The Application of Combined PET/MRI in Staging and Response Assessment of Rectal Cancer
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Technical Aspects of FDG-PET/MRI
3.2. MRI Sequences in Rectal Cancer Imaging
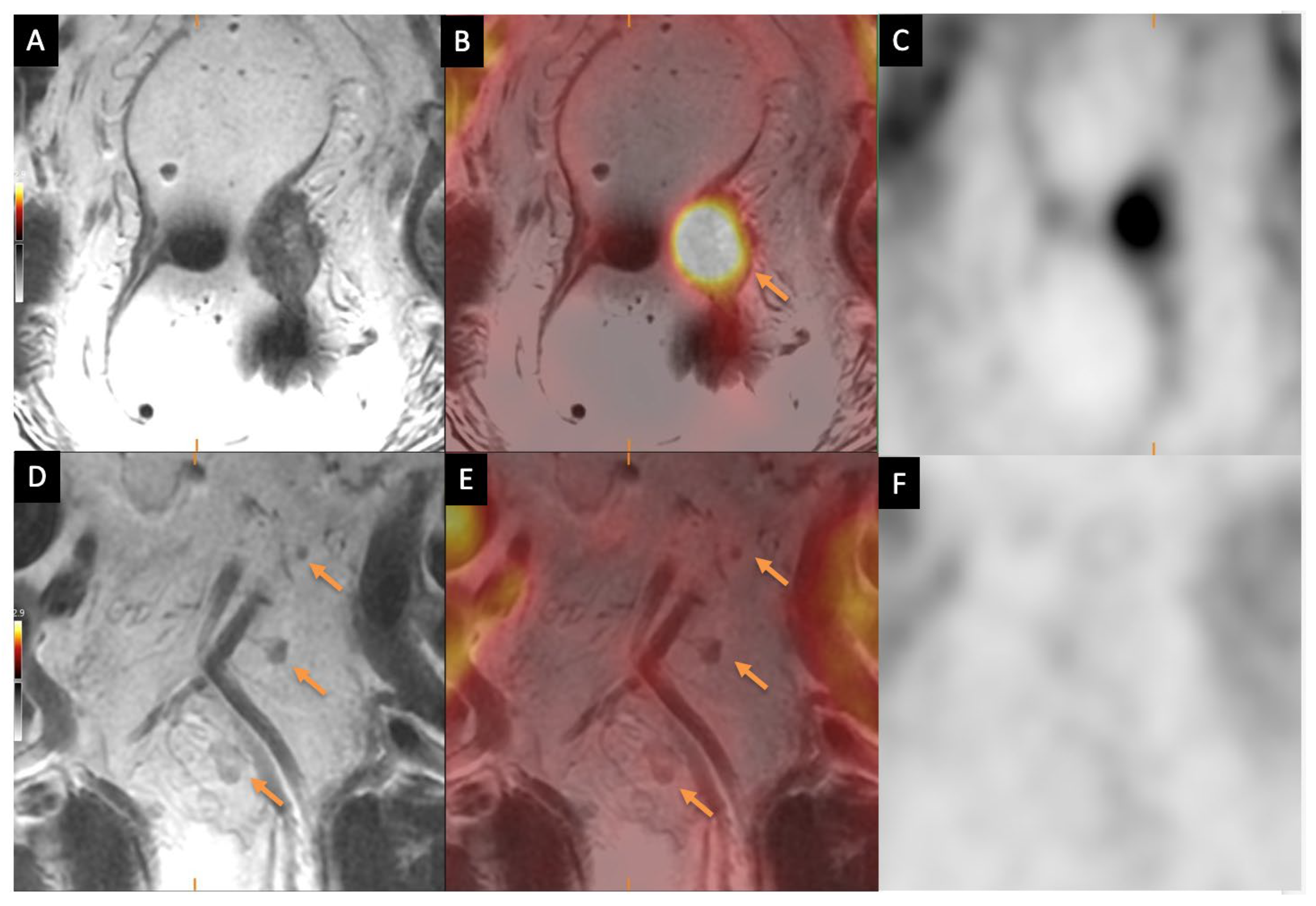
3.3. PET/MRI in Rectal Cancer Staging
3.3.1. Primary Tumor Staging
3.3.2. Nodal Staging
3.3.3. Distant Metastasis Staging
3.4. PET/MRI for Response Assessment in Rectal Cancer
3.5. PET Tracers in Rectal Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Horton, K.M.; Abrams, R.A.; Fishman, E.K. Spiral CT of Colon Cancer: Imaging Features and Role in Management. RadioGraphics 2000, 20, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Ozis, S.; Soydal, C.; Akyol, C.; Can, N.; Kucuk, O.; Yagcı, C.; Erkek, A.; Kuzu, M. The role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the primary staging of rectal cancer. World J. Surg. Oncol. 2014, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Wieder, H.; Wester, H.J.; Piert, M.; Lordick, F.; Ott, K.; Rummeny, E.; Schwaiger, M.; Weber, W.A. PET/CT molecular imaging in abdominal oncology. Abdom. Imaging 2004, 29, 388–397. [Google Scholar] [CrossRef]
- You, Y.N.; Hardiman, K.M.; Bafford, A.; Poylin, V.; Francone, T.D.; Davis, K.; Paquette, I.M.; Steele, S.R.; Feingold, D.L. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer. Dis. Colon Rectum 2020, 63, 1191. [Google Scholar] [CrossRef]
- Samee, A.; Selvasekar, C.R. Current trends in staging rectal cancer. World J. Gastroenterol. 2011, 17, 828–834. [Google Scholar] [CrossRef]
- Agarwal, A.; Marcus, C.; Xiao, J.; Nene, P.; Kachnic, L.A.; Subramaniam, R.M. FDG PET/CT in the Management of Colorectal and Anal Cancers. Am. J. Roentgenol. 2014, 203, 1109–1119. [Google Scholar] [CrossRef]
- Saklani, A.P. Magnetic resonance imaging in rectal cancer: A surgeon’s perspective. World J. Gastroenterol. 2014, 20, 2030. [Google Scholar] [CrossRef] [PubMed]
- Nicastri, D.G.; Doucette, J.T.; Godfrey, T.E.; Hughes, S.J. Is Occult Lymph Node Disease in Colorectal Cancer Patients Clinically Significant? J. Mol. Diagn. 2007, 9, 563–571. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, Z.; Liu, W.; Zeng, X.; Mo, Y. Comparison of whole-body 18F-FDG PET/CT and PET/MRI for distant metastases in patients with malignant tumors: A meta-analysis. BMC Cancer 2023, 23, 37. [Google Scholar] [CrossRef]
- Crimì, F.; Valeggia, S.; Baffoni, L.; Stramare, R.; Lacognata, C.; Spolverato, G.; Albertoni, L.; Spimpolo, A.; Evangelista, L.; Zucchetta, P.; et al. [18F]FDG PET/MRI in rectal cancer. Ann. Nucl. Med. 2021, 35, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Nensa, F.; Beiderwellen, K.; Heusch, P.; Wetter, A. Clinical applications of PET/MRI: Current status and future perspectives. Diagn. Interv. Radiol. 2014, 20, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.; Schaarschmidt, B.M.; Kirchner, J.; Suntharalingam, S.; Grueneisen, J.; Demircioglu, A.; Heusch, P.; Quick, H.H.; Forsting, M.; Antoch, G.; et al. PET/MRI Versus PET/CT for Whole-Body Staging: Results from a Single-Center Observational Study on 1,003 Sequential Examinations. J. Nucl. Med. 2020, 61, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Ince, S.; Itani, M.; Henke, L.E.; Smith, R.K.; Wise, P.E.; Mutch, M.G.; Glasgow, S.C.; Silviera, M.L.; Pedersen, K.S.; Hunt, S.R.; et al. FDG-PET/MRI for Nonoperative Management of Rectal Cancer: A Prospective Pilot Study. Tomography 2022, 8, 2723–2734. [Google Scholar] [CrossRef]
- Nougaret, S.; Rousset, P.; Lambregts, D.M.J.; Maas, M.; Gormly, K.; Lucidarme, O.; Brunelle, S.; Milot, L.; Arrivé, L.; Salut, C.; et al. MRI restaging of rectal cancer: The RAC (Response–Anal canal–CRM) analysis joint consensus guidelines of the GRERCAR and GRECCAR groups. Diagn. Interv. Imaging 2023, 104, 311–322. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; the PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Pichler, B.J.; Wehrl, H.F.; Kolb, A.; Judenhofer, M.S. Positron Emission Tomography/Magnetic Resonance Imaging: The Next Generation of Multimodality Imaging? Semin. Nucl. Med. 2008, 38, 199–208. [Google Scholar] [CrossRef]
- Delso, G.; Fürst, S.; Jakoby, B.; Ladebeck, R.; Ganter, C.; Nekolla, S.G.; Schwaiger, M.; Ziegler, S.I. Performance Measurements of the Siemens mMR Integrated Whole-Body PET/MR Scanner. J. Nucl. Med. 2011, 52, 1914–1922. [Google Scholar] [CrossRef]
- Ladefoged, C.N.; Law, I.; Anazodo, U.; Lawrence, K.S.; Izquierdo-Garcia, D.; Catana, C.; Burgos, N.; Cardoso, M.J.; Ourselin, S.; Hutton, B.; et al. A multi-centre evaluation of eleven clinically feasible brain PET/MRI attenuation correction techniques using a large cohort of patients. NeuroImage 2017, 147, 346–359. [Google Scholar] [CrossRef]
- Catana, C. Principles of Simultaneous PET/MR Imaging. Magn. Reson. Imaging Clin. N. Am. 2017, 25, 231–243. [Google Scholar] [CrossRef]
- Bashir, U.; Mallia, A.; Stirling, J.; Joemon, J.; MacKewn, J.; Charles-Edwards, G.; Goh, V.; Cook, G. PET/MRI in Oncological Imaging: State of the Art. Diagnostics 2015, 5, 333–357. [Google Scholar] [CrossRef] [PubMed]
- Petersen, H.; Holdgaard, P.C.; Madsen, P.H.; Knudsen, L.M.; Gad, D.; Gravergaard, A.E.; Rohde, M.; Godballe, C.; Engelmann, B.E.; Bech, K.; et al. FDG PET/CT in cancer: Comparison of actual use with literature-based recommendations. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 695–706. [Google Scholar] [CrossRef]
- Beets-Tan, R.G.H.; Lambregts, D.M.J.; Maas, M.; Bipat, S.; Barbaro, B.; Curvo-Semedo, L.; Fenlon, H.M.; Gollub, M.J.; Gourtsoyianni, S.; Halligan, S.; et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur. Radiol. 2018, 28, 1465–1475. [Google Scholar] [CrossRef]
- Patel, U.B.; Taylor, F.; Blomqvist, L.; George, C.; Evans, H.; Tekkis, P.; Quirke, P.; Sebag-Montefiore, D.; Moran, B.; Heald, R.; et al. Magnetic Resonance Imaging–Detected Tumor Response for Locally Advanced Rectal Cancer Predicts Survival Outcomes: MERCURY Experience. J. Clin. Oncol. 2011, 29, 3753–3760. [Google Scholar] [CrossRef]
- Hoeffel, C.; Mulé, S.; Laurent, V.; Bouché, O.; Volet, J.; Soyer, P. Primary rectal cancer local staging. Diagn. Interv. Imaging 2014, 95, 485–494. [Google Scholar] [CrossRef]
- Messina, C.; Bignone, R.; Bruno, A.; Bruno, A.; Bruno, F.; Calandri, M.; Caruso, D.; Coppolino, P.; De Robertis, R.; Gentili, F.; et al. Diffusion-Weighted Imaging in Oncology: An Update. Cancers 2020, 12, 1493. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.M.; Bus, E.M.; Hauptmann, M.; Lahaye, M.J.; Maas, M.; Ter Beek, L.C.; Beets, G.L.; Bakers, F.C.H.; Beets-Tan, R.G.H.; Lambregts, D.M.J. Gas-induced susceptibility artefacts on diffusion-weighted MRI of the rectum at 1.5 T—Effect of applying a micro-enema to improve image quality. Eur. J. Radiol. 2018, 99, 131–137. [Google Scholar] [CrossRef]
- Lee, S.J.; Seo, H.J.; Kang, K.W.; Jeong, S.-Y.; Yi, N.-J.; Lee, J.M.; Chung, J.-K.; Edmund Kim, E.; Paeng, J.C.; Cheon, G.J.; et al. Clinical Performance of Whole-Body 18F-FDG PET/Dixon-VIBE, T1-Weighted, and T2-Weighted MRI Protocol in Colorectal Cancer. Clin. Nucl. Med. 2015, 40, e392–e398. [Google Scholar] [CrossRef]
- Sinaei, M.; Swallow, C.; Milot, L.; Moghaddam, P.A.; Smith, A.; Atri, M. Patterns and Signal Intensity Characteristics of Pelvic Recurrence of Rectal Cancer at MR Imaging. RadioGraphics 2013, 33, E171–E187. [Google Scholar] [CrossRef] [PubMed]
- Bissett, I.P.; Chau, K.Y.; Hill, G.L. Extrafascial excision of the rectum: Surgical anatomy of the fascia propria. Dis. Colon Rectum 2000, 43, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, G. Preoperative Staging of Rectal Cancer Using Magnetic Resonance Imaging with External Phase-Arrayed Coils. Arch. Surg. 2002, 137, 447. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.; Radcliffe, A.G.; Newcombe, R.G.; Dallimore, N.S.; Bourne, M.W.; Williams, G.T. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br. J. Surg. 2003, 90, 355–364. [Google Scholar] [CrossRef]
- Poon, F.W.; McDonald, A.; Anderson, J.H.; Duthie, F.; Rodger, C.; McCurrach, G.; McKee, R.F.; Horgan, P.G.; Foulis, A.K.; Chong, D.; et al. Accuracy of thin section magnetic resonance using phased-array pelvic coil in predicting the T-staging of rectal cancer. Eur. J. Radiol. 2005, 53, 256–262. [Google Scholar] [CrossRef]
- Blomqvist, L.; Holm, T.; Rubio, C.; Hindmarsh, T. Rectal tumours—MR imaging with endorectal and/or phased-array coils, and histopathological staging on giant sections: A comparative study. Acta Radiol. 1997, 38, 437–444. [Google Scholar] [CrossRef]
- Rosenkrantz, A.B.; Friedman, K.; Chandarana, H.; Melsaether, A.; Moy, L.; Ding, Y.-S.; Jhaveri, K.; Beltran, L.; Jain, R. Current Status of Hybrid PET/MRI in Oncologic Imaging. Am. J. Roentgenol. 2016, 206, 162–172. [Google Scholar] [CrossRef]
- Catalano, O.A.; Lee, S.I.; Parente, C.; Cauley, C.; Furtado, F.S.; Striar, R.; Soricelli, A.; Salvatore, M.; Li, Y.; Umutlu, L.; et al. Improving staging of rectal cancer in the pelvis: The role of PET/MRI. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1235–1245. [Google Scholar] [CrossRef]
- Schneider, D.A.; Akhurst, T.J.; Ngan, S.Y.; Warrier, S.K.; Michael, M.; Lynch, A.C.; Te Marvelde, L.; Heriot, A.G. Relative Value of Restaging MRI, CT, and FDG-PET Scan After Preoperative Chemoradiation for Rectal Cancer. Dis. Colon Rectum 2016, 59, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bamba, Y.; Itabashi, M.; Kameoka, S. Management of local recurrence of colorectal cancer: The role of PET/CT. Abdom. Imaging 2011, 36, 322–326. [Google Scholar] [CrossRef]
- Li, Y.; Mueller, L.I.; Neuhaus, J.P.; Bertram, S.; Schaarschmidt, B.M.; Demircioglu, A.; Ludwig, J.M.; Kirchner, J.; Rischpler, C.; Herrmann, K.; et al. 18F-FDG PET/MR versus MR Alone in Whole-Body Primary Staging and Restaging of Patients with Rectal Cancer: What Is the Benefit of PET? J. Clin. Med. 2020, 9, 3163. [Google Scholar] [CrossRef]
- Hotta, M.; Minamimoto, R.; Yano, H.; Gohda, Y.; Shuno, Y. Diagnostic performance of 18F-FDG PET/CT using point spread function reconstruction on initial staging of rectal cancer: A comparison study with conventional PET/CT and pelvic MRI. Cancer Imaging 2018, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Herold, A.; Wassipaul, C.; Weber, M.; Lindenlaub, F.; Rasul, S.; Stift, A.; Stift, J.; Mayerhoefer, M.E.; Hacker, M.; Ba-Ssalamah, A.; et al. Added value of quantitative, multiparametric 18F-FDG PET/MRI in the locoregional staging of rectal cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 205–217. [Google Scholar] [CrossRef]
- Cho, Y.B.; Chun, H.; Kim, M.J.; Choi, J.Y.; Park, C.; Kim, B.; Lee, S.J.; Yun, S.H.; Kim, H.C.; Lee, W.Y. Accuracy of MRI and 18F-FDG PET/CT for Restaging After Preoperative Concurrent Chemoradiotherapy for Rectal Cancer. World J. Surg. 2009, 33, 2688–2694. [Google Scholar] [CrossRef]
- Langman, G.; Patel, A.; Bowley, D.M. Size and Distribution of Lymph Nodes in Rectal Cancer Resection Specimens. Dis. Colon Rectum 2015, 58, 406–414. [Google Scholar] [CrossRef]
- Lahaye, M.J.; Beets, G.L.; Engelen, S.M.E.; Kessels, A.G.H.; De Bruïne, A.P.; Kwee, H.W.S.; Van Engelshoven, J.M.A.; Van De Velde, C.J.H.; Beets-Tan, R.G.H. Locally Advanced Rectal Cancer: MR Imaging for Restaging after Neoadjuvant Radiation Therapy with Concomitant Chemotherapy Part II. What Are the Criteria to Predict Involved Lymph Nodes? Radiology 2009, 252, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Kim, J.H.; Ryu, Y.H.; Jeon, T.J.; Yu, J.-S.; Chung, J.-J. Nodal Staging of Rectal Cancer: High-Resolution Pelvic MRI Versus 18F-FDGPET/CT. J. Comput. Assist. Tomogr. 2011, 35, 531–534. [Google Scholar] [CrossRef]
- Cerny, M.; Dunet, V.; Prior, J.O.; Hahnloser, D.; Wagner, A.D.; Meuli, R.A.; Schmidt, S. Initial Staging of Locally Advanced Rectal Cancer and Regional Lymph Nodes. Clin. Nucl. Med. 2016, 41, 289–295. [Google Scholar] [CrossRef]
- Jeong, J.H.; Cho, I.H.; Chun, K.A.; Kong, E.J.; Kwon, S.D.; Kim, J.H. Correlation Between Apparent Diffusion Coefficients and Standardized Uptake Values in Hybrid 18F-FDG PET/MR: Preliminary Results in Rectal Cancer. Nucl. Med. Mol. Imaging 2016, 50, 150–156. [Google Scholar] [CrossRef]
- Heijnen, L.A.; Lambregts, D.M.J.; Mondal, D.; Martens, M.H.; Riedl, R.G.; Beets, G.L.; Beets-Tan, R.G.H. Diffusion-weighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes. Eur. Radiol. 2013, 23, 3354–3360. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.J.; Jordan, E.J.; Burke, C.; Ohliger, M.A.; Wang, Z.J.; Loon, K.V.; Varma, M.G.; Hope, T.A. Does Extended PET Acquisition in PET/MRI Rectal Cancer Staging Improve Results? Am. J. Roentgenol. 2018, 211, 896–900. [Google Scholar] [CrossRef]
- Chen, M.Z.; Zhang, X.; Mui, M.; Kong, J.C.H.; Heriot, A.G.; Ellis-Clark, J. Retrospective audit: Utility of PET scan in routine preoperative rectal cancer staging. ANZ J. Surg. 2023, 93, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Kam, M.H.; Wong, D.C.; Siu, S.; Stevenson, A.R.L.; Lai, J.; Phillips, G.E. Comparison of magnetic resonance imaging–fluorodeoxy- glucose positron emission tomography fusion with pathological staging in rectal cancer. J. Br. Surg. 2010, 97, 266–268. [Google Scholar] [CrossRef]
- Amorim, B.J.; Hong, T.S.; Blaszkowsky, L.S.; Ferrone, C.R.; Berger, D.L.; Bordeianou, L.G.; Ricciardi, R.; Clark, J.W.; Ryan, D.P.; Wo, J.Y.; et al. Clinical impact of PET/MR in treated colorectal cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2260–2269. [Google Scholar] [CrossRef]
- López Llobet, E.; Coronado Poggio, M.; Lancha Hernández, C.; Martín Hervás, C.; Travaglio Morales, D.; Monachello Araujo, D.; Rodado Marina, S.; Domínguez Gadea, L. Controversy in the initial nodal staging of rectal cancer (MRI or PET/CT?). Rev. Española Med. Nucl. Imagen Mol. (Engl. Ed.) 2024, 43, 500004. [Google Scholar] [CrossRef] [PubMed]
- Paspulati, R.M.; Partovi, S.; Herrmann, K.A.; Krishnamurthi, S.; Delaney, C.P.; Nguyen, N.C. Comparison of hybrid FDG PET/MRI compared with PET/CT in colorectal cancer staging and restaging: A pilot study. Abdom. Imaging 2015, 40, 1415–1425. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, S.Y.; Park, S.H.; Kim, K.W.; Lee, J.Y.; Lee, S.S.; Lee, M. Diagnostic performance of CT, gadoxetate disodium-enhanced MRI, and PET/CT for the diagnosis of colorectal liver metastasis: Systematic review and meta-analysis. Magn. Reson. Imaging 2018, 47, 1237–1250. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, S.H.; Lee, S.J.; Jang, K.M.; Rhim, H. Locally Advanced Rectal Cancer: Added Value of Diffusion-weighted MR Imaging for Predicting Tumor Clearance of the Mesorectal Fascia after Neoadjuvant Chemotherapy and Radiation Therapy. Radiology 2011, 260, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Kassam, Z.; Loening, A.; McNamara, M.M.; Paspulati, R. The use of PET/MRI for imaging rectal cancer. Abdom. Radiol. 2019, 44, 3559–3568. [Google Scholar] [CrossRef] [PubMed]
- Goh, V.; Gourtsoyianni, S.; Koh, D.-M. Functional Imaging of the Liver. Semin. Ultrasound CT MRI 2013, 34, 54–65. [Google Scholar] [CrossRef]
- Seto, S.; Tsujikawa, T.; Sawai, K.; Kurebayashi, H.; Morikawa, M.; Okazawa, H.; Goi, T. Feasibility of [18F]FDG PET/MRI with Early-Delayed and Extended PET as One-Stop Imaging for Staging and Predicting Metastasis in Rectal Cancer. Oncology 2022, 100, 212–220. [Google Scholar] [CrossRef]
- Queiroz, M.A.; Ortega, C.D.; Ferreira, F.R.; Nahas, S.C.; Cerri, G.G.; Buchpiguel, C.A. Diagnostic accuracy of FDG-PET/MRI versus pelvic MRI and thoracic and abdominal CT for detecting synchronous distant metastases in rectal cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 186–195. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, J.M.; Chang, W.; Kang, H.-J.; Bandos, A.; Lim, H.-J.; Kang, S.Y.; Kang, K.W.; Ryoo, S.-B.; Jeong, S.-Y.; et al. Initial M Staging of Rectal Cancer: FDG PET/MRI with a Hepatocyte-specific Contrast Agent versus Contrast-enhanced CT. Radiology 2020, 294, 310–319. [Google Scholar] [CrossRef]
- Rutegård, M.K.; Båtsman, M.; Axelsson, J.; Brynolfsson, P.; Brännström, F.; Rutegård, J.; Ljuslinder, I.; Blomqvist, L.; Palmqvist, R.; Rutegård, M.; et al. PET/MRI and PET/CT hybrid imaging of rectal cancer—description and initial observations from the RECTOPET (REctal Cancer trial on PET/MRI/CT) study. Cancer Imaging 2019, 19, 52. [Google Scholar] [CrossRef] [PubMed]
- Rutegård, M.K.; Båtsman, M.; Blomqvist, L.; Rutegård, M.; Axelsson, J.; Ljuslinder, I.; Rutegård, J.; Palmqvist, R.; Brännström, F.; Brynolfsson, P.; et al. Rectal cancer: A methodological approach to matching PET/MRI to histopathology. Cancer Imaging 2020, 20, 80. [Google Scholar] [CrossRef]
- Akkus Gunduz, P.; Ozkan, E.; Kuru Oz, D.; Soydal, C.; Araz, M.; Erden, G.A.; Yavuz, Y.; Kucuk, N.O.; Kir, K.M. Clinical value of fluorine-18-fluorodeoxyglucose PET/MRI for liver metastasis in colorectal cancer: A prospective study. Nucl. Med. Commun. 2023, 44, 150–160. [Google Scholar] [CrossRef]
- Eglinton, T.; Luck, A.; Bartholomeusz, D.; Varghese, R.; Lawrence, M. Positron-emission tomography/computed tomography (PET/CT) in the initial staging of primary rectal cancer. Color. Dis. 2010, 12, 667–673. [Google Scholar] [CrossRef]
- Faneyte, I.F.; Dresen, R.C.; Edelbroek, M.A.L.; Nieuwenhuijzen, G.A.P.; Rutten, H.J.T. Pre-Operative Staging with Positron Emission Tomography in Patients with Pelvic Recurrence of Rectal Cancer. Dig. Surg. 2008, 25, 202–207. [Google Scholar] [CrossRef]
- Zhou, N.; Guo, X.; Sun, H.; Yu, B.; Zhu, H.; Li, N.; Yang, Z. The Value of 18F-FDG PET/CT and Abdominal PET/MRI as a One-Stop Protocol in Patients with Potentially Resectable Colorectal Liver Metastases. Front. Oncol. 2021, 11, 714948. [Google Scholar] [CrossRef]
- Agrawal, A.; Kazi, M.; Gori, J.; Dev, I.; Rangarajan, V.; Veer, A.; Patil, P.; Engineer, R.; Desouza, A.; Saklani, A. Prospective study to assess the role of FDG PET/CT in detecting systemic metastatic spread in rectal cancers with lateral pelvic lymph nodes. Eur. J. Surg. Oncol. 2022, 48, 1093–1099. [Google Scholar] [CrossRef]
- Jemal, A.; Murray, T.; Ward, E.; Samuels, A.; Tiwari, R.C.; Ghafoor, A.; Feuer, E.J.; Thun, M.J. Cancer Statistics, 2005. CA Cancer J. Clin. 2005, 55, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Kokaine, L.; Gardovskis, A.; Gardovskis, J. Evaluation and Predictive Factors of Complete Response in Rectal Cancer after Neoadjuvant Chemoradiation Therapy. Medicina 2021, 57, 1044. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.B.; Blomqvist, L.K.; Taylor, F.; George, C.; Guthrie, A.; Bees, N.; Brown, G. MRI After Treatment of Locally Advanced Rectal Cancer: How to Report Tumor Response—The MERCURY Experience. Am. J. Roentgenol. 2012, 199, W486–W495. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.S.; Hosseini-Nik, H. MRI of Rectal Cancer: An Overview and Update on Recent Advances. Am. J. Roentgenol. 2015, 205, W42–W55. [Google Scholar] [CrossRef]
- Grillo-Ruggieri, F.; Mantello, G.; Berardi, R.; Cardinali, M.; Fenu, F.; Iovini, G.; Montisci, M.; Fabbietti, L.; Marmorale, C.; Guerrieri, M.; et al. Mucinous Rectal Adenocarcinoma Can Be Associated to Tumor Downstaging after Preoperative Chemoradiotherapy. Dis. Colon Rectum 2007, 50, 1594–1603. [Google Scholar] [CrossRef]
- Van Der Paardt, M.P.; Zagers, M.B.; Beets-Tan, R.G.H.; Stoker, J.; Bipat, S. Patients Who Undergo Preoperative Chemoradiotherapy for Locally Advanced Rectal Cancer Restaged by Using Diagnostic MR Imaging: A Systematic Review and Meta-Analysis. Radiology 2013, 269, 101–112. [Google Scholar] [CrossRef]
- Plodeck, V.; Platzek, I.; Streitzig, J.; Nebelung, H.; Blum, S.; Kühn, J.-P.; Hoffmann, R.-T.; Laniado, M.; Michler, E.; Hoberück, S.; et al. Diagnostic performance of 18F-fluorodeoxyglucose-PET/MRI versus MRI alone in the diagnosis of pelvic recurrence of rectal cancer. Abdom. Radiol. 2021, 46, 5086–5094. [Google Scholar] [CrossRef]
- Cerny, M.; Dunet, V.; Rebecchini, C.; Hahnloser, D.; Prior, J.; Sempoux, C.; Schmidt, S. Response of locally advanced rectal cancer (LARC) to radiochemotherapy: DW-MRI and multiparametric PET/CT in correlation with histopathology. Nuklearmedizin 2019, 58, 28–38. [Google Scholar] [CrossRef]
- Capirci, C.; Rampin, L.; Erba, P.A.; Galeotti, F.; Crepaldi, G.; Banti, E.; Gava, M.; Fanti, S.; Mariani, G.; Muzzio, P.C.; et al. Sequential FDG-PET/CT reliably predicts response of locally advanced rectal cancer to neo-adjuvant chemo-radiation therapy. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1583–1593. [Google Scholar] [CrossRef]
- Lambrecht, M.; Deroose, C.; Roels, S.; Vandecaveye, V.; Penninckx, F.; Sagaert, X.; Van Cutsem, E.; De Keyzer, F.; Haustermans, K. The use of FDG-PET/CT and diffusion-weighted magnetic resonance imaging for response prediction before, during and after preoperative chemoradiotherapy for rectal cancer. Acta Oncol. 2010, 49, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Kariv, Y.; Berkovitz, R.; El-On, R.; Barenboim, A.; Tulchinsky, H.; Zemel, M.; Brautbar, O.; Mirelman, D.; Pelles-Avraham, S.; Geva, R.; et al. MRI is more accurate than FDG-PET in assessing complete response in rectal cancer patients after neoadjuvant therapy. Langenbecks Arch. Surg. 2025, 410, 106. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, E.; Lambreton, F.; Yu, J.Q.; Li, T.; Denlinger, C.S.; Meyer, J.E.; Sigurdson, E.R.; Farma, J.M. Impact of PET/CT for Restaging Patients with Locally Advanced Rectal Cancer After Neoadjuvant Chemoradiation. J. Surg. Res. 2019, 243, 242–248. [Google Scholar] [CrossRef]
- Tey, J.; Tan, J.K.; Tan, K.-K.; Soon, Y.Y.; Loi, H.Y.; Mohamed, J.S.A.; Bakulbhai, P.A.; Ang, B.; Liang, T.Y. Restaging of rectal cancer with hybrid positron emission tomography magnetic resonance imaging after preoperative chemoradiotherapy. Ann. Acad. Med. Singap. 2023, 52, 289–295. [Google Scholar] [CrossRef]
- Ippolito, D.; Fior, D.; Trattenero, C.; Ponti, E.D.; Drago, S.; Guerra, L.; Franzesi, C.T.; Sironi, S. Combined value of apparent diffusion coefficient-standardized uptake value max in evaluation of post-treated locally advanced rectal cancer. World J. Radiol. 2015, 7, 509. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Okamoto, M.; Takahashi, T.; Motegi, M.; Ogoshi, K.; Shoji, H.; Onishi, M.; Takakusagi, Y.; Okonogi, N.; Kawamura, H.; et al. SUVmax -based Parameters of FDG-PET/CT Reliably Predict Pathologic Complete Response After Preoperative Hyperthermo-chemoradiotherapy in Rectal Cancer. Anticancer Res. 2018, 38, 5909–5916. [Google Scholar] [CrossRef] [PubMed]
- Aiba, T.; Uehara, K.; Nihashi, T.; Tsuzuki, T.; Yatsuya, H.; Yoshioka, Y.; Kato, K.; Nagino, M. MRI and FDG-PET for Assessment of Response to Neoadjuvant Chemotherapy in Locally Advanced Rectal Cancer. Ann. Surg. Oncol. 2014, 21, 1801–1808. [Google Scholar] [CrossRef]
- Asoglu, O.; Tokmak, H.; Bakir, B.; Demir, G.; Ozyar, E.; Atalar, B.; Goksel, S.; Koza, B.; Guven Mert, A.; Demir, A.; et al. The impact of total neo-adjuvant treatment on nonoperative management in patients with locally advanced rectal cancer: The evaluation of 66 cases. Eur. J. Surg. Oncol. 2020, 46, 402–409. [Google Scholar] [CrossRef]
- Avcı, G.G.; Aral, I.P. The role of MRI and 18F-FDG PET/CT with respect to evaluation of pathological response in the rectal cancer patients after neoadjuvant chemoradiotherapy. Indian J. Cancer 2023, 60, 52–58. [Google Scholar] [CrossRef]
- Denecke, T.; Rau, B.; Hoffmann, K.-T.; Hildebrandt, B.; Ruf, J.; Gutberlet, M.; Hünerbein, M.; Felix, R.; Wust, P.; Amthauer, H. Comparison of CT, MRI and FDG-PET in response prediction of patients with locally advanced rectal cancer after multimodal preoperative therapy: Is there a benefit in using functional imaging? Eur. Radiol. 2005, 15, 1658–1666. [Google Scholar] [CrossRef]
- Ferri, V.; Vicente, E.; Quijano, Y.; Duran, H.; Diaz, E.; Fabra, I.; Malave, L.; Ruiz, P.; Ballelli, L.; Broglio, A.; et al. Predicting treatment response and survival in rectal cancer: Insights from 18 FDG-PET/MRI post-neoadjuvant therapy. Int. J. Color. Dis. 2025, 40, 6. [Google Scholar] [CrossRef] [PubMed]
- Vuijk, F.A.; Feshtali Shahbazi, S.; Noortman, W.A.; Van Velden, F.H.P.; Dibbets-Schneider, P.; Marinelli, A.W.K.S.; Neijenhuis, P.A.; Schmitz, R.; Ghariq, E.; Velema, L.A.; et al. Baseline and early digital [18F]FDG PET/CT and multiparametric MRI contain promising features to predict response to neoadjuvant therapy in locally advanced rectal cancer patients: A pilot study. Nucl. Med. Commun. 2023, 44, 613–621. [Google Scholar] [CrossRef]
- Schurink, N.W.; Van Kranen, S.R.; Berbee, M.; Van Elmpt, W.; Bakers, F.C.H.; Roberti, S.; Van Griethuysen, J.J.M.; Min, L.A.; Lahaye, M.J.; Maas, M.; et al. Studying local tumour heterogeneity on MRI and FDG-PET/CT to predict response to neoadjuvant chemoradiotherapy in rectal cancer. Eur. Radiol. 2021, 31, 7031–7038. [Google Scholar] [CrossRef]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; Van Oosterom, A.T.; Christian, M.C.; et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. JNCI J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef]
- Demetri, G.D.; Von Mehren, M.; Blanke, C.D.; Van Den Abbeele, A.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; et al. Efficacy and Safety of Imatinib Mesylate in Advanced Gastrointestinal Stromal Tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Goshen, E.; Davidson, T.; Zwas, S.T.; Aderka, D. PET/CT in the Evaluation of Response to Treatment of Liver Metastases from Colorectal Cancer with Bevacizumab and Irinotecan. Technol. Cancer Res. Treat. 2006, 5, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.L.; Visvikis, D.; Costa, D.C.; Arulampalam, T.H.A.; Townsend, C.; Luthra, S.K.; Taylor, I.; Ell, P.J. Potential impact of [18F]3′-deoxy-3′-fluorothymidine versus [18F]fluoro-2-deoxy-d-glucose in positron emission tomography for colorectal cancer. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 988–994. [Google Scholar] [CrossRef]
- Puri, T.; Greenhalgh, T.A.; Wilson, J.M.; Franklin, J.; Wang, L.M.; Strauss, V.; Cunningham, C.; Partridge, M.; Maughan, T. [18F]Fluoromisonidazole PET in rectal cancer. EJNMMI Res. 2017, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Wieder, H.A.; Geinitz, H.; Rosenberg, R.; Lordick, F.; Becker, K.; Stahl, A.; Rummeny, E.; Siewert, J.R.; Schwaiger, M.; Stollfuss, J. PET imaging with [18F]3′-deoxy-3′-fluorothymidine for prediction of response to neoadjuvant treatment in patients with rectal cancer. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 878–883. [Google Scholar] [CrossRef]
- Havelund, B.; Holdgaard, P.; Rafaelsen, S.; Ploen, J.; Mortensen, L.; Theil, J.; Bender, D.; Spindler, K.-L.; Jakobsen, A. P-0298 18F-Faza Pet/Ct in Patients with Rectal Cancer. Ann. Oncol. 2012, 23, iv116. [Google Scholar] [CrossRef]
- Uslu-Beşli, L.; Mermut, Ö.; Yardimci, A.H.; Gündoğan, C.; Gürsu, R.U.; Çermik, T.F. Comparison of 18F-FDG PET/CT and DW-MRI in assessment of neoadjuvant radiochemotherapy response in locally advanced rectal cancer patients. Rev. Española Med. Nucl. Imagen Mol. (Engl. Ed.) 2021, 40, 19–29. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Z.; Feng, Y.; Lin, Z.; Tao, K.; Zhang, T.; Lan, X. Predicting Pathologic Complete Response in Locally Advanced Rectal Cancer with [68Ga]Ga-FAPI-04 PET, [18F]FDG PET, and Contrast-Enhanced MRI: Lesion-to-Lesion Comparison with Pathology. J. Nucl. Med. 2024, 65, 1548–1556. [Google Scholar] [CrossRef]


| Sequence | Vendor | Field Strength | TR (ms) | TE (ms) | Other Parameters |
|---|---|---|---|---|---|
| T2W FSE (Axial, Sagittal, Coronal) | GE | 1.5/3 T | 4000–6000 | 100–120 | PROPELLER to reduce motion artifacts |
| Siemens | 1.5/3 T | 3000–5000 | 80–110 | Turbo spin-echo (TSE), no fat suppression | |
| Philips | 1.5/3 T | 4000–6000 | 90–130 | TSE, high-resolution imaging | |
| United Imaging | 1.5/3 T | 4000–6000 | 90–120 | TSE, motion correction (uCS compressed sensing optional) | |
| T2W FSE (Large FOV Axial) | GE | 1.5/3 T | 5000–8000 | 90–130 | Full pelvis coverage |
| Siemens | 1.5/3 T | 4000–7000 | 90–120 | TSE, large field of view | |
| Philips | 1.5/3 T | 5000–8000 | 100–140 | TSE, no fat suppression | |
| United Imaging | 1.5/3 T | 4500–7500 | 90–130 | TSE, large FOV, optional fat sat | |
| DWI (b ≥ 800 sec/mm2) | GE | 1.5/3 T | 3000–5000 | Min Full | EPI-based, Fat suppression |
| Siemens | 1.5/3 T | 3000–4500 | Min Full | RESOLVE for motion correction | |
| Philips | 1.5/3 T | 3000–5000 | Min Full | DIXON for fat suppression | |
| United Imaging | 1.5/3T | 3000–5000 | Min Full | EPI-based DWI, DWI with distortion correction, fat suppression | |
| T1W Imaging (Wide FOV) | GE | 1.5/3 T | 500–800 | 10–15 | Covers distant nodes, incidental findings |
| Siemens | 1.5/3 T | 500–900 | 10–20 | TSE for soft tissue contrast | |
| Philips | 1.5/3 T | 500–900 | 10–18 | dS TSE for optimized signal | |
| United Imaging | 1.5/3 T | 500–850 | 10–18 | TSE, uCS accelerated, wide coverage |
| Author | Year | Study Design | Sample Size | Study Objective | Key Findings Related to MRI Sequences |
|---|---|---|---|---|---|
| Beets et al. [23] | 2018 | Expert-based consensus study | 14 abdominal imaging experts participated in a consensus meeting where 246 questionnaire items were scored and classified as ‘appropriate’ or ‘inappropriate’. | Demonstrates the 2012 ESGAR consensus guidelines on the MRI acquisition and reporting for clinical staging and restaging of rectal cancer. | 85% of the panel agrees for use of DWI in T-stage assessment; 54% agrees for DWI alone for complete response detection; they agreed on no current role of ADC quantification due to lack of standardization; the panel reached full consensus for qualitative assessment of DWI and ADC maps; DCE-MRI should be considered a research tool, not for routine use |
| Hoeffel et al. [25] | 2014 | Systematic review based upon continuing education program | Meta-analysis from 2000–2011 | Focuses on assessment of the initial locoregional extension of rectal tumors. It also highlights spasmolytic agents in case of tumor delineation. | Spasmolytic agents allow better tumor delineation and reduce artifacts in diffusion-weighted acquisition. Rectal tumors are accurate in T2-weighted MR. T2-weighted sequences without fat suppression in the three planes and perpendicular to the axis of the tumor are essential and sufficient |
| Messina et al. [26] | 2020 | Systematic review | An up-to-date review | Focuses on tissue signal attenuation with increasing b values. | DWI with b values 1000 can be helpful in primary staging of rectal cancer in adjunction to conventional sequences |
| Van Griethuysen et al. [27] | 2018 | Retrospective study | 50 rectal cancer patients from 2012 to 2016 who underwent DWI MRI | Focuses on protocol usage of micro-enema in EPI-DWI and its effects related to susceptibility artifacts in DW imaging of the rectum at 1.5 T. | First study to show that application of a micro-enema shortly before image acquisition reduces susceptibility artifacts on rectal DWI |
| Lee et al. [28] | 2015 | Retrospective study | 59 colorectal patients | To investigate whole-body FDG PET/Dixon-VIBE, T1-weighted, and T2-weighted MRI protocols | PET/Dixon-VIBE/T1/T2 MRI protocol is clinically useful for TNM staging (95.7% sensitivity) and chemonaive hepatic metastasis |
| Sinaei et al. [29] | 2013 | Retrospective study | 42 patients with pelvic recurrence of rectal cancer from 1998 to 2009 | Focuses on identification of pelvic recurrence and evaluation of T2 signal intensity to differentiate tumor with fibrosis. | Recurrence commonly occurs in the anterior pelvic structures, sidewall, presacral space, and pelvic floor. An intermediate or high T2 signal and soft-tissue enhancement beyond six months post-surgery should raise suspicion for recurrence |
| Author | Year | Study Design | Sample Size | Study Objective | Key Findings |
|---|---|---|---|---|---|
| Crimì et al. [11] | 2021 | Retrospective study | 36 patients with LARC | To assess FDG-PET/MRI for TNM restaging | [18F]FDG-PET/MRI reported a sensitivity of 94–100%, specificity of 73–94%, and accuracy of 92–100% for T-staging; for N staging, sensitivity, specificity, and accuracy were 90–93%, 92–94%, and 42–90%; for M staging, [18F]FDG-PET/MRI showed 96% sensitivity, 80% specificity, and 74% accuracy |
| Patel et al. [24] | 2011 | Prospective study | 111 rectal cancer patients treated by neoadjuvant therapy between February 2002 and October 2003 | To determine and correlate MRI assessment of TRG and CRM with pathological staging | Both MRI T-staging and tumor regression grade (mTRG) showed a statistical correlation with ypT (post-treatment histopathological T stage). Among 23 of 111 patients with a positive circumferential resection margin (CRM), 21 (91%) had tumors classified as ypT-poor, indicating poor treatment response and higher recurrence risk |
| Gagliardi et al. [31] | 2002 | Retrospective study | 28 rectal cancer patients who did not undergo irradiation | Focuses on comparing MRI with pathological staging | MRI for bowel wall invasion: Sensitivity 89%, specificity 80%, and accuracy 86%, indicating high reliability in detecting tumor extension beyond the bowel wall. MRI for Malignant Lymphadenopathy: sensitivity 67%, specificity 71%, and accuracy 69%, showing moderate performance in identifying metastatic lymph nodes |
| Brown et al. [32] | 2003 | Retrospective study | 98 patients undergoing total mesorectal excision | To determine accuracy of preoperative MRI in evaluating pathological and surgical prognostic factors related to local recurrence | Study highlights clinical case where T2-weighted fast spin-echo can identify extramural vascular expansion which is confirmed in histological WSI. The same has been found for prediction of circumferential resection margin and tumor perforation through peritoneum. MRI showed cases where T1-T2 tumors were staged as T2 and eight pT2 tumors were staged as T3 |
| Poon et al. [33] | 2005 | Retrospective study | 42 rectal cancer patients who underwent surgical resection for primary tumor | To evaluate the accuracy of T2-weighted MRI. | MRI correctly predicted 31 out of 42 histopathological T-stage lesions with an accuracy of 74%. MR sensitivity and specificity was 62% and 79% for pT2 lesions, 84% and 59% for pT3 lesions. and 50% and 76% for pT4 lesions |
| Blomquist et al. [34] | 1997 | Retrospective study | 26 patients who underwent MRI after mesorectal excision | To investigate whether MRI can predict tumor involvement of lateral resection margin | Presence of tumor-free lateral resection margin can be predicted by MRI when it exceeds 1 mm |
| Catalano et al. [36] | 2021 | Retrospective study | 62 rectal cancer patients | To assess [18F]FDG-PET/CT with CT and MRI alone in clinical staging | For local staging, sensitivity, specificity, and accuracy were reported as 79%, 78%, and 73% for CT; 86%, 77%, and 82% for MRI; and 90%, 76%, and 85% for [18F]FDG-PET/CT; for distant staging, sensitivity, specificity, and accuracy were reported as 89%, 94.9%, and 77% for CT; 85%, 99%, and 98% for MRI; and 86%, 97.2%, and 83% for [18F]FDG-PET/CT |
| Schneider et al. [37] | 2016 | Retrospective study | 199 rectal cancer patients | To assess changes in staging observed with PET-CT, CT, and MRI before and after neoadjuvant chemoradiotherapy | T stage was accurate on restaging MRI in 43% patients, with 34% patients overstaged and 18% understaged. In N stage, accuracy of MRI and PET was 56% and 60% |
| Bamba et al. [38] | 2011 | Retrospective study | 256 CRC including preoperative and postoperative patients | To assess sensitivity and specificity of FDG-PET/CT for diagnosing local recurrence compared to CT/MRI | 95.5% patients with local recurrence had positive [18F]FDG uptake by PET/CT with 4.5% negative results; CT/MRI suspected 45.5% cases to be positive and 4.5% negative. PET/CT had 95.5% sensitivity and 100% specificity |
| Li et al. [39] | 2020 | Retrospective study | 34 patients with rectal cancer | To investigate diagnostic performance of [18F]FDG-PET/MRI and MRI alone for staging and restaging of rectal cancer patients | Both [18F]FDG-PET/MRI and MRI alone showed similar accuracies for locoregional T-staging; for N staging, combined [18F]FDG-PET/MRI showed a sensitivity of 93.8% and specificity of 91.7% compared to MRI alone (sensitivity 93.8%, specificity 83.3%); in distant metastases, combined PET/MRI had lower sensitivity (82.6%) and specificity (87%) than MRI alone (sensitivity 87%, specificity 91.3%) |
| Hotta et al. [40] | 2018 | Retrospective study | 59 rectal cancer patients | To assess diagnostic performance of [18F]FDG-PET/CT using point spread function reconstruction over conventional PET/CT on initial staging | For N staging, PSF-PET/CT showed higher sensitivity (78.6%) than conventional PET/CT (64.3%) and MRI (57.1%). Accuracy of T-staging was 69.4% for PSF-PET/CT compared to conventional PET/CT (73.5%) and MRI (73.5%). In M staging, both PSF-PET/CT and conventional PET/CT diagnosed all distant metastases accurately |
| Herold et al. [41] | 2022 | Retrospective study | 46 patients with primary rectal cancer | To determine whether combined PET/MRI can improve LARC and to assess its prognostic value after resection | For T-staging, PET/MRI showed a sensitivity and specificity of 90% and 50% compared to MRI sensitivity (55%) and specificity (65.4%). For N staging, PET/MRI showed a sensitivity and specificity of 63.6% and 76.5% compared to MRI sensitivity (72.7%) and specificity (70.6%) |
| Cho et al. [42] | 2009 | Retrospective study | 30 rectal cancer patients | To investigate accuracy of MRI and [18F]FDG- PET/CT for restaging after preoperative CCRT | Overall accuracy of MRI for T and N staging was 67% and 75%; [18F]FDG-PET/CT was 60% and 71%; [18F]FDG-PET/CT identified distant metastases with 97% accuracy |
| Author | Year | Study Design | Sample Size | Study Objective | Key Findings |
|---|---|---|---|---|---|
| Ince et al. [14] | 2022 | Retrospective study | 14 rectal cancer patients | To assess [18F]FDG-PET/MRI at initial and post-TNT restaging | [18F]FDG-PET/MRI after post TNT can detect more residual diseases than MRI alone and provided added value to 82% restaging cases |
| Lahaye et al. [44] | 2009 | Retrospective study | 39 patients with rectal cancer | To determine diagnostic performance of nodal restaging using MRI after radiation therapy | 2D T2-weighted fast spin-echo images could be accurately matched with 201 lymph nodes (40 malignant; 285 benign) out of 325 lymph nodes |
| Kim et al. [45] | 2011 | Retrospective study | 30 rectal cancer patients who underwent surgery from September 2008 to March 2009 | To compare MRI with [18F]FDG-PET/CT for preoperative assessment of nodal staging | MRI predicted nodal status with 83% accuracy, 94% sensitivity and 67% specificity. [18F]FDG-PET/CT had 70% accuracy, 61% sensitivity, and 83% specificity. Combining MRI and [18F]FDG-PET/CT improved overall accuracy to 90%, with 94% sensitivity and 83% specificity |
| Cerny et al. [46] | 2016 | Retrospective study | 27 patients with locally advanced rectal cancer from October 2012 to September 2014 | To compare DWI-MRI with [18F]FDG-PET/CT in LARC patients | Pathological nodes exhibited higher SUVmax and SUVmean on FDG-PET/CT and lower ADCmean on MRI |
| Jeong et al. [47] | 2016 | Retrospective study | 9 patients with confirmed rectal adenocarcinoma | To assess correlation between SUV and ADC values associated with [18F]FDG-PET/MRI | There is an inverse correlation between SUV of [18F]FDG-PET/MRI and ADC of DWI |
| Heijnen et al. [48] | 2013 | Retrospective study | 21 rectal cancer patients underwent surgery | To compare DWI MRI in lymph node characterization | DWI with b1000 detected 129/212 nodes compared to the 117/212 of T2-weighted MRI but could not identify metastatic nodes |
| Bailey et al. [49] | 2018 | Retrospective study | 22 rectal cancer patients | To assess if extended PET acquisition in pelvis during [18F]FDG-PET/MRI improves metastasis lymph node staging | Out of a total 94 lymph nodes, extended 15 min PET acquisition detected 39.4% more abnormal lymph nodes (≤5 mm); 64.7% were upstaged on increased PET acquisition time |
| Chen et al. [50] | 2023 | Retrospective study | 357 patients with 30.3% stage 3 rectal cancer and 71.7% receiving neoadjuvant therapy | To assess and compare PET findings with CT and MRI for accurate treatment management | PET/MRI helped provide a treatment strategy compared to CT alone or PET/CT in 21.6% patients; both PET/MRI and PET/CT showed overall accuracy in detecting peritoneal and lymph node metastases |
| Kam et al. [51] | 2010 | Retrospective study | 23 patients with rectal adenocarcinoma | To compare [18F]FDG-PET with MRI in rectal cancer primary staging | MRI-PET fusion showed 44% sensitivity and 100% specificity for nodal assessment compared to FDG-PET (21% and 56%) and CT (25% and 60%) |
| Amori et al. [52] | 2019 | Retrospective study | 42 rectal cancer patients | To assess staging performance of [18F]FDG-PET/MRI versus final oncologic stage | Compared with final oncologic stage, [18F]FDG-PET/MRI was correct in detecting 6/42(14%) liver lesions; in 2 patients who were candidates for peritonectomy, already in stage IV, PET/MRI disclosed multiple lesions in other organs, leading to abortion of operation |
| López et al. [53] | 2024 | Retrospective study | 73 LARC patients | To compare MRI and PET/CT in nodal staging | MRI reported a sensitivity and specificity of 80% and 75% compared to PET/CT, with a sensitivity and specificity of 60% and 100%; PET/CT identified pelvic metastatic adenopathies in 8 patients not visible by MRI |
| Paspulati et al. [54] | 2015 | Retrospective study | 12 rectal cancer patients | To compare hybrid [18F]FDG PET/MRI with PET/CT in staging and restaging | In 2 patients with rectal staging, both modalities showed no evidence of locoregional lymph node or distant metastasis. PET/CT showed the least accuracy in N and M staging and restaging—71% over 86% for PET/MRI; PET/CT failed to identify 3 lesions compared to PET/MRI |
| Author | Year | Study Design | Sample Size | Study Objective | Key Findings |
|---|---|---|---|---|---|
| Seto et al. [59] | 2022 | Retrospective study | 23 patients who underwent preoperative treatment | To assess [18F]FDG- PET/MRI without contrast for predicting metastasis | [18F]FDG-PET detected EMVI with irregular vessel and liver and lung metastasis with 100% accuracy |
| Queiroz et al. [60] | 2020 | Retrospective study | 101 rectal cancer patients from November 2016 to April 2018 | To assess diagnostic accuracy of [18F]FDG-PET/MRI for distant metastasis | [18F]FDG-PET/MRI reported 89.2% accuracy for liver and 76.4% accuracy for lung |
| Yoon et al. [61] | 2019 | Retrospective study | 71 patients from January 2016 to August 2017 | To compare contrast agent [18F]FDG-PET/MRI with contrast-enhanced CT | [18F]FDG-PET/MRI showed 98% specificity for metastasis staging |
| Rutegård et al. [62] | 2019 | Retrospective study | 24 patients with 2 diagnosed with hepatic metastasis | To assess [18F]FDG-PET/CT for initial and restaging | The two patients were upstaged to M1 disease from [18F]FDG-PET/CT staging |
| Rutegård et al. [63] | 2020 | Retrospective study | 27 patients | Assess [18F]FDG-PET/MRI results with histopathological findings | [18F]FDG-PET/MRI facilitates mesorectal structures and matches with histopathological findings than MRI alone |
| Akkus et al. [64] | 2023 | Prospective study | 78 patients with CRC and liver metastasis | To evaluate diagnostic performance of [18F]FDG-PET/CT, PET/MRI and MRI alone | Sensitivity of 55.6%, 97.2%, and 100%; specificity of 98.5%, 100%, and 80.5%; and accuracy of 70.7%, 98.2%, and 93.1% were reported for [18F]FDG-PET/CT, PET/MRI, and MRI |
| Eglinton et al. [65] | 2010 | Prospective study | 20 patients from March 2006 to September 2007 with rectal adenocarcinoma | To assess the role of [18F]FDG PET/CT compared to CT and MRI alone in initial staging of primary rectal adenocarcinoma | PET/CT identified primary tumor in all patients with 100% sensitivity and suggested presence of incidental extra-rectal lesions |
| Faneyte et al. [66] | 2008 | Retrospective study | 70 locally recurrent rectal cancer patients between January 2003 to October 2006 | To evaluate the findings of PET, CT, and MRI for lymph node metastases | PET findings differed from CT and MRI in 35% cases; 11% of the cases could have been prevented for unnecessary operations based on PET scans |
| Zhou et al. [67] | 2021 | Prospective study | 56 patients with colorectal liver metastases | To evaluate simultaneous abdominal [18F]FDG-PET/CT and [18F]FDG-PET/MRI in detection of liver metastases | [18F]FDG-PET/MRI showed higher accuracy (99.5%) compared to [18F]FDG-PET/CT (47.5%), except where [18F] FDG-PET/CT showed a valuable result in extrahepatic disease detection |
| Agrawal et al. [68] | 2022 | Prospective study | 44 rectal cancer patients | To assess [18F]FDG-PET/CT in metastatic spread in rectal cancer | 9 patients were detected to have an extra-pelvic site of metastasis; 5 had disease upstaging after PET scan; detection of metastasis led to change in treatment plan in 7 patients |
| Author | Year | Study Design | Sample Size | Study Objective | Key Findings |
|---|---|---|---|---|---|
| Ince et al. [14] | 2022 | Retrospective study | 14 rectal cancer patients | To determine whether [18F]FDG-PET can give clinical complete response (cCR) over MRI alone. | [18F]FDG-PET provided added value in over 80% of restaging cases, based on reader assessments. [18F]FDG-PET/MRI assessments of cCR status at post-TNT restaging had an accuracy of 100%, compared with 71% for MRI alone |
| Ruggieri et al. [73] | 2007 | Retrospective study | 136 rectal cancer patients before surgery | To evaluate downstaging in patients treated with preoperative chemoradiation | Out of 136 patients, 44 patients had complete response, 52 patients had partial response, 37 patients showed no change, and 3 patients had progression |
| Van Der et al. [74] | 2013 | Systematic review and meta-analysis | To assess diagnostic performance of MRI on restaging locally advanced rectal cancer | Restaging with DWI showed sensitivity of 83.6% and specificity of 84.8%, and a moderate result for restaging of CRM | |
| Plodeck et al. [75] | 2021 | Retrospective study | 40 rectal cancer patients | To assess [18F]FDG-PET/MRI in diagnosis of local recurrence of rectal cancer | [18F]FDG-PET/MRI showed sensitivity of 94%, specificity of 88%, and accuracy of 93% in detecting recurrence |
| Capirci et al. [77] | 2007 | Prospective study | 44 rectal cancer patients who underwent chemoradiation therapy between December 2003 and December 2005 | To assess [18F]FDG-PET/CT in prediction of response treatment | [18F]FDG-PET/CT specificity in the detection of neoadjuvant response ranged between 70% and 80% |
| Lambrecht et al. [78] | 2010 | Prospective study | 22 rectal adenocarcinoma patients between May 2005 and August 2009 | To assess and compare [18F]FDG PET with DWI-MRI for response assessment during and after CRT | In patients with pCR after CRT, [18F]FDG-PET showed sensitivity and specificity of 100% and 94%, with DWI-MRI showing 100% sensitivity and 87.5% specificity |
| Kariv et al. [79] | 2025 | Retrospective study | Between January 2015 and September 2022, 60 patients underwent restaging MRI; 54 underwent restaging FDG-PET; and 32 were evaluated by both | To compare [18F]FDG-PET and MRI in response assessment to neoadjuvant therapy | In all restaging cases, MRI interpretation was better than [18F]FDG-PET in terms of accuracy (78.3% vs. 68.5%), sensitivity (58.3% vs. 53.8%), and specificity (83.3% vs. 73.2%) |
| Sorenson et al. [80] | 2019 | Retrospective study | 125 patients with LARC who underwent CRT, pre-CRT, and post-CRT PET-CT | To compare pre- and post-CRT PET-CT for predicting pCR in patients undergoing NAT | Post-CRT with SUVmax < 4.3 showed sensitivity of 65% for predicting pCR |
| Tey et al. [81] | 2023 | Prospective study | 31 patients between April 2015 and August 2019 with stage I-III adenocarcinoma planned for pCR | To assess sensitivity and specificity of combined [18F]FDG-PET/MRI in treatment response | Combined [18F]FDG-PET/MRI shows primary tumor response with SUVmax = 11.2 |
| Ippolito et al. [82] | 2015 | Prospective study | 31 patients with rectal cancer | To assess ADC and SUVmax values with pathologic response (TRG) before and after chemoradiation therapy | Before neoadjuvant CRT, mean ADC of responders and non-responders was 0.93 and 1.03 with post-CRT ADC accuracy of 67% |
| Murata et al. [83] | 2018 | Retrospective study | 44 rectal cancer patients | To determine most reliable pCR in patients who underwent preoperative chemoradiotherapy | Accuracies of predicting pCR using [18F]FDG-PET/CT and MRI were 78% and 61% |
| Aiba et al. [84] | 2014 | Retrospective study | 40 patients with LARC | To assess value of MRI and [18F]FDG-PET/CT for tumor response to neoadjuvant chemotherapy (NAC) | MRI-TV2 and MRI-TV are accurate factors to assess pathological response to NAC. Addition of FDG-PET/CT to MRI did not improve performance |
| Asoglu et al. [85] | 2020 | Retrospective study | 66 rectal patients | To assess [18F]FDG-PET/CT during total neoadjuvant treatment (TNT) | 65% patients with cCR were managed nonoperatively; 35% patients underwent surgery |
| Avci et al. [86] | 2023 | Retrospective study | 71 rectal cancer patients | To evaluate MRI and [18F]FDG-PET/CT in correct staging and predicting pathological response | 57 patients underwent surgery, 84.2% reported downstaging and underwent surgery; sensitivity and specificity of MRI—91.6%, 22.2% and [18F]FDG-PET/CT were 100%, 12.5% |
| Denecke et al. [87] | 2005 | Retrospective study | 23 rectal cancer patients | To assess CT, MRI, [18F]FDG-PET in prediction outcome of neoadjuvant radio chemotherapy | Histopathology showed response to neoadjuvant therapy in 13 patients whereas 10 patients were non-responders; sensitivity and specificity of [18F]FDG-PET-100%, 60%; CT-54%, 80% and MRI-71%, 67% |
| Ferri et al. [88] | 2025 | Prospective study | 33 rectal cancer patients | To evaluate [18F]FDG-PET/MRI and MRI in treatment response | [18F]FDG-PET/MRI showed sensitivity, specificity, and accuracy of 88%, 80%, and 84% compared to MRI (82%, 50%, 67%) |
| Vuijk et al. [89] | 2023 | Prospective study | 19 LARC patients | To evaluate [18F]FDG-PET/CT and multiparametric MRI in predicting response to NAT | 26.3% were good responders; 73.7% were poor responders |
| Schurink et al. [90] | 2021 | Retrospective study | 61 LARC patients | To evaluate and predict response treatment using multiparametric [18F]FDG-PET/CT and MRI | 31 were good responders (TRG1-2); 30 poor responders (TRG3-5) |
| Author | Year | Study Design | Sample Size | Study Objective | Key Findings |
|---|---|---|---|---|---|
| Arulamplam et al. [94] | 2003 | Retrospective study | 17 rectal cancer patients | To compare cellular uptake of 18FLT and 18FDG in rectal cancer patients | Out of 50 malignant lesions, 98% and 83% detected with 18FDG and 18FLT, out of 32 liver metastases, 97% visualized with 18FDG and 34% with 18FLT |
| Puri et al. [95] | 2017 | Retrospective study | 11 rectal cancer patients | To compare ([18F]FMISO)PET scans for predicting response treatment | 8 patients underwent mesorectal surgery out of which 5 were good responders. Tumor hypoxia volume was identified to have increased in 3 patients and reduced in 5 patients in week 2 of chemoradiation therapy |
| Wieder et al. [96] | 2007 | Retrospective study | 10 patients with locally advanced rectal cancer | To find correlation of FLT uptake with histopathological findings | Pre-therapy FLT uptake: 4.2 ± 1.0 SUV 14 days post-chemoradiotherapy: Decreased to 2.9 ± 0.6 SUV (−28.6% ± 10.7%, p = 0.005) Preoperative scan: Further decreased to 1.9 ± 0.4 SUV (−54.7% ± 7.6%, p = 0.005) |
| Havelund et al. [97] | 2012 | Retrospective study | 14 patients with locally advanced rectal cancer | To evaluate feasibility of [18F]FAZA PET/CT in locally advanced rectal cancer patients | [18F]FAZA uptake was higher in rectal tumors compared to both muscles (p < 0.007) or normal intestinal wall (p < 0.002) |
| Uslu et al. [98] | 2021 | Prospective study | 20 patients between June 2016 and August 2017 diagnosed with LARC | To compare [18F]FDG PET/CT and DW-MRI in LARC response assessment | MRI was more promising with a sensitivity and specificity of 75% and 90.9%; [18F]FDG-PET/CT was efficient in differentiating responders |
| Zhang et al. [99] | 2024 | Prospective study | 20 LARC patients from February 2023 to July 2023 | To assess [68Ga]Ga-FAPI-04 PET/MRI, [18F]FDG PET/CT and contrast-enhanced MRI in predicting pathological response (pCR) | All the techniques could detect primary lesions. PET parameters showed that [68Ga]Ga-FAPI-04 performed better than [18F]FDG-PET/CT in distinguishing patients with pCR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, E.; Sheth, V.R. The Application of Combined PET/MRI in Staging and Response Assessment of Rectal Cancer. J. Clin. Med. 2025, 14, 7436. https://doi.org/10.3390/jcm14207436
Hussain E, Sheth VR. The Application of Combined PET/MRI in Staging and Response Assessment of Rectal Cancer. Journal of Clinical Medicine. 2025; 14(20):7436. https://doi.org/10.3390/jcm14207436
Chicago/Turabian StyleHussain, Elima, and Vipul R. Sheth. 2025. "The Application of Combined PET/MRI in Staging and Response Assessment of Rectal Cancer" Journal of Clinical Medicine 14, no. 20: 7436. https://doi.org/10.3390/jcm14207436
APA StyleHussain, E., & Sheth, V. R. (2025). The Application of Combined PET/MRI in Staging and Response Assessment of Rectal Cancer. Journal of Clinical Medicine, 14(20), 7436. https://doi.org/10.3390/jcm14207436






