The Effect of Bazedoxifene and Fulvestrant for Preventing Ovarian Hyperstimulation Syndrome: An Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochemical Evaluation
2.2. Peritoneal Fluid Collection
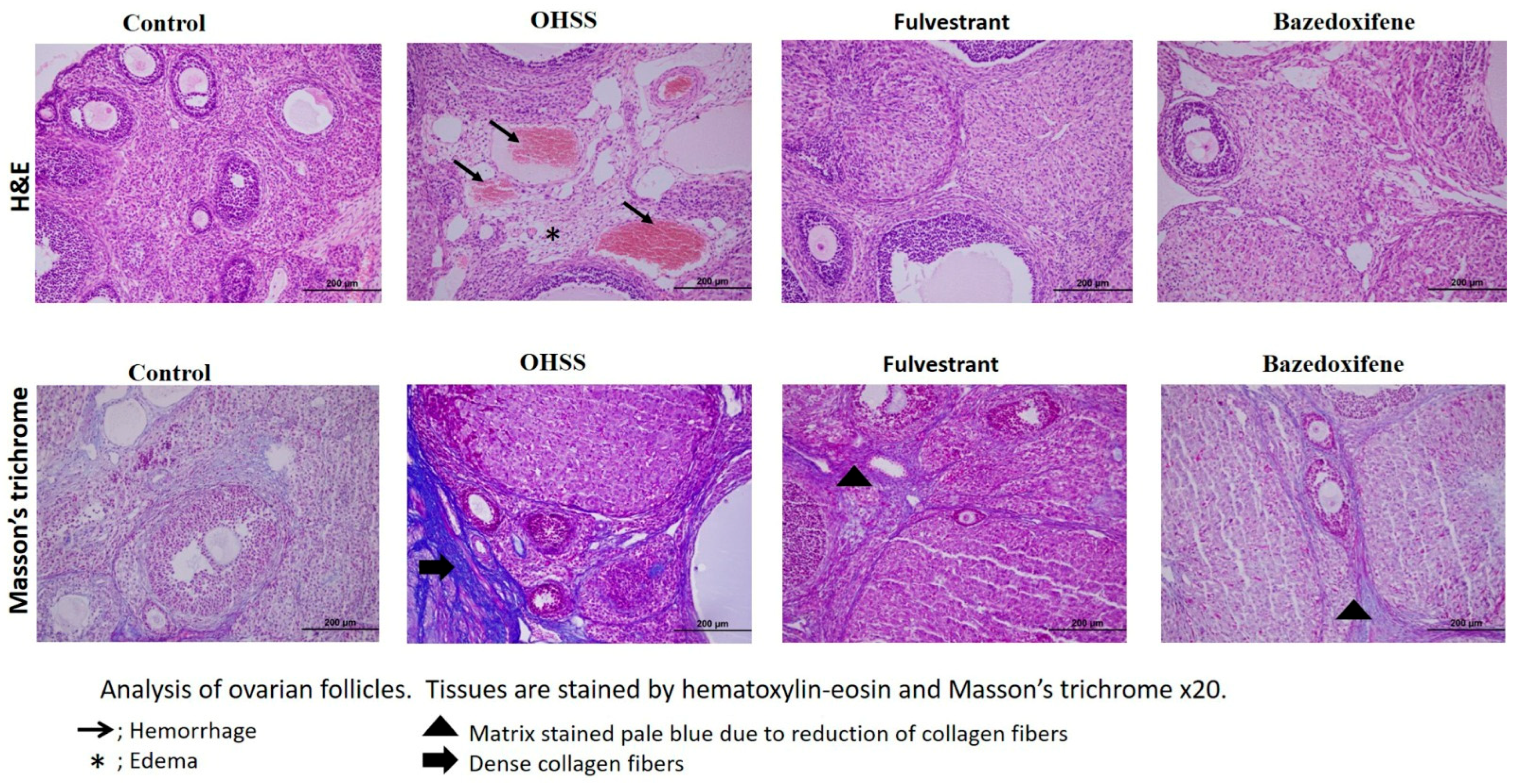
2.3. Evaluation of Histopathology
2.4. Assessment of Ovarian Follicle Numbers
2.5. Immunohistochemistry
2.6. Power Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COS | Controlled ovarian hyperstimulation |
| OHSS | Ovarian hyperstimulation syndrome |
| IVF | In vitro fertilization |
| IUI | In utero insemination |
| HCG | Human chorionic gonadotropin |
| VEGF | Vascular endothelial growth factor |
| TNF-α | Tumor necrosis factor-alpha |
| SERM | Selective estrogen receptor modulators |
| AF-1 | Activating function 1 |
| AF-2 | Activating function 2 |
| ER | Estrogen receptor |
| Sc | subcutaneous |
| PMSG | Pregnant mare serum gonadotropin |
| H&E | Hematoxylin–eosin |
| PBS | Phosphate-buffered saline |
| PASS | Power Analysis Statistical System |
| ANOVA | Analysis of variance |
| VP | Vascular permeability |
| AMP | Adenosine monophosphate |
References
- Nastri, C.O.; Ferriani, R.A.; Rocha, I.A.; Martins, W.P. Ovarian hyperstimulation syndrome: Pathophysiology and prevention. J. Assist. Reprod. Genet. 2010, 27, 121–128. [Google Scholar] [CrossRef]
- Li, H.W.R.; Lee, V.C.Y.; Lau, E.Y.L.; Yeung, W.S.B.; Ho, P.C.; Ng, E.H.Y. Cumulative live-birth rate in women with polycystic ovary syndrome or isolated polycystic ovaries undergoing in-vitro fertilisation treatment. J. Assist. Reprod. Genet. 2014, 31, 205–211. [Google Scholar] [CrossRef]
- Nastri, C.; Teixeira, D.; Moroni, R.; Leitão, V.; Martins, W.d.P. Ovarian hyperstimulation syndrome: Pathophysiology, staging, prediction and prevention. Ultrasound Obstet. Gynecol. 2015, 45, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Ajonuma, L.C. Is vascular endothelial growth factor (VEGF) the main mediator in ovarian hyperstimulation syndrome (OHSS)? Med. Hypotheses 2008, 70, 1174–1178. [Google Scholar] [CrossRef]
- Pala, Ş.; Atilgan, R.; Ozkan, Z.S.; Kavak, S.B.; Ilhan, N.; Akpolat, N.; Sapmaz, E. Effect of varying doses of tamoxifen on ovarian histopathology, serum VEGF, and endothelin 1 levels in ovarian hyperstimulation syndrome: An experimental study. Drug Des. Dev. Ther. 2015, 9, 1761–1766. [Google Scholar] [CrossRef]
- Şanli, C.; Atilgan, R.; Kuloğlu, T.; Pala, Ş.; Türk, B.A.; Keser, H.B.; Ilhan, N. Transient receptor potential melastatin 2 ion channel activity in ovarianhyperstimulation syndrome physiopathology. Turk. J. Med. Sci. 2021, 51, 787–795. [Google Scholar] [CrossRef]
- Atilgan, R.; Pala, Ş.; Yavuzkır, Ş.; Başpınar, M.; Yılmaz, M.; Ilhan, N. What is the impact of short-and long-term supplementation of either cabergoline or clarithromycin on resolving rat ovarian hyperstimulation syndrome (OHSS) model? J. Obstet. Gynaecol. 2019, 39, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Humaidan, P.; Quartarolo, J.; Papanikolaou, E.G. Preventing ovarian hyperstimulation syndrome: Guidance for the clinician. Fertil. Steril. 2010, 94, 389–400. [Google Scholar] [CrossRef]
- Mitwally, M.F. Bazedoxifene: A selective estrogen-receptor modulator. Women’s Health 2008, 4, 319–326. [Google Scholar] [CrossRef]
- Flores, V.A.; Stachenfeld, N.S.; Taylor, H.S. Bazedoxifene–Conjugated Estrogens for Treating Endometriosis. Obstet. Gynecol. 2018, 132, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Bopp, T.; Lo, H.-W.; Tkaczuk, K.; Lin, J. Bazedoxifene as a potential Cancer therapeutic agent targeting IL-6/GP130 signaling. Curr. Oncol. 2024, 31, 5737–5751. [Google Scholar] [CrossRef]
- SMETANA, K.; Rosel, D.; BrÁbek, J. Raloxifene and bazedoxifene could be promising candidates for preventing the COVID-19 related cytokine storm, ARDS and mortality. In Vivo 2020, 34, 3027–3028. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S. Selective estrogen receptor modulators and bone health. Climacteric 2022, 25, 56–59. [Google Scholar] [CrossRef]
- Carlson, R.W. The history and mechanism of action of fulvestrant. Clin. Breast Cancer 2005, 6, S5–S8. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.T.; Heaton, N.S. The impact of estrogens and their receptors on immunity and inflammation during infection. Cancers 2022, 14, 909. [Google Scholar] [CrossRef]
- Ishikawa, K.; Ohba, T.; Tanaka, N.; Iqbal, M.; Okamura, Y.; Okamura, H. Organ-specific production control of vascular endothelial growth factor in ovarian hyperstimulation syndrome-model rats. Endocr. J. 2003, 50, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Sotoudeh, N.; Namavar, M.R. Optimisation of ketamine-xylazine anaesthetic dose and its association with changes in the dendritic spine of CA1 hippocampus in the young and old male and female Wistar rats. Vet. Med. Sci. 2022, 8, 2545–2552. [Google Scholar] [CrossRef]
- Yamamoto, S.; Yamashita, T.; Ito, M.; Caaveiro, J.M.; Egashira, N.; Tozaki-Saitoh, H.; Tsuda, M. New pharmacological effect of fulvestrant to prevent oxaliplatin-induced neurodegeneration and mechanical allodynia in rats. Int. J. Cancer 2019, 145, 2107–2113. [Google Scholar] [CrossRef]
- Komm, B.S.; Kharode, Y.P.; Bodine, P.V.; Harris, H.A.; Miller, C.P.; Lyttle, C.R. Bazedoxifene acetate: A selective estrogen receptor modulator with improved selectivity. Endocrinology 2005, 146, 3999–4008. [Google Scholar] [CrossRef]
- Ozdemir, F.; Acmaz, G.; Yay, A.; Akgun, H.; Cengiz, O.M.; Zararsız, G.; Acmaz, B.; Muderris, I.; Muhtaroglu, S.; Karaman, E. The Effect of Retinoic Acid and Bevacizumab on Ovarian Hyperstimulation Syndrome. J. Clin. Pract. Res. 2025, 47, 148–155. [Google Scholar] [CrossRef]
- Kalkan, K.T.; Yalcin, B.; Yildiz, H.T.; Katirci, E.; Koseoglu, E.; Bolat, D.; Yay, A. Royal jelly alleviates gemcitabine-induced ovarian toxicity: An investigation on rat models. BMC Complement. Med. Ther. 2025, 25, 264. [Google Scholar] [CrossRef]
- Sarma, U.C.; Winship, A.L.; Hutt, K.J. Comparison of methods for quantifying primordial follicles in the mouse ovary. J. Ovarian Res. 2020, 13, 121. [Google Scholar] [CrossRef]
- Yalcın, B.; Kalkan, T.K.; Gonen, Z.B.; Koseoglu, E.; Onder, G.O.; Gokdemir, N.S.; Baran, M.; Yay, A. Role of adipose-derived stem cell exosomes in paclitaxel-induced acute ovarian injury: An experimental approach. Reprod. Toxicol. 2025, 137, 109033. [Google Scholar] [CrossRef]
- Taskin, M.I.; Topcu, O.; Yay, A.; Erken, G.; Balcioğlu, E.; Adali, E.; Hismiogulları, A.A. Prevention of ovarian hyperstimulation syndrome in a rat model: Comparison of the efficacy of tocilizumab with that of ranibizumab, cabergoline, and a gonadotropin-releasing hormone antagonist. Gynecol. Endocrinol. 2015, 31, 949–954. [Google Scholar] [CrossRef]
- Gergin, Ö.Ö.; Pehlivan, S.S.; Ulger, M.; Mat, O.C.; Bayram, A.; Gönen, Z.B.; Gökdemir, N.S.; Biçer, C.; Yildiz, K.; Yay, A.H. Efficacy of stem cell-based therapies for colistin-induced nephrotoxicity. Environ. Toxicol. Pharmacol. 2022, 94, 103933. [Google Scholar] [CrossRef]
- Pfeifer, S.; Butts, S.; Dumesic, D.; Fossum, G.; Gracia, C.; La Barbera, A.; Mersereau, J.; Odem, R.; Paulson, R.; Penzias, A. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: A guideline. Fertil. Steril. 2016, 106, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Wardell, S.E.; Yllanes, A.P.; Chao, C.A.; Bae, Y.; Andreano, K.J.; Desautels, T.K.; Heetderks, K.A.; Blitzer, J.T.; Norris, J.D.; McDonnell, D.P. Pharmacokinetic and pharmacodynamic analysis of fulvestrant in preclinical models of breast cancer to assess the importance of its estrogen receptor-α degrader activity in antitumor efficacy. Breast Cancer Res. Treat. 2020, 179, 67–77. [Google Scholar] [CrossRef]
- McKeand, W.; Baird-Bellaire, S.; Ermer, J.; Patat, A. Pharmacokinetics and safety of bazedoxifene in hepatically impaired and healthy postmenopausal women. Clin. Pharmacol. Drug Dev. 2018, 7, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.-L.; Shioda, K.; Coser, K.R.; Rivizzigno, D.; McSweeney, K.R.; Shioda, T. Fulvestrant-induced cell death and proteasomal degradation of estrogen receptor α protein in MCF-7 cells require the CSK c-Src tyrosine kinase. PLoS ONE 2013, 8, e60889. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Park, S.-A.; Park, H.; Kim, H.; Heo, T.-H. Bazedoxifene, a GP130 inhibitor, modulates EMT signaling and exhibits antitumor effects in HPV-positive cervical cancer. Int. J. Mol. Sci. 2021, 22, 8693. [Google Scholar] [CrossRef]
- Hortu, I.; Karadadas, E.; Ozceltik, G.; Tavmergen, E.; Tavmergen Goker, E.N.; Yigitturk, G.; Erbas, O. Oxytocin and cabergoline alleviate ovarian hyperstimulation syndrome (OHSS) by suppressing vascular endothelial growth factor (VEGF) in an experimental model. Arch. Gynecol. Obstet. 2021, 303, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Liu, J.; Zhao, S.; Zhao, H.; Chen, Z.-J.; Du, Y.; Li, W. Kisspeptin-10 inhibits OHSS by suppressing VEGF secretion. Reproduction 2017, 154, 355–362. [Google Scholar] [CrossRef]
- Cheng, J.-C.; Fang, L.; Li, Y.; Wang, S.; Li, Y.; Yan, Y.; Jia, Q.; Wu, Z.; Wang, Z.; Han, X. Melatonin stimulates aromatase expression and estradiol production in human granulosa-lutein cells: Relevance for high serum estradiol levels in patients with ovarian hyperstimulation syndrome. Exp. Mol. Med. 2020, 52, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.; Orisaka, M.; Wang, H.; Orisaka, S.; Thompson, W.; Zhu, C.; Kotsuji, F.; Tsang, B.K. Gonadotropin and intra-ovarian signals regulating follicle development and atresia: The delicate balance between life and death. Front. Biosci. 2007, 12, 3628–3639. [Google Scholar] [CrossRef] [PubMed]
- Samuel, E.; Chiang, C.; Jennens, R.; Faulkner, D.; Francis, P.A. Fulvestrant falsely elevates oestradiol levels in immunoassays in postmenopausal women with breast cancer. Eur. J. Cancer 2020, 126, 104–105. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Nie, R.; Carnes, K.; Franca, L.R.; Prins, G.S.; Saunders, P.T.; Hess, R.A. The antiestrogen ICI 182,780 decreases the expression of estrogen receptor-alpha but has no effect on estrogen receptor-beta and androgen receptor in rat efferent ductules. Reprod. Biol. Endocrinol. 2003, 1, 75. [Google Scholar] [CrossRef]
- Guan, J.; Zhou, W.; Hafner, M.; Blake, R.A.; Chalouni, C.; Chen, I.P.; De Bruyn, T.; Giltnane, J.M.; Hartman, S.J.; Heidersbach, A.; et al. Therapeutic Ligands Antagonize Estrogen Receptor Function by Impairing Its Mobility. Cell 2019, 178, 949–963.e18. [Google Scholar] [CrossRef]
- Robertson, J.F.; Bondarenko, I.M.; Trishkina, E.; Dvorkin, M.; Panasci, L.; Manikhas, A.; Shparyk, Y.; Cardona-Huerta, S.; Cheung, K.-L.; Philco-Salas, M.J. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): An international, randomised, double-blind, phase 3 trial. Lancet 2016, 388, 2997–3005. [Google Scholar] [CrossRef]
- Di Leo, A.; Jerusalem, G.; Petruzelka, L.; Torres, R.; Bondarenko, I.N.; Khasanov, R.; Verhoeven, D.; Pedrini, J.L.; Smirnova, I.; Lichinitser, M.R. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor–positive advanced breast cancer. J. Clin. Oncol. 2010, 28, 4594–4600. [Google Scholar] [CrossRef]
- Robertson, J.F.; Llombart-Cussac, A.; Rolski, J.; Feltl, D.; Dewar, J.; Macpherson, E.; Lindemann, J.; Ellis, M.J. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: Results from the FIRST study. J. Clin. Oncol. 2009, 27, 4530–4535. [Google Scholar] [CrossRef]
- Silverman, S.L.; Christiansen, C.; Genant, H.K.; Vukicevic, S.; Zanchetta, J.R.; de Villiers, T.J.; Constantine, G.D.; Chines, A.A. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: Results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J. Bone Miner. Res. 2008, 23, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, J.V.; Abraham, L.; Bushmakin, A.G.; Cappelleri, J.C.; Racketa, J.; Shi, H.; Chines, A.A.; Mirkin, S. Evaluation of the efficacy and safety of bazedoxifene/conjugated estrogens for secondary outcomes including vasomotor symptoms in postmenopausal women by years since menopause in the Selective estrogens, Menopause and Response to Therapy (SMART) trials. J. Women’s Health 2014, 23, 18–28. [Google Scholar] [CrossRef] [PubMed]


| OHSS Variables | Experimental Groups | p-Value | |||
|---|---|---|---|---|---|
| Negative Control (n = 10) | Positive Control (n = 10) | Fulvestrant Treatment (n = 10) | Bazedoxifene Treatment (n = 10) | ||
| Rat Weight | |||||
| Before | 59.50 ± 1.65 | 58.20 ± 2.30 | 59.50 ± 2.84 | 58.90 ± 2.88 | 0.602 |
| After | 62.00 ± 2.40 a | 75.90 ± 1.97 b | 66.20 ± 4.05 c | 67.10 ± 4.72 c | <0.001 |
| Difference | 2.50 ± 1.43 a | 17.70 ± 1.64 b | 6.70 ± 3.62 c | 8.20 ± 5.31 c | <0.001 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Peritoneal fluid (present) | 0 (0.0) a | 8 (80.0) b | 4 (40.0) b | 4 (40.0) b | 0.004 |
| Follicle Count | Experimental Groups | p-Value | |||
|---|---|---|---|---|---|
| Negative Control (n = 10) | Positive Control (n = 10) | Fulvestrant Treatment (n = 10) | Bazedoxifene Treatment (n = 10) | ||
| Primordial | 29.50 ± 15.32 | 22.10 ± 9.78 | 16.50 ± 6.96 | 21.86 ± 10.96 | 0.114 |
| Primer | 50.00 ± 13.09 | 40.70 ± 14.69 | 43.60 ± 13.35 | 38.71 ± 12.65 | 0.292 |
| Seconder | 19.25 ± 8.28 | 19.70 ± 8.03 | 15.90 ± 6.81 | 17.14 ± 6.21 | 0.614 |
| Tertiary | 5.88 ± 3.60 a | 15.30 ± 9.33 b | 11.30 ± 6.29 ab | 10.50 ± 3.13 ab | 0.019 |
| Atresia | 15.00 ± 8.55 a | 32.80 ± 13.64 b | 20.70 ± 10.31 ab | 24.93 ± 9.07 ab | 0.007 |
| Experimental Groups | p-Value | ||||
|---|---|---|---|---|---|
| Negative Control | Positive Control | Fulvestrant Treatment | Bazedoxifene Treatment | ||
| ER variables (Stromal) Mean ± SD | 169.24 ± 3.38 a | 174.32 ± 4.83 b | 170.82 ± 5.00 a | 176.02 ± 3.77 b | <0.001 |
| ER variables (Corpus Luteum) Mean ± SD | 172.36 ± 5.53 ab | 172.79 ± 5.03 ab | 171.35 ± 5.51 a | 174.70 ± 5.48 b | 0.014 |
| VEGF tissue | 164.69 ± 9.57 ab | 160.94 ± 13.24 a | 166.10 ± 11.80 b | 166.06 ± 12.58 ab | 0.037 |
| VEGF blood | 17.60 (15.86–26.69) a | 60.97 (37.15–84.47) b | 38.03 (29.80–49.12) b | 53.87 (31.00–106.59) b | 0.008 |
| E2 blood | 110.91 (81.67–131.62) a | 137.00 (103.15–158.38) a | 244.92 (179.11–358.77) b | 107.62 (88.84–145.45) a | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozdemir, F.; Acmaz, G.; Yay, A.H.; Mat, O.C.; Zararsiz, G.E.; Acmaz, B.; Muderris, I.; Muhtaroglu, S.; Karakas, E.; Inanc, M. The Effect of Bazedoxifene and Fulvestrant for Preventing Ovarian Hyperstimulation Syndrome: An Experimental Study. J. Clin. Med. 2025, 14, 7435. https://doi.org/10.3390/jcm14207435
Ozdemir F, Acmaz G, Yay AH, Mat OC, Zararsiz GE, Acmaz B, Muderris I, Muhtaroglu S, Karakas E, Inanc M. The Effect of Bazedoxifene and Fulvestrant for Preventing Ovarian Hyperstimulation Syndrome: An Experimental Study. Journal of Clinical Medicine. 2025; 14(20):7435. https://doi.org/10.3390/jcm14207435
Chicago/Turabian StyleOzdemir, Fatma, Gokhan Acmaz, Arzu Hanim Yay, Ozge Cengiz Mat, Gozde Erturk Zararsiz, Banu Acmaz, Ipek Muderris, Sabahattin Muhtaroglu, Erol Karakas, and Mevlude Inanc. 2025. "The Effect of Bazedoxifene and Fulvestrant for Preventing Ovarian Hyperstimulation Syndrome: An Experimental Study" Journal of Clinical Medicine 14, no. 20: 7435. https://doi.org/10.3390/jcm14207435
APA StyleOzdemir, F., Acmaz, G., Yay, A. H., Mat, O. C., Zararsiz, G. E., Acmaz, B., Muderris, I., Muhtaroglu, S., Karakas, E., & Inanc, M. (2025). The Effect of Bazedoxifene and Fulvestrant for Preventing Ovarian Hyperstimulation Syndrome: An Experimental Study. Journal of Clinical Medicine, 14(20), 7435. https://doi.org/10.3390/jcm14207435






