Post-Traumatic Orbital Reconstruction Using Titanium Patient-Specific Implants: A Clinical and Radiological Cohort Study Focusing on Paranasal Sinuses Physiology
Abstract
1. Introduction
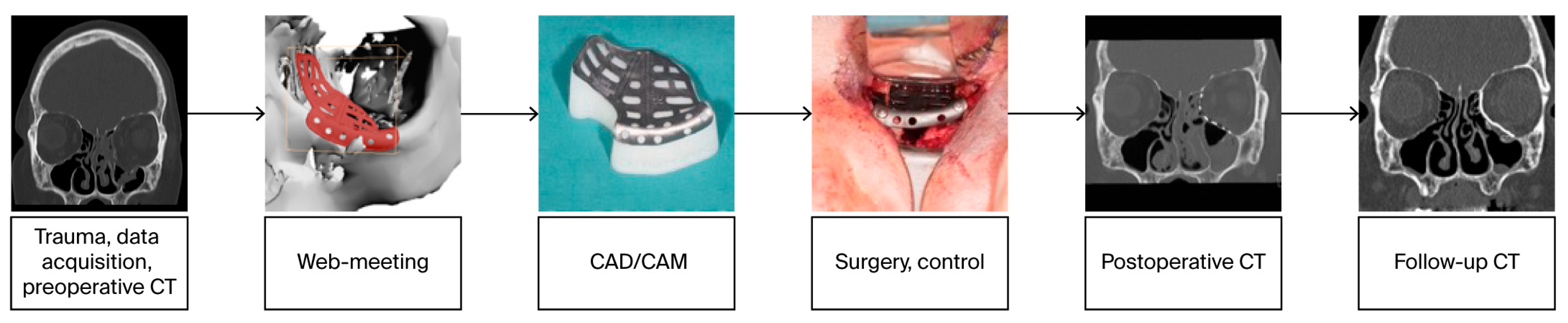
1.1. Virtual Surgical Planning and CAD/CAM Technologies in Craniomaxillofacial Surgery
1.2. Research Background and Study Objective
2. Materials and Methods
2.1. Study Participants
- ○
- Enophthalmos greater than 2 mm
- ○
- Diplopia
- ○
- Entrapment of the inferior rectus muscle
- Radiological criteria [20]
- ○
- Defect size ≥ 1 cm2 or defect over 50% of the orbital floor
- ○
- Herniation or incarceration of orbital contents, in particular degree of dislocation of the inferior rectus muscle.
- Adult patients aged ≥ 18 years
- 3D bony defect in the orbital region
- Complex unilateral orbital fractures—category ≥ II according to Jaquiery [22]
- Indication for a PSI as part of the primary or secondary reconstruction based on the defect size according to Dubois [23].
- Availability of the follow-up examination data.
- Previous midface trauma
- Tumor patients
- Cystic fibrosis
- History of radiotherapy in the midface area
- Trauma patients in whom osteosynthesis can be performed exclusively with ready-made material
- Category I orbital floor fractures according to Jaquiery [22].
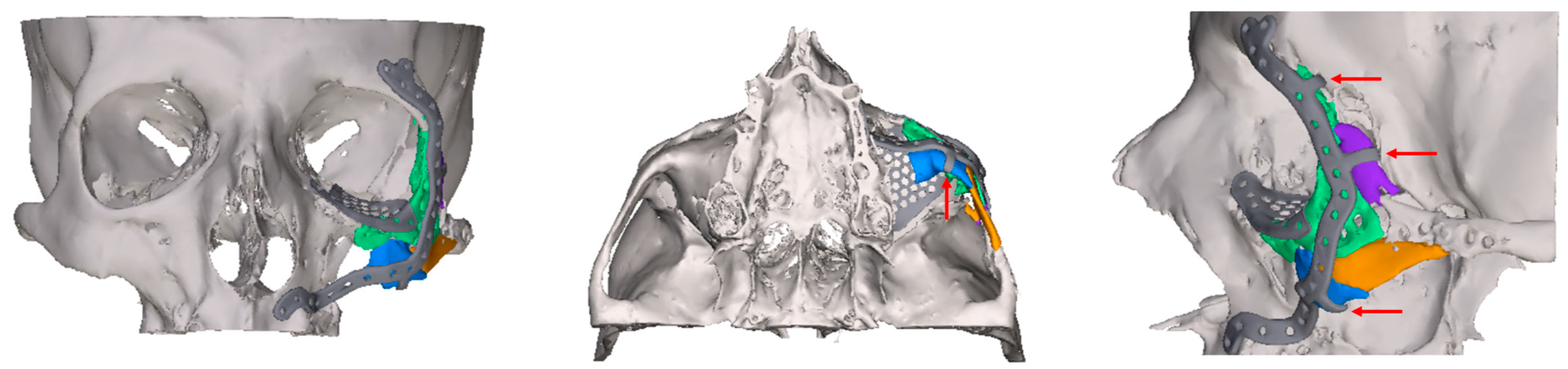
2.2. Digital Workflow and Implants
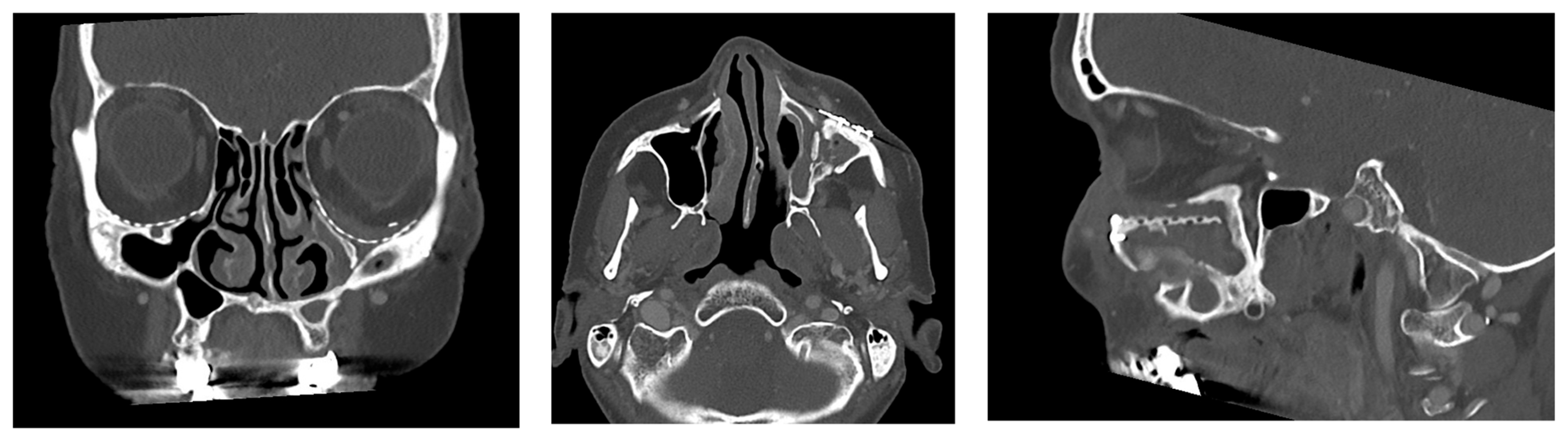
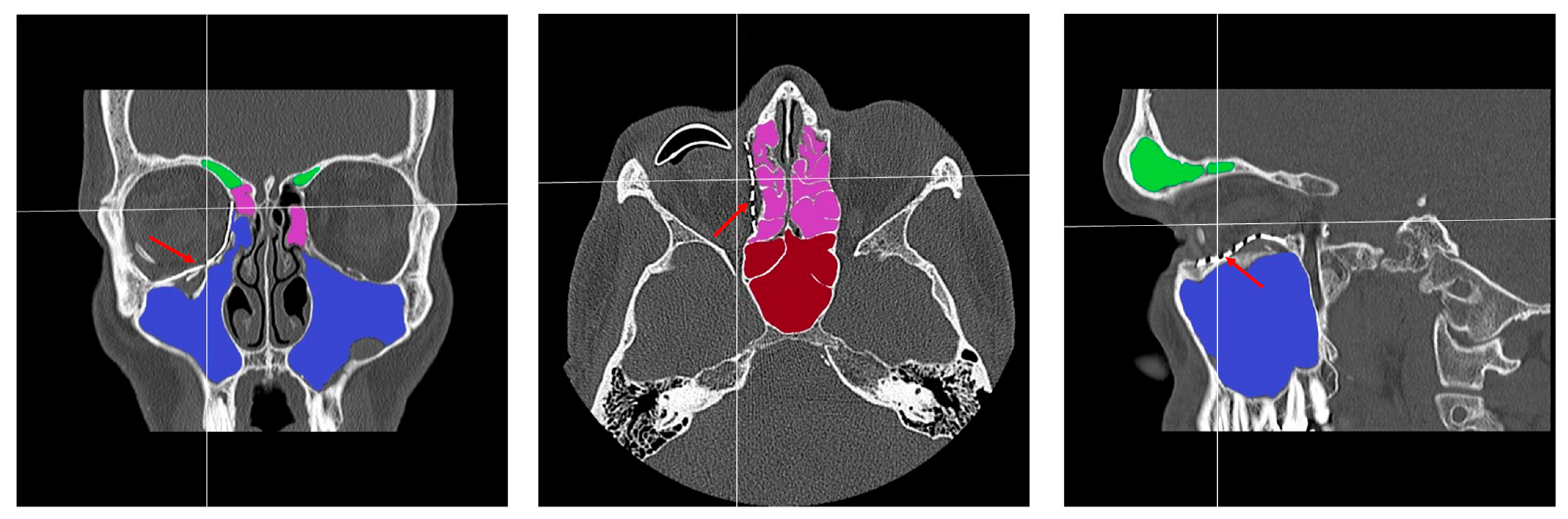
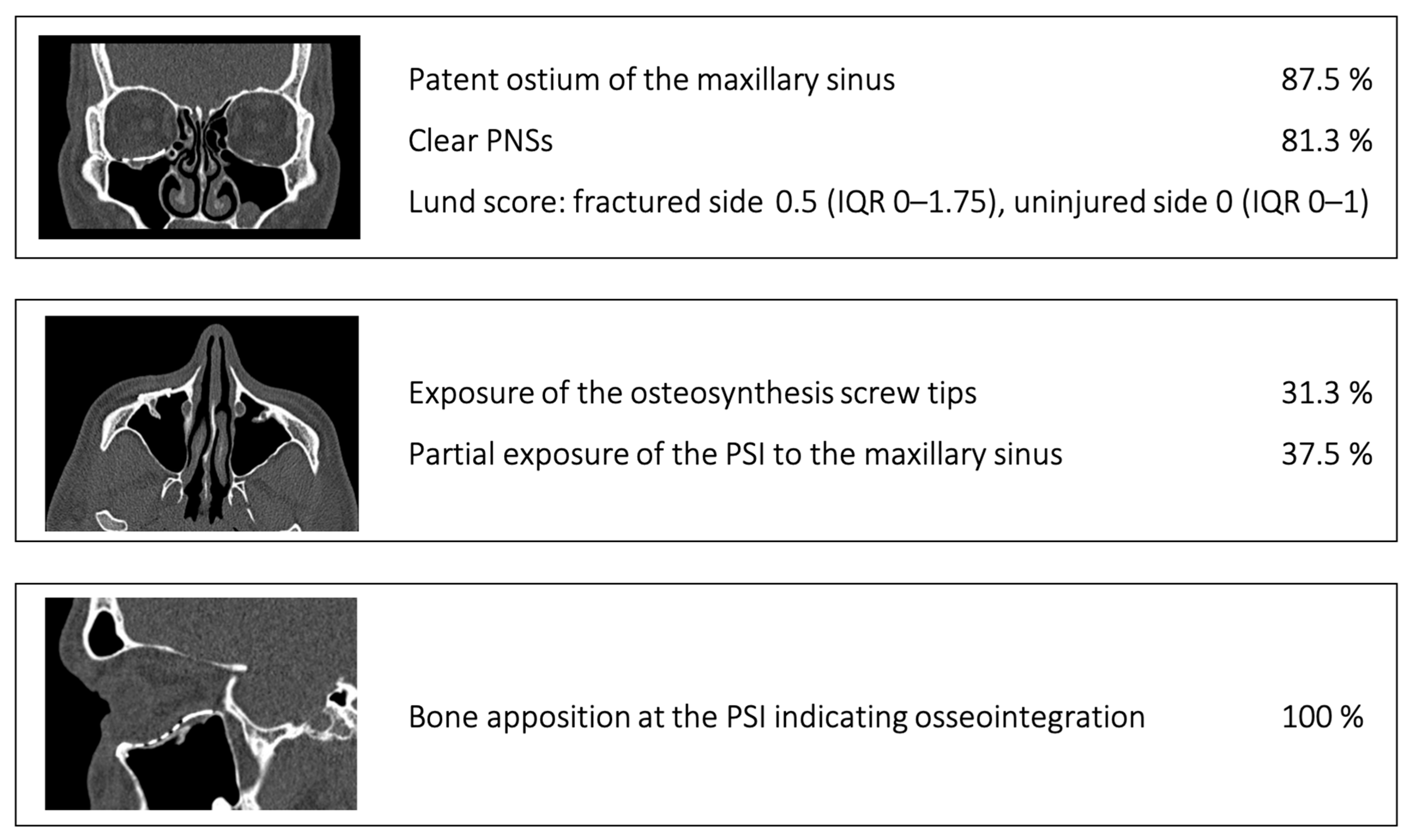
2.3. Data Collection and Defect Morphology
- Patent ostium of the maxillary sinus (coronal plane)
- Non-inflammatory PNSs (three planes)
- Exposure of the osteosynthesis screws (axial and sagittal planes)
- Exposure of the PSI to the maxillary sinus (coronal and sagittal planes)
- Remodeling of the fractured orbital walls and bone apposition at the PSI indicating osseointegration (three planes)
2.4. Consent, Data Management
2.5. Statistics
3. Results
- Fracture of the orbital floor (n = 3)
- Fracture of the orbital floor + medial orbital wall/zygomatic bone (n = 7)
- Complex centrolateral midface fracture, in some cases with involvement of the skull base (n = 6).
- Patent outflow tract of the maxillary sinus (n = 14) and physiologically ventilated PNSs (maxillary sinus, ethmoid cells, n = 13)
- Exposure of the fixation screws (infraorbital recess of the maxillary sinus) and limited exposure of the PSI (transition zone, ethmoid cells) without signs of mucosal swelling in these regions (n = 6 each)
- (Basal) mucosal swelling in the maxillary sinus indicative of chronic maxillary sinusitis, irrespective of the complexity of the primary injury (n = 4)
- Bone apposition at the PSI as a sign of osseointegration (n = 16)
- Remodeling of the dislocated bone fragments of the orbital walls (n = 16), including in the area of the infraorbital canal.
4. Discussion
4.1. Orbital Floor Fractures and Complex Centrolateral Midface Fractures
4.2. Paranasal Sinuses
4.3. Remodeling
5. Conclusions
5.1. Study Limitations
5.2. Clinical Application and Further Research Direction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nilsson, J.; Hindocha, N.; Thor, A. Time matters—Differences between computer-assisted surgery and conventional planning in cranio-maxillofacial surgery: A systematic review and meta-analysis. J. Cranio-Maxillofac. Surg. 2020, 48, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Gerbino, G.; Zavattero, E.; Zenga, F.; Bianchi, F.A.; Garzino-Demo, P.; Berrone, S. Primary and secondary reconstruction of complex craniofacial defects using polyetheretherketone custom-made implants. J. Cranio-Maxillofac. Surg. 2015, 43, 1356–1363. [Google Scholar] [CrossRef]
- Peel, S.; Bhatia, S.; Eggbeer, D.; Morris, D.S.; Hayhurst, C. Evolution of design considerations in complex craniofacial reconstruction using patient-specific implants. Proc. Inst. Mech. Eng. H 2017, 231, 509–524. [Google Scholar] [CrossRef]
- Rana, M.; Gellrich, M.M.; Gellrich, N.C. Customised reconstruction of the orbital wall and engineering of selective laser melting (SLM) core implants. Br. J. Oral Maxillofac. Surg. 2015, 53, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Kamyszek, T.; Weihe, S.; Scholz, M.; Wehmoller, M.; Eufinger, H. Management of craniofacial bone defects with individually prefabricated titanium implants. Follow-up and evaluation of 78 patients with 78 titanium implants 1994–1998. Mund Kiefer Gesichtschir. 2001, 5, 233–238. [Google Scholar] [CrossRef]
- Huang, G.J.; Zhong, S.; Susarla, S.M.; Swanson, E.W.; Huang, J.; Gordon, C.R. Craniofacial reconstruction with poly(methyl methacrylate) customized cranial implants. J. Craniofac. Surg. 2015, 26, 64–70. [Google Scholar] [CrossRef]
- Zhang, D.; Ni, N.; Su, Y.; Miao, H.; Tang, Z.; Ji, Y.; Wang, Y.; Gao, H.; Ju, Y.; Sun, N.; et al. Targeting Local Osteogenic and Ancillary Cells by Mechanobiologically Optimized Magnesium Scaffolds for Orbital Bone Reconstruction in Canines. ACS Appl. Mater. Interfaces 2020, 12, 27889–27904. [Google Scholar] [CrossRef]
- Vasile, V.A.; Istrate, S.; Iancu, R.C.; Piticescu, R.M.; Cursaru, L.M.; Schmetterer, L.; Garhofer, G.; Cherecheanu, A.P. Biocompatible Materials for Orbital Wall Reconstruction—An Overview. Materials 2022, 15, 2183. [Google Scholar] [CrossRef]
- Garcia-Mato, D.; Ochandiano, S.; Garcia-Sevilla, M.; Navarro-Cuellar, C.; Darriba-Alles, J.V.; Garcia-Leal, R.; Calvo-Haro, J.A.; Perez-Mananes, R.; Salmeron, J.I.; Pascau, J. Craniosynostosis surgery: Workflow based on virtual surgical planning, intraoperative navigation and 3D printed patient-specific guides and templates. Sci. Rep. 2019, 9, 17691. [Google Scholar] [CrossRef]
- Nyberg, E.L.; Farris, A.L.; Hung, B.P.; Dias, M.; Garcia, J.R.; Dorafshar, A.H.; Grayson, W.L. 3D-Printing Technologies for Craniofacial Rehabilitation, Reconstruction, and Regeneration. Ann. Biomed. Eng. 2017, 45, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef]
- Zimmerer, R.M.; Gellrich, N.C.; von Bulow, S.; Strong, E.B.; Ellis, E., 3rd; Wagner, M.E.H.; Sanchez Aniceto, G.; Schramm, A.; Grant, M.P.; Thiam Chye, L.; et al. Is there more to the clinical outcome in posttraumatic reconstruction of the inferior and medial orbital walls than accuracy of implant placement and implant surface contouring? A prospective multicenter study to identify predictors of clinical outcome. J. Cranio-Maxillofac. Surg. 2018, 46, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Welkoborsky, H.J.; Pitz, S.; Grass, S.; Breuer, B.; Holte, A.P.V.; Bertram, O.; Wiechens, B. Sinogenic Orbital Complications. Dtsch. Ärzteblatt Int. 2022, 119, 31–37. [Google Scholar] [CrossRef]
- Jones, N. The nose and paranasal sinuses physiology and anatomy. Adv. Drug Deliv. Rev. 2001, 51, 5–19. [Google Scholar] [CrossRef]
- Menezes, J.D.; Moura, L.B.; Pereira-Filho, V.A.; Hochuli-Vieira, E. Maxillary Sinus Mucocele as a Late Complication in Zygomatic-Orbital Complex Fracture. Craniomaxillofacial Trauma Reconstr. 2016, 9, 342–344. [Google Scholar] [CrossRef]
- Yelverton, J.C.; Jackson, P.; Schmidt, R.S. Chronic rhinosinusitis in patients requiring surgical repair of a midface fracture. Ear Nose Throat J. 2014, 93, E26–E28. [Google Scholar] [CrossRef]
- Reich, W.; Seidel, D.; Bredehorn-Mayr, T.; Eckert, A.W. Reconstruction of isolated orbital floor fractures with a prefabricated titanium mesh. Klin. Monatsblatter Fur Augenheilkd. 2014, 231, 246–255. [Google Scholar] [CrossRef]
- Becker, S.T.; Terheyden, H.; Fabel, M.; Kandzia, C.; Moller, B.; Wiltfang, J. Comparison of collagen membranes and polydioxanone for reconstruction of the orbital floor after fractures. J. Craniofacial Surg. 2010, 21, 1066–1068. [Google Scholar] [CrossRef]
- Poeschl, P.W.; Baumann, A.; Dorner, G.; Russmueller, G.; Seemann, R.; Fabian, F.; Ewers, R. Functional outcome after surgical treatment of orbital floor fractures. Clin. Oral Investig. 2012, 16, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Schouman, T.; Courvoisier, D.S.; Imholz, B.; Van Issum, C.; Scolozzi, P. Computational area measurement of orbital floor fractures: Reliability, accuracy and rapidity. Eur. J. Radiol. 2012, 81, 2251–2254. [Google Scholar] [CrossRef] [PubMed]
- Dubois, L.; Steenen, S.A.; Gooris, P.J.; Mourits, M.P.; Becking, A.G. Controversies in orbital reconstruction—II. Timing of post-traumatic orbital reconstruction: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Jaquiery, C.; Aeppli, C.; Cornelius, P.; Palmowsky, A.; Kunz, C.; Hammer, B. Reconstruction of orbital wall defects: Critical review of 72 patients. Int. J. Oral Maxillofac. Surg. 2007, 36, 193–199. [Google Scholar] [CrossRef]
- Dubois, L.; Steenen, S.A.; Gooris, P.J.; Mourits, M.P.; Becking, A.G. Controversies in orbital reconstruction—I. Defect-driven orbital reconstruction: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 308–315. [Google Scholar] [CrossRef]
- Lund, V.J.; Kennedy, D.W. Quantification for staging sinusitis. The Staging and Therapy Group. Ann. Otol. Rhinol. Laryngol. Suppl. 1995, 167, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Holtmann, H.; Rana, M.; Kanatas, A.N.; Singh, D.D.; Sproll, C.K.; Kubler, N.R.; Ipaktchi, R.; Hufendiek, K.; Gellrich, N.C. Primary orbital reconstruction with selective laser melted core patient-specific implants: Overview of 100 patients. Br. J. Oral Maxillofac. Surg. 2019, 57, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Coroneos, C.J.; Ignacy, T.A.; Thoma, A. Designing and reporting case series in plastic surgery. Plast. Reconstr. Surg. 2011, 128, 361e–368e. [Google Scholar] [CrossRef]
- Pant, H.; Ferguson, B.; Hughes, A.; Schembri, M. Confounding factors in rhinological research. Curr. Opin. Otolaryngol. Head. Neck Surg. 2013, 21, 282–292. [Google Scholar] [CrossRef]
- Jansen, J.; Dubois, L.; Maal, T.J.J.; Mourits, M.P.; Jellema, H.M.; Neomagus, P.; de Lange, J.; Hartman, L.J.C.; Gooris, P.J.J.; Becking, A.G. A nonsurgical approach with repeated orthoptic evaluation is justified for most blow-out fractures. J. Cranio-Maxillofac. Surg. 2020, 48, 560–568. [Google Scholar] [CrossRef]
- Oluwole, M.; Russell, N.; Tan, L.; Gardiner, Q.; White, P. A comparison of computerized tomographic staging systems in chronic sinusitis. Clin. Otolaryngol. Allied Sci. 1996, 21, 91–95. [Google Scholar]
- Hastan, D.; Fokkens, W.J.; Bachert, C.; Newson, R.B.; Bislimovska, J.; Bockelbrink, A.; Bousquet, P.J.; Brozek, G.; Bruno, A.; Dahlen, S.E.; et al. Chronic rhinosinusitis in Europe—An underestimated disease. A GA2LEN study. Allergy 2011, 66, 1216–1223. [Google Scholar] [CrossRef]
- Ashraf, N.; Bhattacharyya, N. Determination of the “incidental” Lund score for the staging of chronic rhinosinusitis. Otolaryngol. Head. Neck Surg. 2001, 125, 483–486. [Google Scholar] [CrossRef]
- Gander, T.; Essig, H.; Metzler, P.; Lindhorst, D.; Dubois, L.; Rucker, M.; Schumann, P. Patient specific implants (PSI) in reconstruction of orbital floor and wall fractures. J. Cranio-Maxillofac. Surg. 2015, 43, 126–130. [Google Scholar] [CrossRef]
- Zimmerer, R.M.; Ellis, E., 3rd; Aniceto, G.S.; Schramm, A.; Wagner, M.E.; Grant, M.P.; Cornelius, C.P.; Strong, E.B.; Rana, M.; Chye, L.T.; et al. A prospective multicenter study to compare the precision of posttraumatic internal orbital reconstruction with standard preformed and individualized orbital implants. J. Cranio-Maxillofac. Surg. 2016, 44, 1485–1497. [Google Scholar] [CrossRef]
- Probst, F.A.; Cornelius, C.P.; Otto, S.; Malenova, Y.; Probst, M.; Liokatis, P.; Haidari, S. Accuracy of free-hand positioned patient specific implants (PSI) in primary reconstruction after inferior and/or medial orbital wall fractures. Comput. Biol. Med. 2021, 137, 104791. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, R.; Klop, C.; Gooris, P.J.J.; Maal, T.J.J.; Becking, A.G.; Dubois, L. Critical appraisal of patient-specific implants for secondary post-traumatic orbital reconstruction. Int. J. Oral Maxillofac. Surg. 2021, 51, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Welkoborsky, H.J.; Plontke, S.K. Possible surgical approaches to the orbit. HNO 2018, 66, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Paluzzi, A.; Gardner, P.A.; Fernandez-Miranda, J.C.; Tormenti, M.J.; Stefko, S.T.; Snyderman, C.H.; Maroon, J.C. “Round-the-Clock” Surgical Access to the Orbit. J. Neurol. Surg. B Skull Base 2015, 76, 12–24. [Google Scholar] [CrossRef]
- Hajibandeh, J.; Be, A.; Lee, C. Custom Interlocking Implants for Primary and Secondary Reconstruction of Large Orbital Floor Defects: Case Series and Description of Workflow. J. Oral Maxillofac. Surg. 2021, 79, 2539.e1–2539.e10. [Google Scholar] [CrossRef]
- Mommaerts, M.Y.; Buttner, M.; Vercruysse, H., Jr.; Wauters, L.; Beerens, M. Orbital Wall Reconstruction with Two-Piece Puzzle 3D Printed Implants: Technical Note. Craniomaxillofac. Trauma. Reconstr. 2016, 9, 55–61. [Google Scholar] [CrossRef]
- Sabelis, J.F.; Youssef, S.; Hoefnagels, F.W.A.; Becking, A.G.; Schreurs, R.; Dubois, L. Technical Note on Three- and Four-Wall Orbital Reconstructions with Patient-Specific Implants. J. Craniofac. Surg. 2022, 33, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Sabelis, J.F.; Schreurs, R.; Essig, H.; Becking, A.G.; Dubois, L. Personalized Medicine Workflow in Post-Traumatic Orbital Reconstruction. J. Pers. Med. 2022, 12, 1366. [Google Scholar] [CrossRef]
- Lehtinen, V.; Salli, M.; Pyotsia, K.; Toivari, M.; Snall, J. Primary reconstruction of combined orbital and zygomatic complex fractures with patient-specific milled titanium implants—A retrospective study. J. Cranio-Maxillofac. Surg. 2022, 50, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Betz, M.W.; Caccamese, J.F.; Coletti, D.P.; Sauk, J.J.; Fisher, J.P. Challenges associated with regeneration of orbital floor bone. Tissue Eng. Part B Rev. 2010, 16, 541–550. [Google Scholar] [CrossRef]
- Bitter, T.; Guntinas-Lichius, O. Funktionelle endoskopische Nasennebenhöhlenchirurgie (FESS). Laryngo-Rhino-Otol. 2019, 98, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Beule, A.G. and W. Hosemann; Wound healing after endoscopic sinus surgery and postoperative management. HNO 2009, 57, 763–771. [Google Scholar] [CrossRef]
- Weber, R. Endonasal frontal sinus surgery. Part 1: Frontal sinus drainage, types I and II. HNO 2009, 57, 739–750. [Google Scholar] [CrossRef]
- Suresh, V.; Anolik, R.; Powers, D. The Utility of Polyether-Ether-Ketone Implants Adjacent to Sinus Cavities After Craniofacial Trauma. J. Oral Maxillofac. Surg. 2018, 76, 2361–2369. [Google Scholar] [CrossRef]
- Sarfraz, S.; Mantynen, P.H.; Laurila, M.; Rossi, S.; Leikola, J.; Kaakinen, M.; Suojanen, J.; Reunanen, J. Comparison of Titanium and PEEK Medical Plastic Implant Materials for Their Bacterial Biofilm Formation Properties. Polymers 2022, 14, 3862. [Google Scholar] [CrossRef]
- Punchak, M.; Chung, L.K.; Lagman, C.; Bui, T.T.; Lazareff, J.; Rezzadeh, K.; Jarrahy, R.; Yang, I. Outcomes following polyetheretherketone (PEEK) cranioplasty: Systematic review and meta-analysis. J. Clin. Neurosci. 2017, 41, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wu, M.; Li, H.; Liang, J.; Chen, J.; Liu, L. Risk Factors for Maxillary Sinus Pathology after Surgery for Midfacial Fracture: A Multivariate Analysis. J. Clin. Med. 2022, 11, 6299. [Google Scholar] [CrossRef]
- Amin, D.; Mandloi, S.; Nunes, K.; Garg, N.; Kahn, C.; Duffy, A.; Toskala, E.; Rabinowitz, M.; Rosen, M.; Nyquist, G. A novel staging system to consolidate silent sinus syndrome and chronic maxillary atelectasis: A systematic review and case series. Int. Forum Allergy Rhinol. 2024, 14, 1378–1381. [Google Scholar] [CrossRef]
- Strabbing, E.M.; Engin, O.; Telleman, M.A.J.; Nagtegaal, A.P.; Wolvius, E.B. Post-traumatic and iatrogenic silent sinus syndrome: A case series. Oral Maxillofac. Surg. 2025, 29, 106. [Google Scholar] [CrossRef]
- Crockett, J.C.; Rogers, M.J.; Coxon, F.P.; Hocking, L.J.; Helfrich, M.H. Bone remodelling at a glance. J. Cell Sci. 2011, 124, 991–998. [Google Scholar] [CrossRef]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular mechanosensors in osteocytes. Bone Res. 2020, 8, 23. [Google Scholar] [CrossRef]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aznar, J.M.; Nasello, G.; Hervas-Raluy, S.; Perez, M.A.; Gomez-Benito, M.J. Multiscale modeling of bone tissue mechanobiology. Bone 2021, 151, 116032. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.J.; Li, F.W.; Zhan, W.F.; Lin, F.C.; Luo, S.K. Three-Dimensional Analysis of Age-Related Orbital and Midfacial Bone Remodeling in Asians. Dermatol. Surg. 2020, 46, e139–e145. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.Y.Q.; Leong, Y.Y.; Lang, S.S.; Htoon, Z.M.; Young, S.M.; Sundar, G. Radiologic Parameters of Orbital Bone Remodeling in Thyroid Eye Disease. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2527–2533. [Google Scholar] [CrossRef]
- Fisher, T.; Nugent, R.; Rootman, J. Arachnoid cysts with orbital bone remodeling—Two interesting cases. Orbit 2005, 24, 59–62. [Google Scholar] [CrossRef]
- Sigron, G.R.; Britschgi, C.L.; Gahl, B.; Thieringer, F.M. Insights into Orbital Symmetry: A Comprehensive Retrospective Study of 372 Computed Tomography Scans. J. Clin. Med. 2024, 13, 1041. [Google Scholar] [CrossRef]
- Gellrich, N.C.; Dittmann, J.; Spalthoff, S.; Jehn, P.; Tavassol, F.; Zimmerer, R. Current Strategies in Post-traumatic Orbital Reconstruction. J. Maxillofac. Oral Surg. 2019, 18, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.D.; Kang, D.H.; Kim, H.S. Orbital wall restoring surgery with resorbable mesh plate. Arch. Craniofac. Surg. 2018, 19, 264–269. [Google Scholar] [CrossRef]
- Tomic, J.; Wiederstein-Grasser, I.; Schanbacher, M.; Weinberg, A.M. Newly Developed Resorbable Magnesium Biomaterials for Orbital Floor Reconstruction in Caprine and Ovine Animal Models-A Prototype Design and Proof-of-Principle Study. J. Funct. Biomater. 2023, 14, 339. [Google Scholar] [CrossRef]
- Sun, X.; Lin, X.; Zhang, C.; Huang, R.; Liu, Y.; Zhang, G.; Di, S. Improved Osseointegration of Selective Laser Melting Titanium Implants with Unique Dual Micro/Nano-Scale Surface Topography. Materials 2022, 15, 7811. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, R.; Yu, X.; Chen, J.; Wan, S.; Ouyang, J.; Deng, F. Enhanced antibacterial efficacy of selective laser melting titanium surface with nanophase calcium phosphate embedded to TiO2 nanotubes. Biomed. Mater. 2018, 13, 045015. [Google Scholar] [CrossRef] [PubMed]

| Field | Population | Intervention | Control | Outcome | Setting | Time |
|---|---|---|---|---|---|---|
| Craniomaxillofacial surgery | Inpatients who required orbital reconstruction due to trauma | Virtual surgical planning and reconstruction with patient-specific CAD/CAM implants (PSIs) | Contralateral uninjured structures of the midface | Primary outcome measure: status of the paranasal sinuses in relation to the PSI (Lund score [24]). Secondary outcome measures: post-operative (implant-associated) complications, other adverse events | Group of consecutively enrolled patients (February/2019 to May/2024) Department of Oral, Maxillofacial and Plastic Surgery (University Hospital Halle) | Post-operative follow-up period ≥ 6 months to wait for stable wound healing and to record possible complications; the clinical and radiological findings at the last outpatient follow-up were decisive |
| Classification | Diplopia, Enophthalmos, Other Complications or Consequences |
|---|---|
| Excellent | No diplopia, enophthalmos 0–2 mm, no other complications or consequences |
| Good | No diplopia, enophthalmos 0–2 mm, other minor complications or consequences |
| Acceptable | Diplopia outside the field of vision (no compensatory head rotation), enophthalmos 0–2 mm |
| Poor | Diplopia within the field of vision (no compensatory head rotation), enophthalmos > 2 mm |
| Failure | Revision due to persistent dysfunctions, diplopia, enophthalmos, other serious complications or consequences |
| Patient | Gender | Age | Primary Trauma Diagnosis | Primary Treatment | Year of Surgery | Classification [22] | Access | Duration of Surgery (min) | Ophthalmological Status (Post-Operative, Tensio mmHg) | Complications (Posttraumatic/Post-Operative) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 71 | Orbital floor | - | 2020 | Category II, 1 PSI | Mediopalpebral | 60 | Visus right 0.8 s.c, left 1.0 s.c | None |
| 2 | F | 51 | Orbital floor | Monitoring | 2020 | Category III, 1 PSI | Transconjunctival-transcaruncular | 105 | Visus right 0.2, left 0.8, Tensio right/left normal (10–21 mm Hg) | Lower lid entropion, trichiasis |
| 3 | M | 48 | Orbital floor | Soft tissue reconstruction, removal of foreign bodies | 2022 | Category III, 1 PSI | Mediopalpebral | 64 | Visus right 0.6, left 0.6, Tensio right 18, left 14 | Initial diplopia; prisms |
| 4 | F | 49 | Orbital floor, open, infraorbital soft tissue laceration | Soft tissue debridement | 2023 | Category II, 1 PSI | Accidental wounds, subcilliar | 107 | Visus right 0.6, left 0.8, Tensio right 14, left 12 | Lower lid ectropion (result of trauma), preexisting strabismus convergens |
| 5 | F | 41 | Orbital floor, zygomatic bone | Wound care | 2020 | Category III, 1 PSI | Mediopalpebral | 116 | Visus right 0.8, left 0.8, Tensio right 18, left 22, swelling | None |
| 6 | M | 55 | Orbital floor, medial orbital wall | - | 2020 | Category III, 1 PSI | Transconjunctival-transcaruncular | 70 | - | None |
| 7 | M | 34 | Orbital floor, zygomatic bone | Osteosynthesis upper extremities | 2020 | Category III, #, 1 PSI | Mediopalpebral | 120 | Visus right 1.0, left 1.0, Tensio right 12, left 12 | None |
| 8 | F | 71 | Orbital floor, infraorbital rim, paranasal buttress comminuted | Osteosynthesis, Infraorbital and paranasal | 2023 | Category III, #, 1 PSI | Infraorbital | 88 | Visus right 1.0/left 0.6, Tensio right 15, left 16 | Infraorbital dysesthesia |
| 9 | F | 45 | Centrolateral midface (orbital floor, medial orbital wall, NOE complex), frontal base both sides, open, comminuted | Frontobasal coverage, osteosynthesis, ethmoid bone debridement | 2019 | Category V+, 2 PSIs | Accidental wounds, transconjunctival | 295 | Visus right 0.3, left 1.0., Tensio r/l normal | Diplopia outside the field of vision, potential opticus lesion |
| 10 | M | 56 | Centrolateral midface both sides, open, comminuted | Soft tissue wound care | 2020 | Category IV+, ##, 1 PSI | Transconjunctival | 213 | Visus right 0.8, left 0.8. Tensio r/l 13, Contusio bulbi | Traumatic crooked saddle nose with nasal obstruction, no diplopia |
| 11 | F | 26 | Centrolateral midface (orbital floor, NOE complex, zygomatic bone, zygomatic arch, maxilla), comminuted, (condylar process, right mandible) | Monitoring, soft tissue reconstruction, initial osteosynthesis of midfacial buttresses | 2023 | Category III, 1 PSI | Mediopalpebral | 110 (initial surgery 464) | Visus right 0.8, left 1.0, Tensio right 15, left 15 | Diplopia outside the field of vision (upgaze), infraorbital hypoesthesia |
| 12 | M | 32 | Centrolateral midface right, comminuted | Soft tissue reconstruction, eyeball reconstruction, monitoring | 2021 | Not classifiable (blow-out fracture of all 4 orbital walls, category V+), 3 PSIs | Accidental wounds, combination | 360 | Visus-, Tensio normal, Hyposphagma right | Amaurosis (questionable lux, blast trauma), soft tissue deficit paranasal and on the nasal wing |
| 13 | M | 70 | Centrolateral midface both sides, frontal base, comminuted | Soft tissue reconstruction, intracranial pressure probe, monitoring | 2021 | Category III, 1 PSI | Mediopalpebral | 187 | Visus right 1.0, left 0.4, Tensio right 15, left 12, Contusio bulbi | None |
| 14 | M | 34 | Centrolateral midface (orbital floor, zygomatic bone, zygomatic arch, nasal framework, maxilla) right | Soft tissue reconstruction, tracheotomy, monitoring | 2021 | Category III, 1 PSI | Accidental wounds, mediopalpebral | 80 | - | Lesion of the brachial plexus (result of trauma—traffic accident); neurosurgical reconstruction |
| 15 | M | 28 | Centrolateral midface (zygomatic bone, zygomatic arch, maxilla, nasal framework, orbital floor, medial orbital wall) both sides | Soft tissue reconstruction, foreign body removal, monitoring, pre-formed titanium mesh implant for orbital floor and medial orbital wall | 2022 | Category V+, ##, 2 PSIs | Transconjunctival-transcaruncular with canthotomy | 302 | Visus right 1.0, left 0.2. Tensio right 15, left 17 | Visual acuity 0.25, traumatic mydriasis (iris sphincter tear), scarred lower lid ectropion, left eyebrow ptosis (result of trauma) |
| 16 | M | 62 | Centrolateral midface (zygomatic bone, zygomatic arch, maxilla, nasal framework, orbital floor, orbital roof) | Monitoring, soft tissue reconstruction, initial osteosynthesis of midfacial buttresses | 2024 | Category V+, ##, 1 PSI | Mediopalpebral | 83 (initial surgery 196) | Visus right 0.4, left 0.6. Tensio right 18, left 17 | Infraorbital paresthesia and lymphoedema |
| Patient | Follow-Up Interval | Clinical Findings | Radiological Findings | ||||
|---|---|---|---|---|---|---|---|
| Local Complications, Correction | Patent Ostiomeatal Complex | Lund Score (Injured/Uninjured Orbit) | Exposure of Screw Tips | Exposure of the PSI | Bone Apposition at the PSI | ||
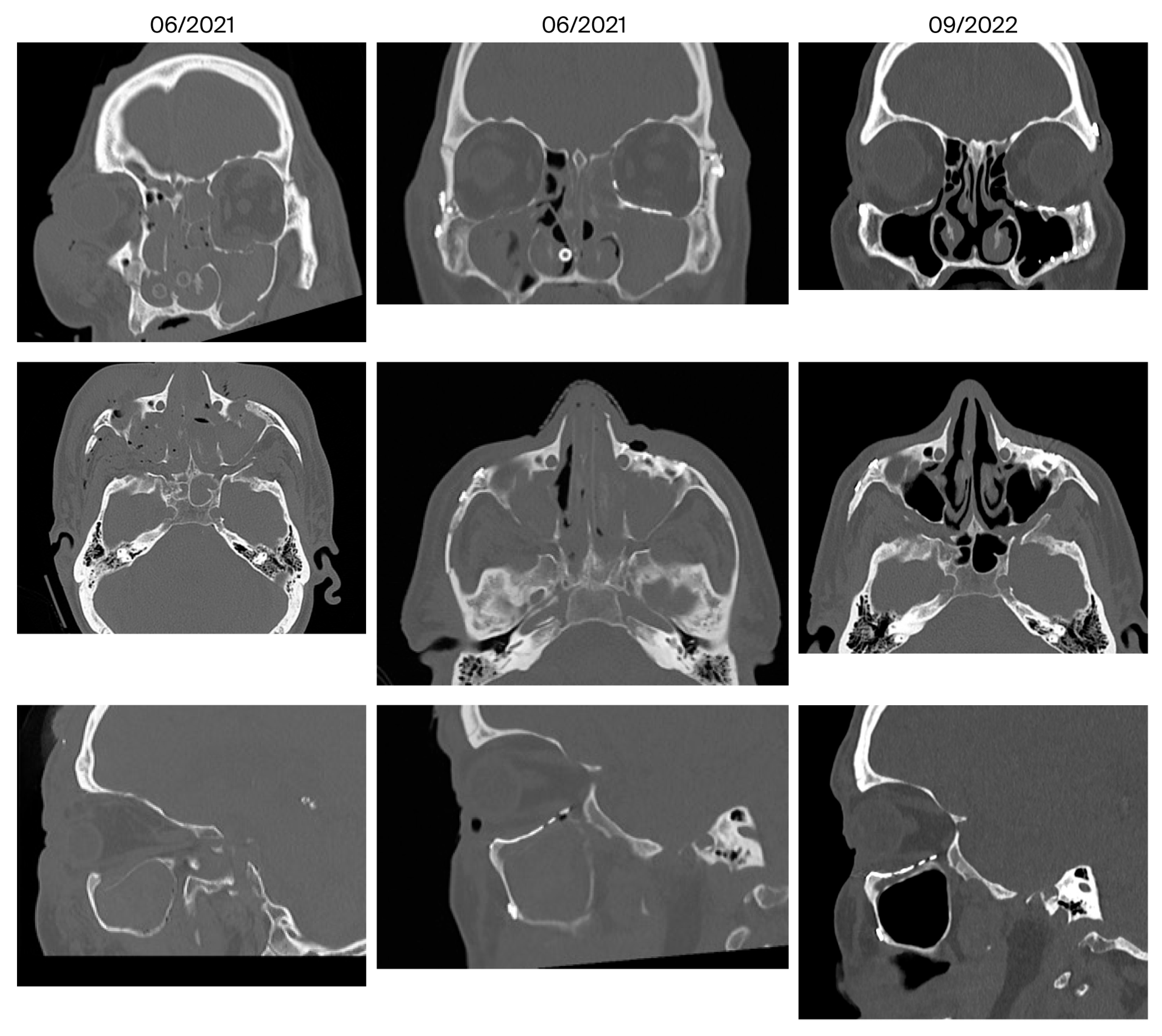
| 1 | July 2020 September 2022 (26 months) | Occasional serous rhinorrhea, infraorbital paresthesia | Yes | 0/0 | Yes | No | Yes |
| 2 | October 2020 November 2022 (25 months) | None | Yes | 0/0 | Yes | No | Yes, partially on the medial wall, dorsally protruding PSI end (4 mm) |
| 3 | March 2022 January 2023 (10 months) | Occasional serous rhinorrhea | Yes | 0/1 | 1 screw tip | No | Yes |
| 4 | August 2023 February 2024 (6 months) | Lower lid ectopion, “finger flap” | Yes | 1/0 | No | No | Yes |
| 5 | June 2020 October 2022 (28 months) | Infraorbital hyperesthesia | Yes | 0/0 | No | No | Yes |
| 6 | July 2020 November 2022 (28 months) | None | Yes | 0/0 | No | Minor, ethmoidal, transition zone | Yes |
| 7 | August 2020 October 2022 (26 months) | - | Yes | 1/0 | No | No | Yes |
| 8 | October 2023 October 2025 (24 months) | Infraorbital dysesthesia | Yes | 0/0 | No | No | Yes |
| 9 | February 2019 April 2021 (26 months) | Asymmetry, telecanthus on the right, scars in the nasal region | Yes | 5/5 | No | Ethmoidal | Yes |
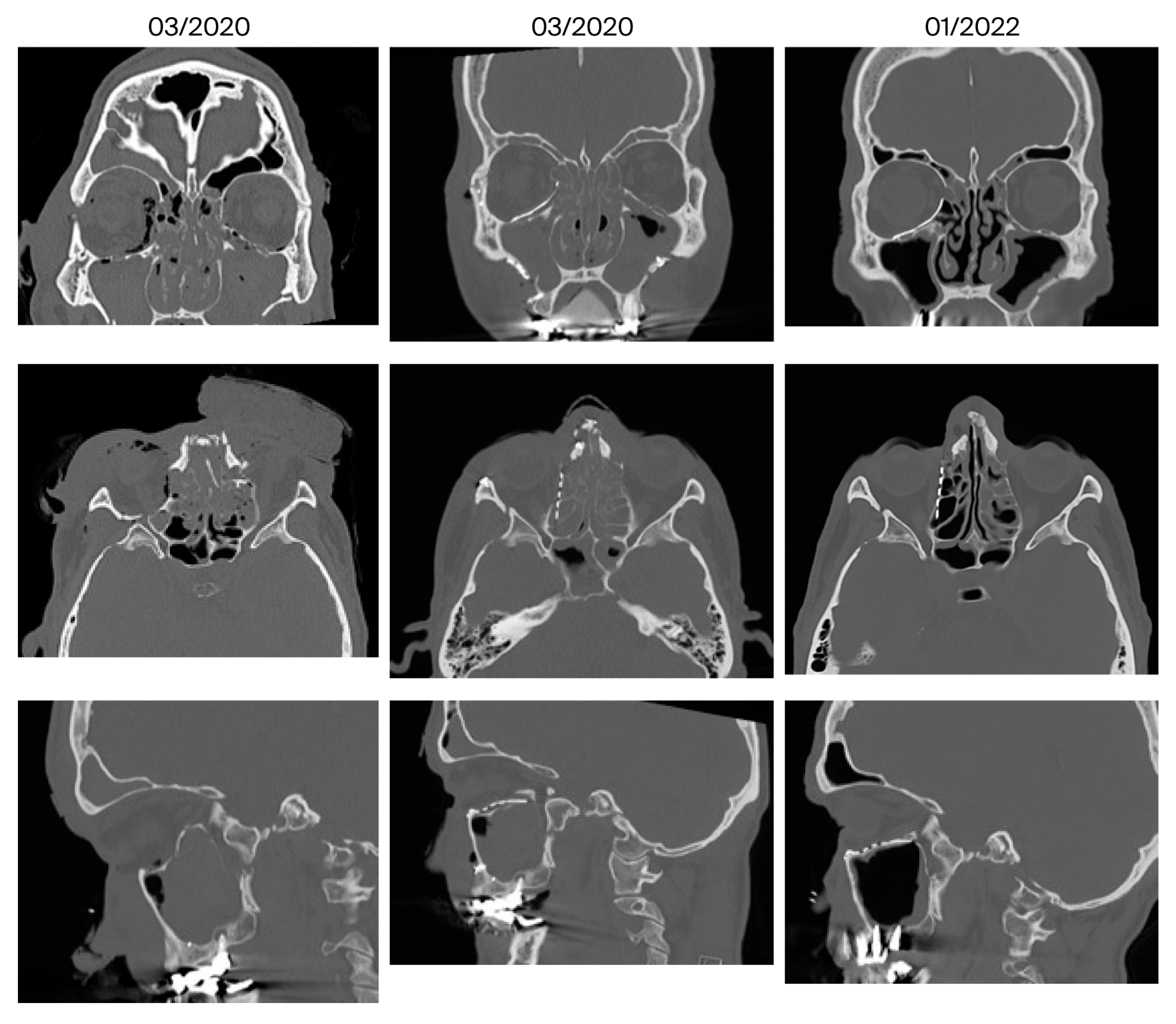
| 10 | March 2020 January 2022 (22 months) | Anosmia, migraine | Mucosal swelling | 3/5 | No | Ethmoidal | Yes |
| 11 | December 2023 February 2025 (14 months) | Infraorbital hypesthesia | No | 6/3 | Yes | No | Yes |
| 12 | February 2021 June 2022 (16 months) | Amaurosis, blepharophimosis, soft tissue deficit of the nasal wings; forehead flap planed | Yes, additionally neo-infundibulum | 2/1 | No | Minor, ethmoidal transition zone | Yes |
| 13 | June 2021 September 2022 (15 months) | None | Yes | 1/0 | No | No | Yes |
| 14 | July 2021 September 2022 (14 months) | Infraorbital hypoesthesia | No | 5/4 | No | Covered with mucosa | Minor |
| 15 | August 2021 December 2022 (16 months) | Discrete hypoesthesia, corneal scar, traumatic cataract, mydriasis, eyebrow ptosis, antimongoloid eyelid axis position; eyebrow lift, tarsal strip procedure | Yes | 0/0 | No | No | Yes |
| 16 | May 2024 January 2025 (8 months) | None | Yes neo-infundibulum | 2/0 | No | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reich, W.; Widmaier, L.; Kisser, U.; Heichel, J.; Otto, S.; Tavassol, F. Post-Traumatic Orbital Reconstruction Using Titanium Patient-Specific Implants: A Clinical and Radiological Cohort Study Focusing on Paranasal Sinuses Physiology. J. Clin. Med. 2025, 14, 7439. https://doi.org/10.3390/jcm14207439
Reich W, Widmaier L, Kisser U, Heichel J, Otto S, Tavassol F. Post-Traumatic Orbital Reconstruction Using Titanium Patient-Specific Implants: A Clinical and Radiological Cohort Study Focusing on Paranasal Sinuses Physiology. Journal of Clinical Medicine. 2025; 14(20):7439. https://doi.org/10.3390/jcm14207439
Chicago/Turabian StyleReich, Waldemar, Louis Widmaier, Ulrich Kisser, Jens Heichel, Sven Otto, and Frank Tavassol. 2025. "Post-Traumatic Orbital Reconstruction Using Titanium Patient-Specific Implants: A Clinical and Radiological Cohort Study Focusing on Paranasal Sinuses Physiology" Journal of Clinical Medicine 14, no. 20: 7439. https://doi.org/10.3390/jcm14207439
APA StyleReich, W., Widmaier, L., Kisser, U., Heichel, J., Otto, S., & Tavassol, F. (2025). Post-Traumatic Orbital Reconstruction Using Titanium Patient-Specific Implants: A Clinical and Radiological Cohort Study Focusing on Paranasal Sinuses Physiology. Journal of Clinical Medicine, 14(20), 7439. https://doi.org/10.3390/jcm14207439






