Short-Term Outcomes of Micropulse Transscleral Cyclophotocoagulation Using the VITRA 810 and Postoperative Ciliochoroidal Effusion in Japanese Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Surgical Technique
2.3. Data Collection and Statistical Analysis
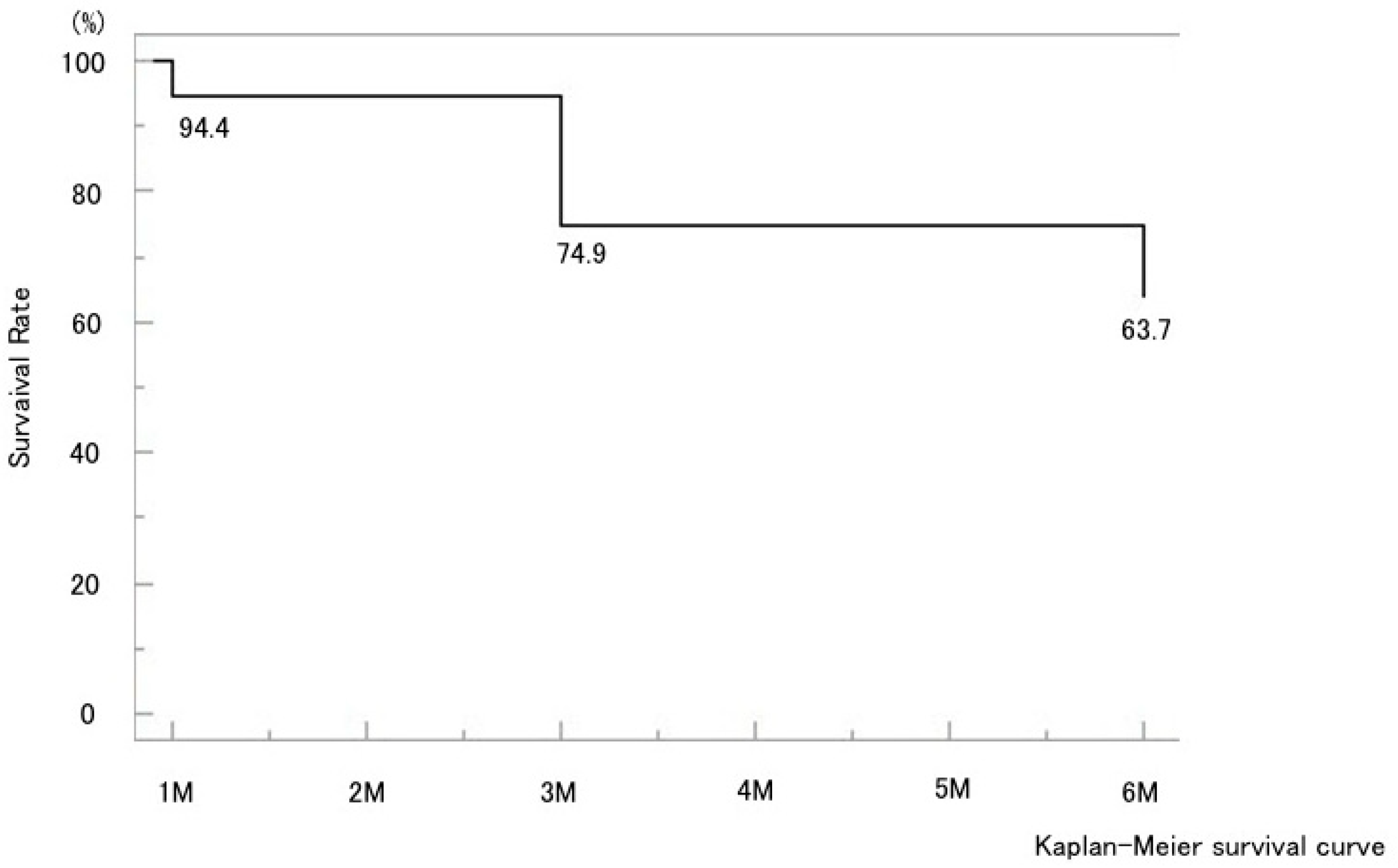
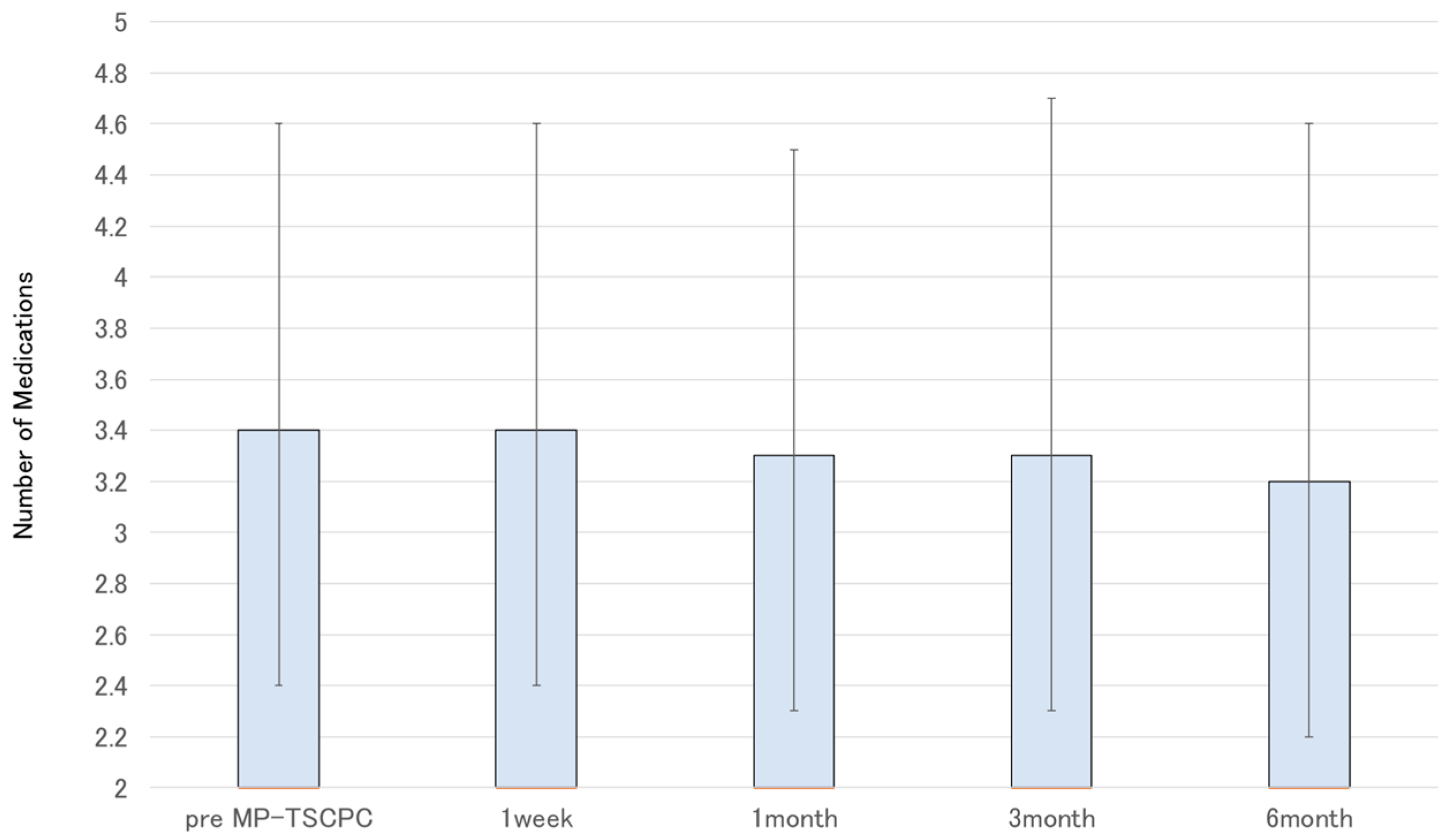
3. Results
3.1. Overall Outcomes
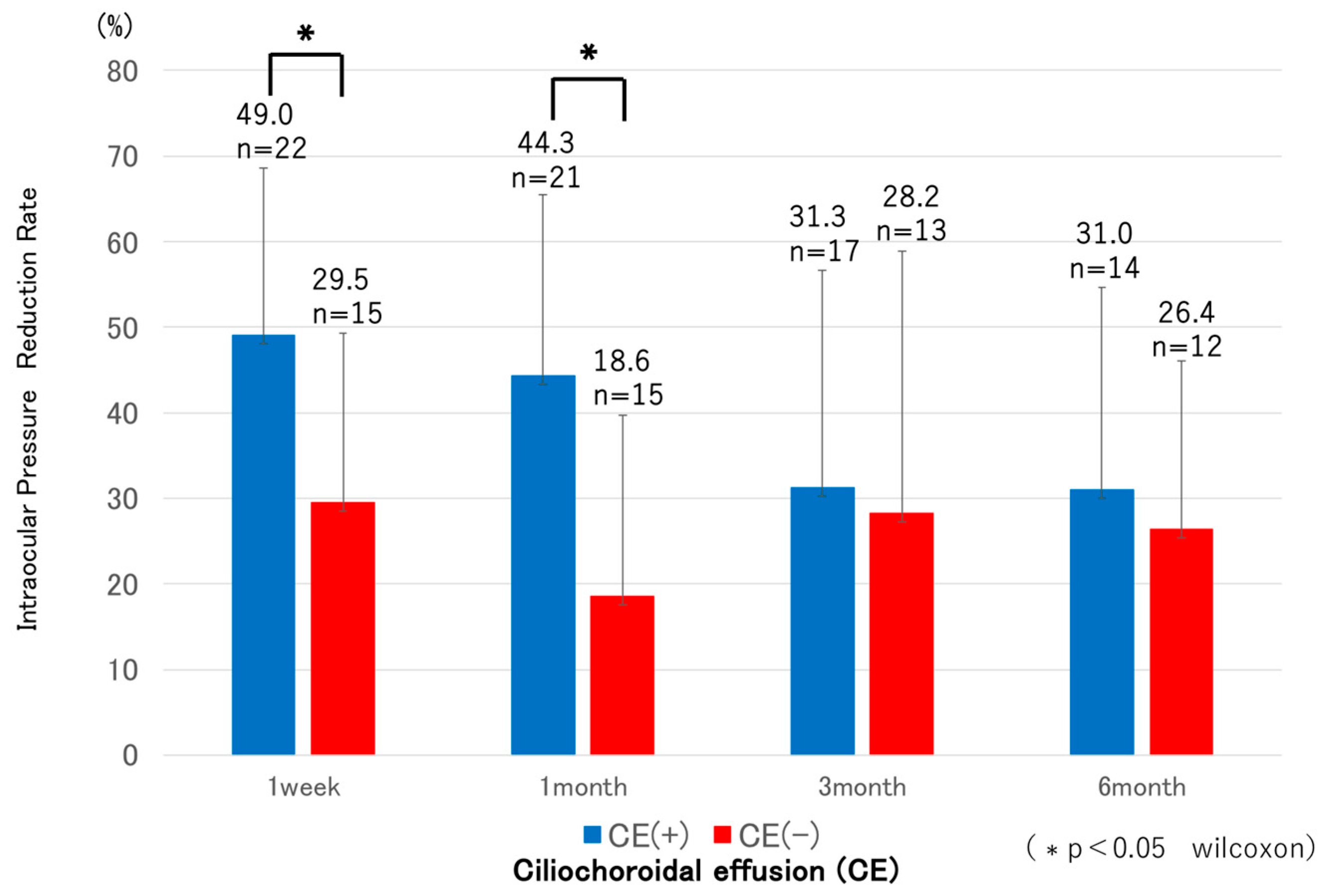
3.2. Comparison Between CE(+) and CE(−) Groups
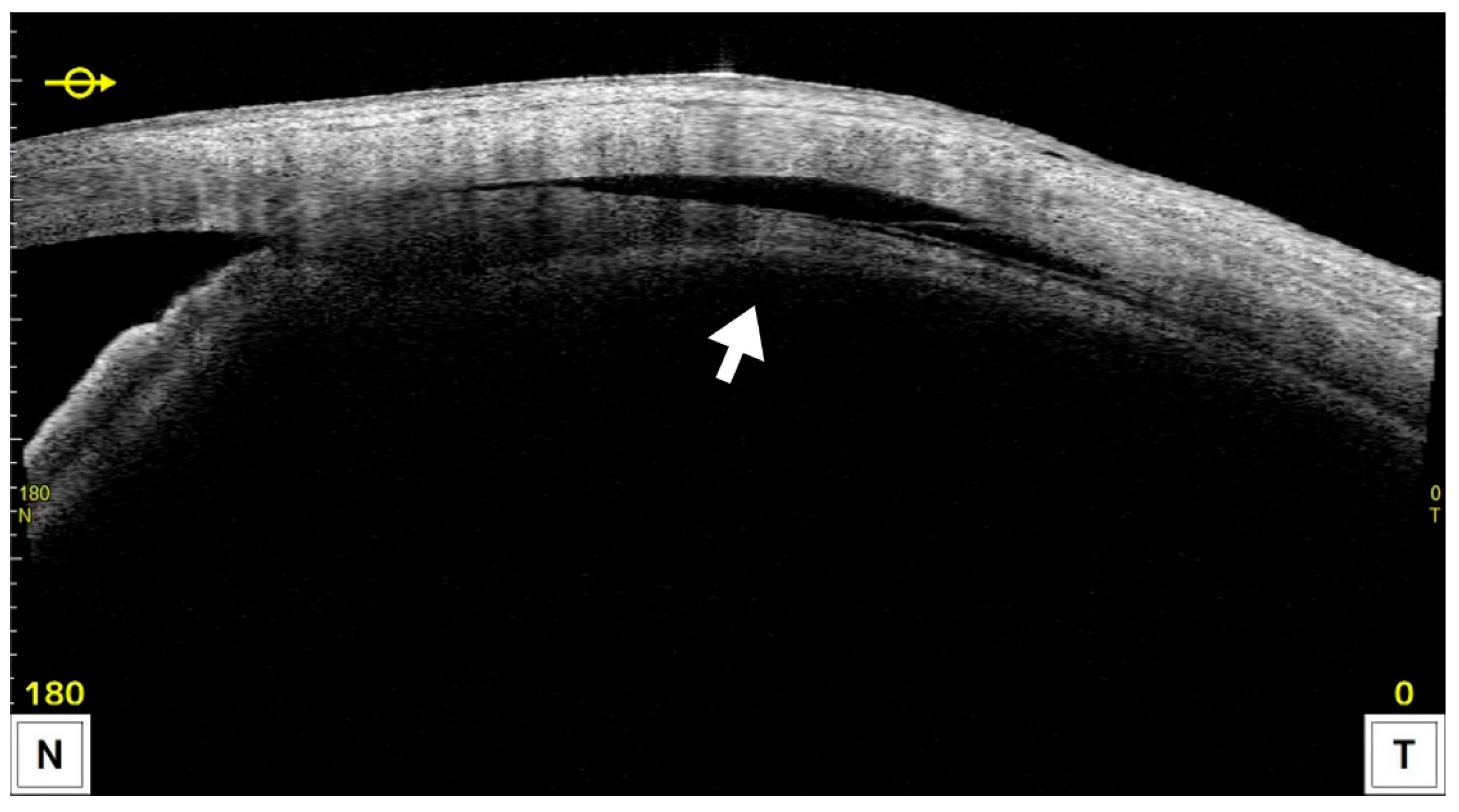
3.3. Postoperative Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quigley, H.A. Glaucoma. Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Matoba, R.; Morimoto, N.; Kawasaki, R.; Fujiwara, M.; Kanenaga, K.; Yamashita, H.; Sakamoto, T.; Morizane, Y. A nationwide survey of newly certified visually impaired individuals in Japan for the fiscal year 2019: Impact of the revision of criteria for visual impairment certification. Jpn. J. Ophthalmol. 2023, 67, 346–352. [Google Scholar] [CrossRef]
- The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am. J. Ophthalmol. 2000, 130, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Bloom, P.A.; Tsai, J.C.; Sharma, K.; Miller, M.H.; Rice, N.S.; Hitchings, R.A.; Khaw, P.T. “Cyclodiode”. Transscleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology 1997, 104, 1508–1520. [Google Scholar] [CrossRef] [PubMed]
- Gazzard, G.; Konstantakopoulou, E.; Garway-Heath, D.; Adeleke, M.; Vickerstaff, V.; Ambler, G.; Hunter, R.; Bunce, C.; Nathwani, N.; Barton, K.; et al. Laser in Glaucoma and Ocular Hypertension (LiGHT) Trial: Six-Year Results of Primary Selective Laser Trabeculoplasty versus Eye Drops for the Treatment of Glaucoma and Ocular Hypertension. Ophthalmology 2023, 130, 181–190. [Google Scholar] [CrossRef]
- De Vries, V.A.; Pals, J.; Poelman, H.J.; Rostamzad, P.; Wolfs, R.C.W.; Ramdas, W.D. Efficacy and safety of micropulse transscleral cyclophotocoagulation. J. Clin. Med. 2022, 11, 3447. [Google Scholar] [CrossRef]
- Grippo, T.M.; de Crom, R.M.P.C.; Giovingo, M.; Töteberg-Harms, M.; Francis, B.A.; Jerkins, B.; Brubaker, J.W.; Radcliffe, N.; An, J.; Noecker, R. Evidence-based consensus guidelines series for micropulse transscleral laser therapy: Dosimetry and patient selection. Clin. Ophthalmol. 2022, 16, 1837–1846. [Google Scholar] [CrossRef]
- Valle, I.T.; Bazarra, S.P.; Taboas, M.F.; Cid, S.R.; Diaz, M.D.A. Medium-term outcomes of micropulse transscleral cyclophotocoagulation in refractory glaucoma. J. Curr. Glaucoma Pract. 2022, 16, 91–95. [Google Scholar] [CrossRef]
- Chen, H.S.; Yeh, P.H.; Yeh, C.T.; Su, W.W.; Lee, Y.S.; Chuang, L.H.; Shen, S.H.; Wu, W.C. Micropulse transscleral cyclophotocoagulation in a Taiwanese population: 2-year clinical outcomes and prognostic factors. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 1265–1273. [Google Scholar] [CrossRef]
- Vig, N.; Ameen, S.; Bloom, P.; Crawley, L.; Normando, E.; Porteous, A.; Ahmed, F. Micropulse transscleral cyclophotocoagulation: Initial results using a reduced energy protocol in refractory glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1073–1079. [Google Scholar] [CrossRef]
- Yelenskiy, A.; Gillette, T.B.; Arosemena, A.; Stern, A.G.; Garris, W.J.; Young, C.T.; Hoyt, M.; Worley, N.; Zurakowski, D.; Ayyala, R.S. Patient outcomes following micropulse transscleral cyclophotocoagulation: Intermediate-term results. J. Glaucoma 2018, 27, 920–925. [Google Scholar] [CrossRef]
- Chamard, C.; Bachouchi, A.; Daien, V.; Villain, M. Efficacy, safety, and retreatment benefit of micropulse transscleral cyclophotocoagulation in glaucoma. J. Glaucoma 2021, 30, 781–788. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Wan, J.; Tran, B.; Thai, A.; Hernandez-Siman, J.; Chen, K.; Nguyen, N.; Pickering, T.D.; Tanaka, H.G.; Lieberman, M.; et al. Micropulse cyclophotocoagulation: A multicenter study of efficacy, safety, and factors associated with increased risk of complications. J. Glaucoma 2020, 29, 1126–1131. [Google Scholar] [CrossRef]
- Issiaka, M.; Zrikem, K.; Mchachi, A.; Benhmidoune, L.; Rachid, R.; El Belhadji, M.; Youssoufou, S.A.S.; Amza, A. Micropulse diode laser therapy in refractory glaucoma. Adv. Ophthalmol. Pract. Res. 2022, 3, 23–28. [Google Scholar] [CrossRef]
- Williams, A.L.; Moster, M.R.; Rahmatnejad, K.; Reynolds, M.; Horan, T.; Waisbourd, M. Clinical efficacy and safety profile of micropulse transscleral cyclophotocoagulation in refractory glaucoma. J. Glaucoma 2018, 27, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.J.Y.; Cecilia, A.M.; Lim, D.K.A.; Sng, C.C.A.; Loon, S.C.; Lun, K.W.X.; Chew, P.T.K.; Koh, V.T.C. Clinical efficacy and safety outcomes of micropulse transscleral diode cyclophotocoagulation in patients with advanced glaucoma. J. Glaucoma 2021, 30, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Kelada, M.; Normando, E.M.; Cordeiro, F.M.; Crawley, L.; Ahmed, F.; Ameen, S.; Vig, N.; Bloom, P. Cyclodiode versus micropulse transscleral laser treatment. Eye 2024, 38, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Varikuti, V.N.V.; Shah, P.; Rai, O.; Chaves, A.C.; Miranda, A.; Lim, B.A.; Dorairaj, S.K.; Sieminski, S.F. Outcomes of micropulse transscleral cyclophotocoagulation in eyes with good central vision. J. Glaucoma 2019, 28, 901–905. [Google Scholar] [CrossRef]
- Nemoto, H.; Honjo, M.; Okamoto, M.; Sugimoto, K.; Aihara, M. Potential mechanisms of intraocular pressure reduction by micropulse transscleral cyclophotocoagulation in rabbit eyes. Investig. Ophthalmol. Vis. Sci. 2022, 63, 3. [Google Scholar] [CrossRef]
- Desmettre, T.J.; Mordon, S.R.; Buzawa, D.M.; Mainster, M.A. Micropulse and continuous wave diode retinal photocoagulation: Visible and subvisible lesion parameters. Br. J. Ophthalmol. 2006, 90, 709–712. [Google Scholar] [CrossRef]
- Liu, G.J.; Mizukawa, A.; Okisaka, S. Mechanism of intraocular pressure decrease after contact transscleral continuous-wave Nd:YAG laser cyclophotocoagulation. Ophthalmic Res. 1994, 26, 65–79. [Google Scholar] [CrossRef]
- Tsujisawa, T.; Ishikawa, H.; Uga, S.; Asakawa, K.; Kono, Y.; Mashimo, K.; Shoji, N. Morphological changes and potential mechanisms of intraocular pressure reduction after micropulse transscleral cyclophotocoagulation in rabbits. Ophthalmic Res. 2022, 65, 595–602. [Google Scholar] [CrossRef]
- Sanchez, F.G.; Perirano-Bonomi, J.C.; Grippo, T.M. Micropulse transscleral cyclophotocoagulation: A hypothesis for the ideal parameters. Med. Hypothesis Discov. Innov. Ophthalmol. 2018, 7, 94–100. [Google Scholar]
- Johnstone, M.A. Microscope real-time video (MRTV), highresolution OCT (HR-OCT) & histopathology (HP) to assess how transscleral micropulse laser (TML) affects the sclera, ciliary body (CB), muscle (CM), secretory epithelium (CBSE), suprachoroidal space (SCS) & aqueous. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2825. [Google Scholar]
- Ahn, S.M.; Choi, M.; Kim, S.W.; Kim, Y.Y. Changes after a month following micropulse cyclophotocoagulation in normal porcine eyes. Transl. Vis. Sci. Technol. 2021, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Barac, R.; Vuzitas, M.; Balta, F. Choroidal thickness increase after micropulse transscleral cyclophotocoagulation. Rom. J. Ophthalmol. 2018, 62, 144–148. [Google Scholar] [CrossRef]
- Chansangpetch, S.; Taechajongjintana, N.; Ratanawongphaibul, K.; Itthipanichpong, R.; Manassakarn, A.; Tantiseve, V.; Rojanapongpun, P.; Lin, S.C. Ciliochoroidal effusion and its association with the outcomes of micropulse transscleral laser therapy in glaucoma patients: A pilot study. Sci. Rep. 2022, 12, 16403. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.J.C.; Li, K.K.K.; Tang, S.W.K. Retrospective review on the outcome and safety of transscleral diode laser cyclophotocoagulation in refractory glaucoma in Chinese patients. Int. Ophthalmol. 2019, 39, 41–46. [Google Scholar] [CrossRef]
- Kaushik, S.; Pandav, S.S.; Jain, R.; Ansal, S.; Gupta, A. Lower energy levels adequate for effective transscleral diode laser cyclophotocoagulation in Asian eyes with refractory glaucoma. Eye 2008, 22, 398–405. [Google Scholar] [CrossRef]
- Cantor, L.B.; Nichols, D.A.; Katz, L.J.; Moster, M.R.; Poryzees, E.; Shields, J.A.; Spaeth, G.L. Neodymium-YAG transscleral cyclophotocoagulation. The role of pigmentation. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1834–1837. [Google Scholar]



| Parameter | Value |
|---|---|
| Number of eyes, n | 37 |
| Age, years | 79.3 ± 9.5 |
| Male/Female, n | 18/19 |
| BCVA, logMAR | 0.4 ± 0.5 |
| Visual field MD, dB | −18.8 ± 9.7 |
| IOP, mmHg | 22.8 ± 7.1 |
| Number of medications | 3.4 ± 1.2 |
| Endothelial cell density (ECD), cells/mm2 | 2014.2 ± 718.2 |
| Intraocular lens eyes/Phakic eyes, n | 32/5 |
| Number of previous glaucoma surgeries | 1.2 ± 1.1 |
| Type of previous glaucoma surgeries, n | |
| Selective laser trabeculoplasty | 11 |
| MP-TSCPC | 13 |
| Conventional trabeculotomy (ab externo) | 2 |
| Trabeculotomy (ab interno) | 11 |
| Trabeculectomy | 3 |
| Ex-PRESS implantation | 3 |
| Ahmed glaucoma valve implantation | 1 |
| Revision of trabeculectomy | 1 |
| Type of glaucoma, n | |
| −POAG | 11 |
| −PXG | 22 |
| −SOAG | 4 |
| Complication | No. of Eyes, n (%) |
|---|---|
| Mydriasis | 9 (24.3%) |
| Anterior chamber inflammation | 9 (24.3%) |
| Hyphema | 1 (2.7%) |
| Cystoid macular edema | 2 (5.4%) |
| Decreased visual acuity | 3 (8.1%) |
| Phthisis bulbi | 1 (2.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handa, S.; Kawaji, T.; Otsuka, S.-i. Short-Term Outcomes of Micropulse Transscleral Cyclophotocoagulation Using the VITRA 810 and Postoperative Ciliochoroidal Effusion in Japanese Patients. J. Clin. Med. 2025, 14, 7453. https://doi.org/10.3390/jcm14217453
Handa S, Kawaji T, Otsuka S-i. Short-Term Outcomes of Micropulse Transscleral Cyclophotocoagulation Using the VITRA 810 and Postoperative Ciliochoroidal Effusion in Japanese Patients. Journal of Clinical Medicine. 2025; 14(21):7453. https://doi.org/10.3390/jcm14217453
Chicago/Turabian StyleHanda, So, Takahiro Kawaji, and Shin-ichi Otsuka. 2025. "Short-Term Outcomes of Micropulse Transscleral Cyclophotocoagulation Using the VITRA 810 and Postoperative Ciliochoroidal Effusion in Japanese Patients" Journal of Clinical Medicine 14, no. 21: 7453. https://doi.org/10.3390/jcm14217453
APA StyleHanda, S., Kawaji, T., & Otsuka, S.-i. (2025). Short-Term Outcomes of Micropulse Transscleral Cyclophotocoagulation Using the VITRA 810 and Postoperative Ciliochoroidal Effusion in Japanese Patients. Journal of Clinical Medicine, 14(21), 7453. https://doi.org/10.3390/jcm14217453






