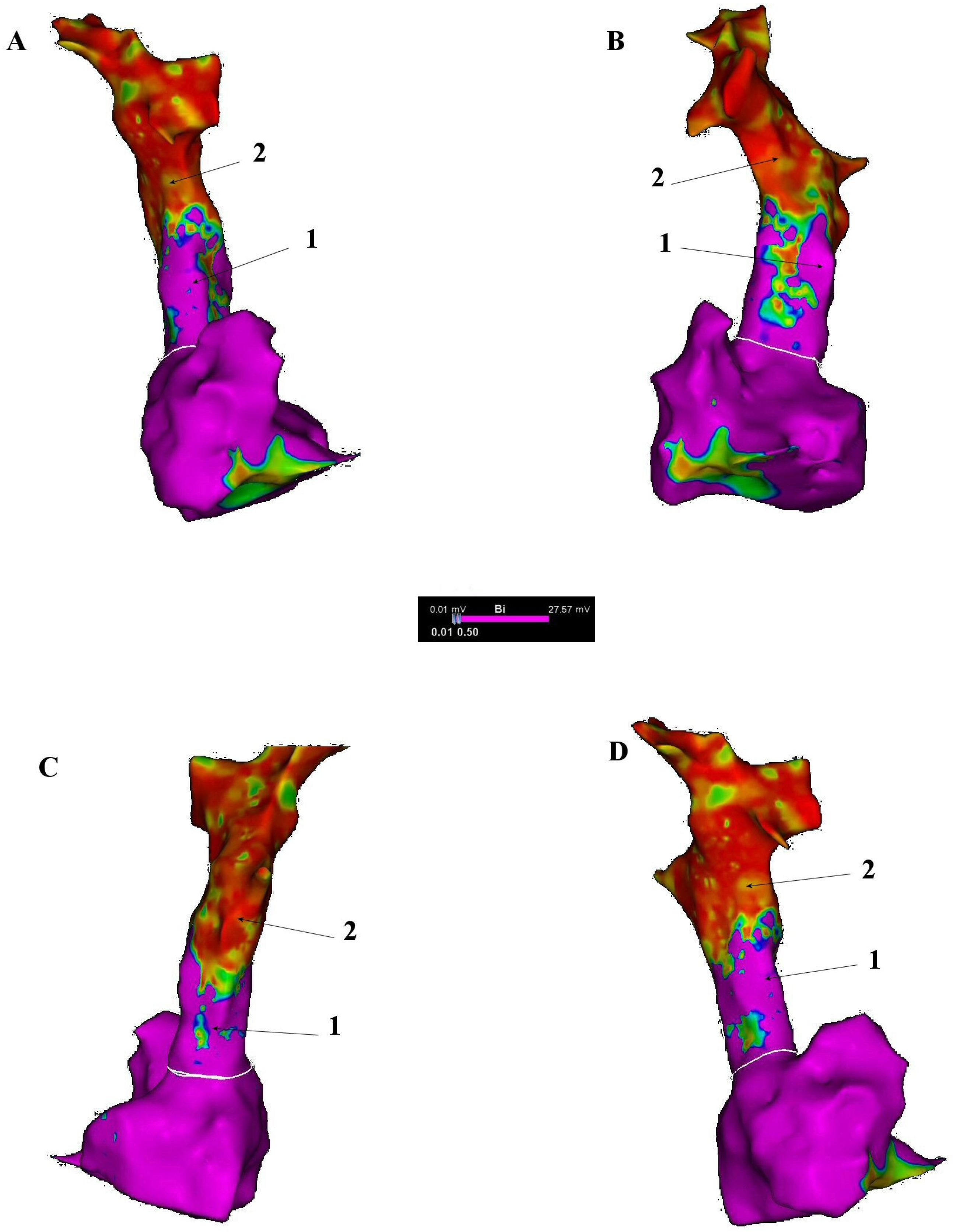
6. Electrophysiological Characteristics of the SVC and Venoatrial Junction
7. Arrhythmogenic Mechanisms Associated with the SVC
8. Electroanatomical Correlations and Clinical Implications
9. Superior Vena Cava Isolation (SVCI)
10. RF Ablation
10.1. SVC Isolation Approaches
10.2. Very High-Power, Short Duration (vHPSD) RF Ablation
10.3. Pulsed-Field Ablation (PFA)
10.4. Cryoballoon Ablation (CBA)
10.5. Complications Associated with Superior Vena Cava Isolation
10.6. Phrenic Nerve Injury (PNI)
10.7. Sinus Node Injury
10.8. SVC Stenosis
11. Conclusions
12. Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AF | atrial fibrillation |
| PV | pulmonary vein |
| SVC | superior vena cava |
| PVI | pulmonary vein isolation |
| LA | left atrium |
| RA | right atrium |
| VAJ | venoatrial junction |
| RAA | right atrial appendage |
| HRA | high right atrium |
| ERP | effective refractory period |
| EADs | early afterdepolarizations |
| DADs | delayed afterdepolarizations |
| SVC-Ao-GP | SVC-aorta ganglionated plexus |
| ICANS | intrinsic cardiac autonomic nervous system |
| ARGP | anterior right GP |
| IRGP | inferior right GP |
| SLGP | superior left GP |
| ILGP | inferior left GP |
| LOM | ligament of Marshall |
| L-SVC | myocardial sleeve in the SVC |
| LAT | local activation time |
| RUPV | right upper pulmonary vein |
| SVCI | superior vena cava isolation |
| RF | radio frequency |
| vHPSD | very high-power, short duration |
| PFA | pulsed-field ablation |
| CBA | cryoballoon ablation |
| PNI | phrenic nerve injury |
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.N.; Shipp, N.J.; Brooks, A.G.; Kuklik, P.; Lau, D.H.; Lim, H.S.; Sullivan, T.; Roberts-Thomson, K.C.; Sanders, P. Long-term outcomes of catheter ablation of atrial fibrillation: A systematic review and meta-analysis. J. Am. Heart Assoc. 2013, 2, e004549. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef]
- Mulder, M.J.; Kemme, M.J.B.; Allaart, C.P. Radiofrequency ablation to achieve durable pulmonary vein isolation. Europace 2022, 24, 874–886. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Oginosawa, Y.; Fujino, Y.; Yagyu, K.; Miyamoto, T.; Tsukahara, K.; Ohe, H.; Kohno, R.; Kataoka, M.; Abe, H. Relationship between Effective Refractory Period and Inducibility of Atrial Fibrillation from the Superior Vena Cava after Pulmonary Vein Isolation. Int. Heart J. 2022, 63, 498–503. [Google Scholar] [CrossRef]
- Della Rocca, D.G.; Tarantino, N.; Trivedi, C.; Mohanty, S.; Anannab, A.; Salwan, A.S.; Gianni, C.; Bassiouny, M.; Al-Ahmad, A.; Romero, J.; et al. Non-pulmonary vein triggers in nonparoxysmal atrial fibrillation: Implications of pathophysiology for catheter ablation. J. Cardiovasc. Electrophysiol. 2020, 31, 2154–2167. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, D.G.; Di Biase, L.; Mohanty, S.; Trivedi, C.; Gianni, C.; Romero, J.; Tarantino, N.; Magnocavallo, M.; Bassiouny, M.; Natale, V.N.; et al. Targeting non-pulmonary vein triggers in persistent atrial fibrillation: Results from a prospective, multicentre, observational registry. Europace 2021, 23, 1939–1949. [Google Scholar] [CrossRef]
- Lin, W.S.; Tai, C.T.; Hsieh, M.H.; Tsai, C.F.; Lin, Y.K.; Tsao, H.M.; Huang, J.L.; Yu, W.C.; Yang, S.P.; Ding, Y.A.; et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation 2003, 107, 3176–3183. [Google Scholar] [CrossRef]
- Takigawa, M.; Takahashi, A.; Kuwahara, T.; Okubo, K.; Takahashi, Y.; Watari, Y.; Nakashima, E.; Nakajima, J.; Yamao, K.; Takagi, K.; et al. Long-term outcome after catheter ablation of paroxysmal atrial fibrillation: Impact of different atrial fibrillation foci. Int. J. Cardiol. 2017, 227, 407–412. [Google Scholar] [CrossRef]
- Kawai, S.; Sakamoto, K.; Takase, S.; Noma, A.; Kisanuki, H.; Nakashima, H.; Watanabe, T.; Sakemi, T.; Okabe, K.; Okahara, A.; et al. Prevalence and distribution of non-pulmonary vein atrial fibrillation triggers in real-world clinical settings. Eur. Heart J. 2022, 43 (Suppl. S2), ehac544.535. [Google Scholar] [CrossRef]
- Miyata, T.; Suzuki, A.; Fujiwara, R.; Sonoda, Y.; Sakai, J.; Kiuchi, K.; Matsumoto, D.; Kijima, Y.; Ogura, E.; Takaishi, H.; et al. Prevalance of incessant form of atrial fibrillation by superior vena cava (svc) firing:simplified svc and sinus node mapping(sss map) to avoid sinus node injury. Eur. Heart J. 2024, 45 (Suppl. S1), ehae666.576. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, D.; Chen, X.; Shi, L.; Chen, Q.; Zhang, H.; Yu, Y.; Ullah, I.; Kojodjojo, P.; Zhang, F. Role of electroanatomical mapping–guided superior vena cava isolation in paroxysmal atrial fibrillation patients without provoked superior vena cava triggers: A randomized controlled study. Europace 2024, 26, euae039. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Chen, S.A.; Tai, C.T.; Tsai, C.F.; Prakash, V.S.; Yu, W.C.; Liu, C.C.; Ding, Y.A.; Chang, M.S. Double multielectrode mapping catheters facilitate radiofrequency catheter ablation of focal atrial fibrillation originating from pulmonary veins. J. Cardiovasc. Electrophysiol. 1999, 10, 136–144. [Google Scholar] [CrossRef]
- Lin, F.Y.; Devereux, R.B.; Roman, M.J.; Meng, J.; Jow, V.M.; Simprini, L.; Jacobs, A.; Weinsaft, J.W.; Shaw, L.J.; Berman, D.S.; et al. The Right Sided Great Vessels by Cardiac Multidetector Computed Tomography. Normative Reference Values among Healthy Adults Free of Cardiopulmonary Disease, Hypertension, and Obesity. Acad. Radiol. 2009, 16, 981–987. [Google Scholar] [CrossRef]
- Sonavane, S.K.; Milner, D.M.; Singh, S.P.; Aal, A.K.A.; Shahir, K.S.; Chaturvedi, A. Comprehensive imaging review of the superior vena cava. Radiographics 2015, 35, 1873–1892. [Google Scholar] [CrossRef]
- DeSimone, C.V.; Noheria, A.; Lachman, N.; Edwards, W.D.; Gami, A.S.; Maleszewski, J.J.; Friedman, P.A.; Munger, T.M.; Hammill, S.C.; Packer, D.L.; et al. Myocardium of the Superior Vena Cava, Coronary Sinus, Vein of Marshall, and the Pulmonary Vein Ostia: Gross Anatomic Studies in 620 Hearts. J. Cardiovasc. Electrophysiol. 2012, 23, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H.; Ushiki, T.; Abe, K. A Histological Study of the Cardiac Muscle of the Human Superior and Inferior Venae Cave. Arch. Histol. Cytol. 1995, 58, 457–464. [Google Scholar] [CrossRef] [PubMed]
- MacEdo, P.G.; Kapa, S.; Mears, J.A.; Fratianni, A.; Asirvatham, S.J. Correlative anatomy for the electrophysiologist: Ablation for atrial fibrillation. Part I: Pulmonary vein ostia, superior vena cava, vein of Marshall. J. Cardiovasc. Electrophysiol. 2010, 21, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Kugler, S.; Tőkés, A.M.; Nagy, N.; Fintha, A.; Danics, K.; Sághi, M.; Törő, K.; Rácz, G.; Nemeskéri, Á. Strong desmin immunoreactivity in the myocardial sleeves around pulmonary veins, superior caval vein and coronary sinus supports the presumed arrhythmogenicity of these regions. J. Anat. 2024, 244, 120–132. [Google Scholar] [CrossRef]
- Kholová, I.; Kautzner, J. Morphology of atrial myocardial extensions into human caval veins: A postmortem study in patients with and without atrial fibrillation. Circulation 2004, 110, 483–488. [Google Scholar] [CrossRef]
- Spach, M.S.; Barr, R.C.; Jewett, P.H. Spread of excitation from the atrium into thoracic veins in human beings and dogs. Am. J. Cardiol. 1972, 30, 844–854. [Google Scholar] [CrossRef]
- Fukumoto, K.; Takatsuki, S.; Kimura, T.; Nishiyama, N.; Tanimoto, K.; Aizawa, Y.; Tanimoto, Y.; Fukuda, Y.; Miyoshi, S.; Fukuda, K. Electrophysiological properties of the superior vena cava and venoatrial junction in patients with atrial fibrillation: Relevance to catheter ablation. J. Cardiovasc. Electrophysiol. 2014, 25, 16–22. [Google Scholar] [CrossRef]
- Yeh, H.I.; Lai, Y.J.; Lee, S.H.; Lee, Y.N.; Ko, Y.S.; Chen, S.A.; Severs, N.J.; Tsai, C.H. Heterogeneity of myocardial sleeve morphology and gap junctions in canine superior vena cava. Circulation 2001, 104, 3152–3157. [Google Scholar] [CrossRef]
- Lee, K.T.; Chu, C.S.; Lin, T.H.; Yen, H.W.; Voon, W.C.; Sheu, S.H.; Lai, W.T. Effects of verapamil on superior vena cava electrical remodeling induced by short-term pacing from right atrium and superior vena cava in human. Int. J. Cardiol. 2007, 120, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.A.; Hsieh, M.H.; Tai, C.T.; Tsai, C.F.; Prakash, V.S.; Yu, W.C.; Hsu, T.L.; Ding, Y.A.; Chang, M.S. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: Electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 1999, 100, 1879–1886. [Google Scholar] [CrossRef]
- Lee, S.H.; Chen, Y.J.; Tai, C.T.; Yeh, H.I.; Cheng, J.J.; Hung, C.R.; Chen, S.A. Electrical remodeling of the canine superior vena cava after chronic rapid atrial pacing. Basic Res. Cardiol. 2005, 100, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, H.L.; Yousufuddin, M.; Lieving, W.R.; Watson, B.E.; Malas, A.; Rosencrance, G.; McCowan, R.J. Persistent left superior vena cava: Case reports and clinical implications. Int. J. Cardiol. 2006, 113, 242–246. [Google Scholar] [CrossRef]
- James, T.N.; Marshall, T.K.; Edwards, J.E. De subitaneis mortibus. XX. Cardiac electrical instability in the presence of a left superior vena cava. Circulation 1976, 54, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Ogawa, M.; Noguchi, H.; Yasuda, T.; Nakashima, H.; Saku, K. Electrophysiologic properties of pulmonary veins assessed using a multielectrode basket catheter. J. Am. Coll. Cardiol. 2004, 43, 2281–2289. [Google Scholar] [CrossRef]
- Tada, H.; Oral, H.; Ozaydin, M.; Greenstein, R.; Pelosi, F., Jr.; Knight, B.P.; Strickberger, S.A.; Morady, F. Response of Pulmonary Vein Potentials to Premature Stimulation. J. Cardiovasc. Electrophysiol. 2002, 13, 33–37. [Google Scholar] [CrossRef]
- Jaïs, P.; Hocini, M.; Macle, L.; Choi, K.J.; Deisenhofer, I.; Weerasooriya, R.; Shah, D.C.; Garrigue, S.; Raybaud, F.; Scavee, C.; et al. Distinctive electrophysiological properties of pulmonary veins in patients with atrial fibrillation. Circulation 2002, 106, 2479–2485. [Google Scholar] [CrossRef]
- Lewalter, T.; Burkhardt, D.; Chun, S.; Schimpf, R.; Bielik, H.; Schrickell, J.; Shlevkov, N.; Yang, A.; Lüderitz, B. Decremental pulmonary venous pulse propagation: Impact for catheter ablation in focal atrial fibrillation. J. Interv. Card. Electrophysiol. 2003, 9, 269–273. [Google Scholar] [CrossRef]
- Sicouri, S.; Blazek, J.; Belardinelli, L.; Antzelevitch, C. Electrophysiological characteristics of canine superior vena cava sleeve preparations: Effect of ranolazine. Circ. Arrhythm. Electrophysiol. 2012, 5, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Chen, S.A.; Chang, M.S.; Lin, C.I. Arrhythmogenic activity of cardiac muscle in pulmonary veins of the dog: Implication for the genesis of atrial fibrillation. Cardiovasc. Res. 2000, 48, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Chen, S.A.; Chen, Y.C.; Yeh, H.I.; Chan, P.; Chang, M.S.; Lin, C.I. Effects of rapid atrial pacing on the arrhythmogenic activity of single cardiomyocytes from pulmonary veins: Implication in initiation of atrial fibrillation. Circulation 2001, 104, 2849–2854. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Chen, Y.C.; Yeh, H.I.; Lin, C.I.; Chen, S.A. Electrophysiology and arrhythmogenic activity of single cardiomyocytes from canine superior vena cava. Circulation 2002, 105, 2679–2685. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Vinogradova, T.; Lyashkov, A.; Sirenko, S.; Zhu, W.; Ruknudin, A.; Maltsev, V.A. The integration of spontaneous intracellular Ca2+ cycling and surface membrane ion channel activation entrains normal automaticity in cells of the heart’s pacemaker. Ann. N. Y. Acad. Sci. 2006, 1080, 178–206. [Google Scholar] [CrossRef]
- Lu, Z.; Scherlag, B.J.; Niu, G.; Lin, J.; Fung, K.M.; Zhao, L.; Yu, L.; Jackman, W.M.; Lazzara, R.; Jiang, H.; et al. Functional properties of the superior vena cava (SVC)-aorta ganglionated plexus: Evidence suggesting an autonomic basis for rapid SVC firing. J. Cardiovasc. Electrophysiol. 2010, 21, 1392–1399. [Google Scholar] [CrossRef]
- Lu, Z.; Scherlag, B.J.; Lin, J.; Yu, L.; Guo, J.H.; Niu, G.; Jackman, W.M.; Lazzara, R.; Jiang, H.; Po, S.S. Autonomic mechanism for initiation of rapid firing from atria and pulmonary veins: Evidence by ablation of ganglionated plexi. Cardiovasc. Res. 2009, 84, 245–252. [Google Scholar] [CrossRef]
- Nyuta, E.; Takemoto, M.; Sakai, T.; Mito, T.; Masumoto, A.; Todoroki, W.; Yagyu, K.; Ueno, J.; Antoku, Y.; Koga, T.; et al. Importance of the length of the myocardial sleeve in the superior vena cava in patients with atrial fibrillation. J. Arrhythm. 2021, 37, 43–51. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Liu, Y.; Chen, Q.; Chen, H.; Ju, W.; Yang, G.; Zhu, Y.; Zhao, P.; Zhang, J.; et al. Three-dimensional electroanatomic mapping characteristics of superior vena cava myocardial sleeve and sinoatrial node in patients with atrial fibrillation. Front. Cardiovasc. Med. 2022, 9, 902828. [Google Scholar] [CrossRef] [PubMed]
- Kiedrowicz, R.M.; Wielusiński, M.; Peregud-Pogorzelska, M.; Kaźmierczak, J. Conduction from the arrhythmogenic right upper pulmonary vein to superior vena cava can induce atrial fibrillation. Kardiol. Pol. 2019, 77, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.V.; Palombi, M.; Jabbour, J.P.; Pierucci, N.; Cipollone, P.; Piro, A.; Chimenti, C.; Miraldi, F.; Vizza, C.D.; Lavalle, C.; et al. Usefulness of empiric superior vena cava isolation in paroxysmal atrial fibrillation ablation: A meta-analysis of randomized clinical trials. J. Interv. Card. Electrophysiol. 2025, 68, 93–100. [Google Scholar] [CrossRef]
- Gu, Z.; Yang, G.; Ju, W.; Li, M.; Chen, H.; Gu, K.; Liu, H.; Chen, M. Empirical superior vena cava isolation improves outcomes of radiofrequency re-ablation in pulmonary vein isolation non-responders: A 2-center retrospective study in China. Front. Cardiovasc. Med. 2022, 9, 1049414. [Google Scholar] [CrossRef]
- Oguri, N.; Okubo, Y.; Ishibashi, N.; Maeda, J.; Sakai, T.; Uotani, Y.; Furutani, M.; Miyamoto, S.; Miyauchi, S.; Okamura, S.; et al. Novel omnipolar mapping technology for effective superior vena cava isolation: A randomized clinical trial. J. Arrhythm. 2025, 41, e70007. [Google Scholar] [CrossRef]
- Haghjoo, M. Efficacy, safety, and role of segmental superior vena cava isolation in the treatment of atrial fibrillation. J. Electrocardiol. 2007, 40, 327.e1. [Google Scholar] [CrossRef]
- Chen, C.K.; Yu, C.C. Effective superior vena cava isolation using a novel C-shaped approach. Front. Cardiovasc. Med. 2023, 10, 1253912. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Okajima, S.; Hachisuka, M.; Hagiwara, K.; Murata, H.; Aizawa, Y.; Yodogawa, K.; Shimizu, W.; Asai, K.; Iwasaki, Y.; et al. Safety and efficacy of very high-power short ablation in superior vena cava isolation in patients with atrial fibrillation. EP Eur. 2024, 26 (Suppl. S1), euae102.188. [Google Scholar] [CrossRef]
- Ollitrault, P.; Chaumont, C.; Font, J.; Manninger, M.; Conti, S.; Matusik, P.T.; Mulder, B.A.; Ferchaud, V.; Pellissier, A.; Al Khoury, M.; et al. Superior vena cava isolation using a pentaspline pulsed-field ablation catheter: Feasibility and safety in patients undergoing atrial fibrillation catheter ablation. Europace 2024, 26, euae160. [Google Scholar] [CrossRef]
- Mansour, M.; Mohanty, S.; Natale, A. Quest for safe and feasible isolation of superior vena cava by pulsed-field ablation: Are we there yet? Europace 2024, 26, euae159. [Google Scholar] [CrossRef]
- Castro-Urda, V.; Segura-Dominguez, M.; Jiménez-Sánchez, D.; Aguilera-Agudo, C.; Vela-Martín, P.; Lorente-Ros, A.; García-Rodriguez, D.; Sánchez-Ortiz, D.; Pham-Trung, C.; García-Izquierdo, E.; et al. Superior Vena Cava Isolation with Cryoballoon in AF Ablation: Randomized CAVAC AF Trial. Circ. Arrhythm. Electrophysiol. 2025, 18, e012917. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, A.; Levallois, M.; Romeyer-Bouchard, C.; Bisch, L.; Gate-Martinet, A.; Isaaz, K. Remote-controlled magnetic pulmonary vein isolation combined with superior vena cava isolation for paroxysmal atrial fibrillation: A prospective randomized study. Arch. Cardiovasc. Dis. 2015, 108, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, R.; Peng, X.; Wang, X.; Li, Y.; Liu, X.; Wang, W.; Yu, R.; Bai, R.; Ma, C.; et al. Visualization and mapping of the right phrenic nerve by intracardiac echocardiography during atrial fibrillation ablation. Europace 2023, 25, 1352–1360. [Google Scholar] [CrossRef]
- Yamaji, H.; Higashiya, S.; Murakami, T.; Kawamura, H.; Murakami, M.; Kamikawa, S.; Kusachi, S. Optimal prevention method of phrenic nerve injury in superior vena cava isolation: Efficacy of high-power, short-duration radiofrequency energy application on the risk points. J. Interv. Card. Electrophysiol. 2023, 66, 1465–1475. [Google Scholar] [CrossRef]
- Killu, A.M.; Fender, E.A.; Deshmukh, A.J.; Munger, T.M.; Araoz, P.; Brady, P.A.; Cha, Y.M.; Packer, D.L.; Friedman, P.A.; Asirvatham, S.J.; et al. Acute Sinus Node Dysfunction after Atrial Ablation: Incidence, Risk Factors, and Management. Pacing Clin. Electrophysiol. 2016, 39, 1116–1125. [Google Scholar] [CrossRef]
- Kühne, M.; Schaer, B.; Osswald, S.; Sticherling, C. Superior vena cava stenosis after radiofrequency catheter ablation for electrical isolation of the superior vena cava. Pacing Clin. Electrophysiol. 2010, 33, e36–e38. [Google Scholar] [CrossRef]
- Callans, D.J.; Ren, J.F.; Schwartzman, D.; Gottlieb, C.D.; Chaudhry, F.A.; Marchlinski, F.E. Narrowing of the superior vena cava-right atrium junction during radiofrequency catheter ablation for inappropriate sinus tachycardia: Analysis with intracardiac echocardiography. J. Am. Coll. Cardiol. 1999, 33, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Patients Included | Global Number of Non-PV Triggers | SVC Arrhythmogenic Foci in Non-PV Triggers Cohort | SVC Arrhythmogenic Foci in Overall Cohort |
|---|---|---|---|---|
| Lin et al. (2003) [8] | 240 (paroxysmal AF) | 73/240 (30.4%) | 27/73 (37.0%) | 27/240 (11.3%) |
| Takigawa et al. (2017) [9] | 865 (paroxysmal AF) | 125/865 (14.5%) | 68/125 (45.6%) | 68/865 (7.9%) |
| Kawai et al. (2022) [10] | 1020 (paroxysmal + non-paroxysmal AF) | 126/1020 (12.4%) | 29/126 (23%) | 29/1020 (2.8%) |
| Miyata et al. (2024) [11] | 1089 patients/1160 procedures (paroxysmal + non-paroxysmal AF) | 160/1160 (13.8%) | 64/160 (40%) | 64/1160 (5.5%) |
| Dong et al. (2024) [12] | 130 (paroxysmal AF) | 43/130 (33.1%) | 30/43 (69.8%) | 30/130 (23%) |
| Ablation Technique | Clinical Data | ||||||
|---|---|---|---|---|---|---|---|
| Procedural Time | Radiation Exposure | Acute Success Rate | Long-Term Success Rate | Phrenic Nerve Palsy | Sinus Node Dysfunction | SVC Stenosis | |
| Point-by-point low-power RF ablation integrated with 3d mapping system | +++ | + | + | +++ | +++ | +++ | + |
| Point-by-point very high-power short duration RF ablation integrated with 3d mapping system | ++ | + | ++ | ++ | ++ | ++ | + |
| Single Shot Pulsed Field Ablation under fluoroscopy | + | +++ | +++ | data pending | + | + | + |
| Cryoballoon ablation | + | ++ | + | + | ++++ | ++++ | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakrzewski, S.; Wielusinski, M.; Kazmierczak, J.; Duda, L.; Hoppe, W.; Bazylewicz-Zakrzewska, K.; Kladna, A.; Kiedrowicz, R.M. Superior Vena Cava as an Arrhythmogenic Substrate in Atrial Fibrillation: Anatomical, Electrophysiological and Clinical Perspectives. J. Clin. Med. 2025, 14, 7456. https://doi.org/10.3390/jcm14217456
Zakrzewski S, Wielusinski M, Kazmierczak J, Duda L, Hoppe W, Bazylewicz-Zakrzewska K, Kladna A, Kiedrowicz RM. Superior Vena Cava as an Arrhythmogenic Substrate in Atrial Fibrillation: Anatomical, Electrophysiological and Clinical Perspectives. Journal of Clinical Medicine. 2025; 14(21):7456. https://doi.org/10.3390/jcm14217456
Chicago/Turabian StyleZakrzewski, Szymon, Maciej Wielusinski, Jaroslaw Kazmierczak, Lukasz Duda, Wiktoria Hoppe, Katarzyna Bazylewicz-Zakrzewska, Aleksandra Kladna, and Radoslaw Marek Kiedrowicz. 2025. "Superior Vena Cava as an Arrhythmogenic Substrate in Atrial Fibrillation: Anatomical, Electrophysiological and Clinical Perspectives" Journal of Clinical Medicine 14, no. 21: 7456. https://doi.org/10.3390/jcm14217456
APA StyleZakrzewski, S., Wielusinski, M., Kazmierczak, J., Duda, L., Hoppe, W., Bazylewicz-Zakrzewska, K., Kladna, A., & Kiedrowicz, R. M. (2025). Superior Vena Cava as an Arrhythmogenic Substrate in Atrial Fibrillation: Anatomical, Electrophysiological and Clinical Perspectives. Journal of Clinical Medicine, 14(21), 7456. https://doi.org/10.3390/jcm14217456






