Implementation of Remote Patient Monitoring and Earlier CERT Activation: Effects on ICU Transfer and Mortality
Abstract
1. Introduction
2. Study Setting
3. Objective
4. Methods
4.1. Study Design and Population
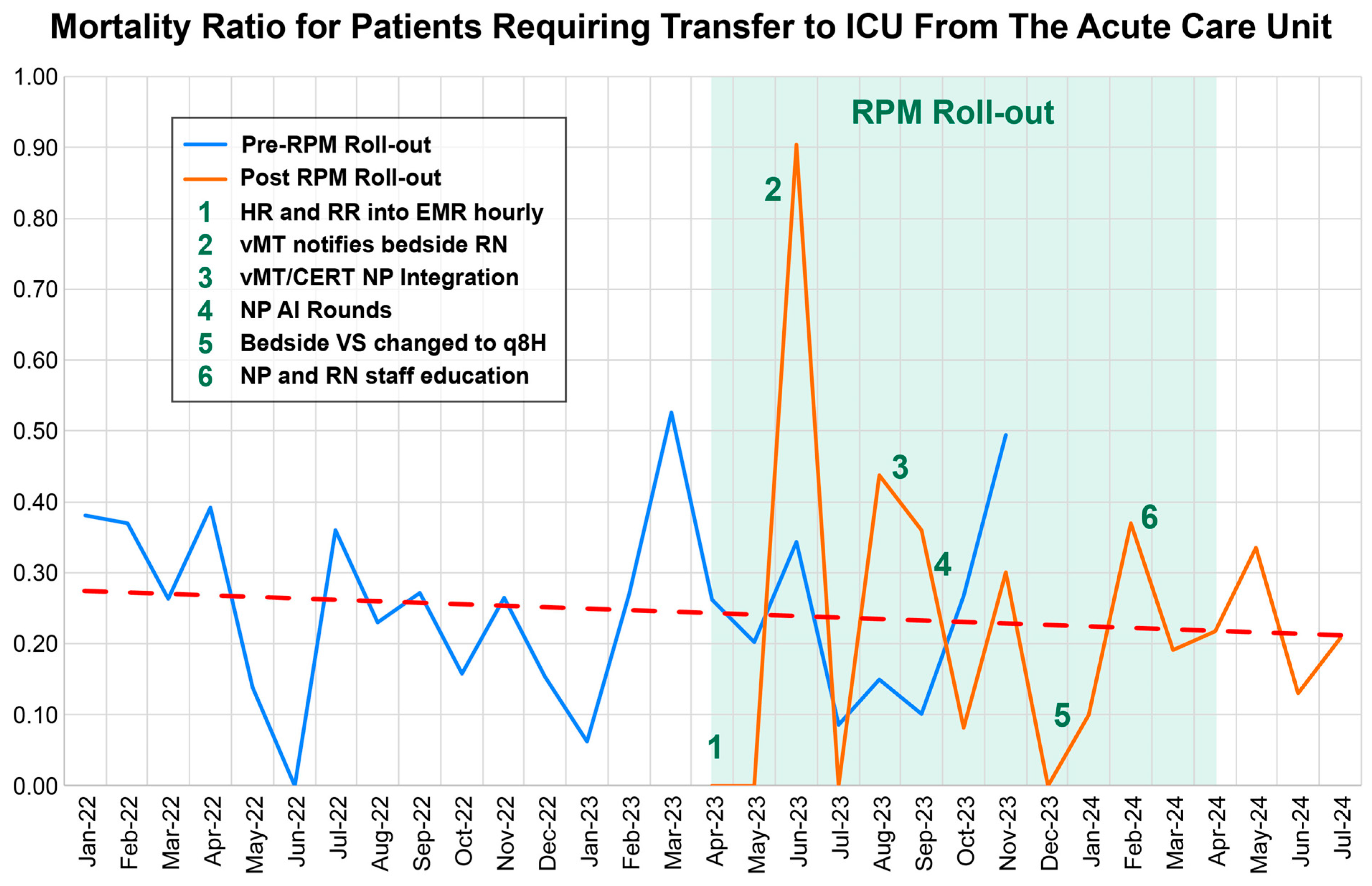
4.2. Intervention Components
4.3. Statistical Analysis
5. Results
5.1. Demographics
5.2. Illness Severity Scores
5.3. ICU Mortality
5.4. Pre-ICU Length of Stay
5.5. ICU Length of Stay
5.6. Total Length of Stay (LOS)
6. Discussion
6.1. Strengths
6.2. Limitations
6.3. Clinical Implications
6.4. Future Directions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VS | Vital signs |
| RPM | Remote patient monitoring |
| RRS | Rapid response system |
| CERT | Clinical emergency response team |
| LOS | Length of stay |
| ICU | Intensive care unit |
| vICU | Virtual intensive care unit |
References
- Liu, V.X.; Lu, Y.; Carey, K.A.; Gilbert, E.R.; Afshar, M.; Akel, M.; Shah, N.S.; Dolan, J.; Winslow, C.; Kipnis, P.; et al. Comparison of Early Warning Scoring Systems for Hospitalized Patients With and Without Infection at Risk for In-Hospital Mortality and Transfer to the Intensive Care Unit. JAMA Netw. Open 2020, 3, e205191. [Google Scholar] [CrossRef]
- Mitsunaga, T.; Hasegawa, I.; Uzura, M.; Okuno, K.; Otani, K.; Ohtaki, Y.; Sekine, A.; Takeda, S. Comparison of the National Early Warning Score (NEWS) and the Modified Early Warning Score (MEWS) for predicting admission and in-hospital mortality in elderly patients in the pre-hospital setting and in the emergency department. PeerJ 2019, 7, e6947. [Google Scholar] [CrossRef]
- Wilson, A.J.; Parker, A.J.; Kitchen, G.B.; Martin, A.; Hughes-Noehrer, L.; Nirmalan, M.; Peek, N.; Martin, G.P.; Thistlethwaite, F.C. The completeness, accuracy and impact on alerts, of wearable vital signs monitoring in hospitalised patients. BMC Digit. Health 2025, 3, 13. [Google Scholar] [CrossRef]
- Mitchell, O.J.L.; Neefe, S.; Ginestra, J.C.; Schweickert, W.D.; Falk, S.; Weissman, G.E.; Covin, D.; Shults, J.; Abella, B.S.; Shashaty, M.G.S. Association of Time to Rapid Response Team Activation With Patient Outcomes Using a Range of Physiologic Deterioration Thresholds. Crit. Care Explor. 2022, 4, e0786. [Google Scholar] [CrossRef]
- Solomon, R.S.; Corwin, G.S.; Barclay, D.C.; Quddusi, S.F.; Dannenberg, M.D. Effectiveness of rapid response teams on rates of in-hospital cardiopulmonary arrest and mortality: A systematic review and meta-analysis. J. Hosp. Med. 2016, 11, 438–445. [Google Scholar] [CrossRef]
- Cardona-Morrell, M.; Prgomet, M.; Turner, R.M.; Nicholson, M.; Hillman, K. Effectiveness of continuous or intermittent vital signs monitoring in preventing adverse events on general wards: A systematic review and meta-analysis. Int. J. Clin. Pract. 2016, 70, 806–824. [Google Scholar] [CrossRef]
- Weller, G.B.; Mault, J.; Ventura, M.E.; Adams, J.; Campbell, F.J.; Tremper, K.K. A Retrospective Observational Study of Continuous Wireless Vital Sign Monitoring via a Medical Grade Wearable Device on Hospitalized Floor Patients. J. Clin. Med. 2024, 13, 4747. [Google Scholar] [CrossRef]
- Herlitz, J.; Rundqvist, S.; Bång, A.; Aune, S.; Lundström, G.; Ekström, L.; Lindkvist, J. Is there a difference between women and men in characteristics and outcome after in hospital cardiac arrest? Resuscitation 2001, 49, 15–23. [Google Scholar] [CrossRef]
- Lyons, P.G.; Edelson, D.P.; Churpek, M.M. Rapid response systems. Resuscitation 2018, 128, 191–197. [Google Scholar] [CrossRef]
- Jones, C.H.; Dolsten, M. Healthcare on the brink: Navigating the challenges of an aging society in the United States. npj Aging 2024, 10, 22. [Google Scholar] [CrossRef]
- Chalwin, R.; Salter, A.; Karnon, J.; Eaton, V.; Giles, L. Effect of a multi-faceted rapid response system re-design on repeat calling of the rapid response team. PLoS ONE 2022, 17, e0265485. [Google Scholar] [CrossRef]
- Brown, H.; Terrence, J.; Vasquez, P.; Bates, D.W.; Zimlichman, E. Continuous monitoring in an inpatient medical-surgical unit: A controlled clinical trial. Am. J. Med. 2014, 127, 226–232. [Google Scholar] [CrossRef]
- Fernando, S.M.; Reardon, P.M.; Bagshaw, S.M.; Scales, D.C.; Murphy, K.; Shen, J.; Tanuseputro, P.; Heyland, D.K.; Kyeremanteng, K. Impact of nighttime Rapid Response Team activation on outcomes of hospitalized patients with acute deterioration. Crit. Care 2018, 22, 67. [Google Scholar] [CrossRef] [PubMed]
- Dall’Ora, C.; Griffiths, P.; Hope, J.; Briggs, J.; Jeremy, J.; Gerry, S.; Redfern, O.C. How long do nursing staff take to measure and record patients’ vital signs observations in hospital? A time-and-motion study. Int. J. Nurs. Stud. 2021, 118, 103921. [Google Scholar] [CrossRef]
- Downey, C.; Randell, R.; Brown, J.; Jayne, D.G. Continuous Versus Intermittent Vital Signs Monitoring Using a Wearable, Wireless Patch in Patients Admitted to Surgical Wards: Pilot Cluster Randomized Controlled Trial. J. Med. Internet Res. 2018, 20, e10802. [Google Scholar] [CrossRef]
- Kadar, R.B.; Amici, D.R.; Hesse, K.; Bonder, A.; Ries, M. Impact of Telemonitoring of Critically Ill Emergency Department Patients Awaiting ICU Transfer. Crit. Care Med. 2019, 47, 1201–1207. [Google Scholar] [CrossRef]
- Eddahchouri, Y.; Peelen, R.V.; Koeneman, M.; Touw, H.R.W.; van Goor, H.; Bredie, S.J.H. Effect of continuous wireless vital sign monitoring on unplanned ICU admissions and rapid response team calls: A before-and-after study. Br. J. Anaesth. 2022, 128, 857–863. [Google Scholar] [CrossRef]
- Balshi, A.N.; Al-Odat, M.A.; Alharthy, A.M.; Alshaya, R.A.; Alenzi, H.M.; Dambung, A.S.; Mhawish, H.; Altamimi, S.M.; Aletreby, W.T. Tele-Rapid Response Team (Tele-RRT): The effect of implementing patient safety network system on outcomes of medical patients-A before and after cohort study. PLoS ONE 2022, 17, e0277992. [Google Scholar] [CrossRef]
- Watanabe, T.; Ohsugi, K.; Suminaga, Y.; Somei, M.; Kikuyama, K.; Mori, M.; Maruo, H.; Kono, N.; Kotani, T. An evaluation of the impact of the implementation of the Tele-ICU: A retrospective observational study. J. Intensive Care 2023, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Rowland, B.; Saha, A.; Motamedi, V.; Bundy, R.; Winsor, S.; McNavish, D.; Lippert, W.; Khanna, A.K. Impact on Patient Outcomes of Continuous Vital Sign Monitoring on Medical Wards: Propensity-Matched Analysis. J. Med. Internet Res. 2025, 27, e66347. [Google Scholar] [CrossRef]
- Beard, J.W.; Sethi, A.; Jiao, W.; Hyatt, H.W.; Yapici, H.O.; Erslon, M.; Overdyk, F.J. Cost savings through continuous vital sign monitoring in the medical-surgical unit. J. Med. Econ. 2023, 26, 760–768. [Google Scholar] [CrossRef] [PubMed]

| Variable | Post (n = 464) | Pre (n = 656) | Total (N = 1120) | Chi2 p-Value |
|---|---|---|---|---|
| Gender | ||||
| Female | 213 (45.9%) | 302 (46.0%) | 515 | 0.965 |
| Male | 251 (54.1%) | 354 (54.0%) | 605 | |
| Race | ||||
| White | 295 (63.6%) | 414 (63.1%) | White | 0.104 |
| Black | 136 (29.3%) | 168 (25.6%) | Black | |
| Asian | 18 (3.9%) | 43 (6.6%) | Asian | |
| Other/Unknown ‡ | 15 (3.2%) | 30 (4.6%) | Other/Unknown ‡ | |
| Comorbidities | ||||
| CHF | 214 (40.07%) | 320 (59.93%) | 534 | 0.380 |
| COPD | 68 (45.33%) | 82 (54.67%) | 150 | 0.297 |
| DM | 203 (40.85%) | 294 (59.15%) | 497 | 0.723 |
| Hypertension | 352 (41.56%) | 495 (58.44%) | 847 | 0.876 |
| Liver Disease | 112 (43.75%) | 144 (56.25%) | 256 | 0.391 |
| CHF | 214 (40.07%) | 320 (59.93%) | 534 | 0.380 |
| Mean ± SD (Pre)
N: 656 | Mean ± SD (Post)
N: 464 | Mean Difference (95% CI) | p-Value | |
|---|---|---|---|---|
| Total Population | ||||
| APACHE IV | 90.01 ± 33.12 | 83.96 ± 30.23 | −6.05 (2.30 to 9.79) | 0.0016 |
| APS | 73.73 ± 30.56 | 67.76 ± 28.41 | −5.97 (2.48 to 9.46) | 0.0008 |
| Median IQR | Median IQR | |||
| Pre-ICU LOS | 4.1 (1.6–9.7) | 3.6 (1.35–8.9) | 0.1866 | |
| ICU LOS | 5.44 (2.68–11.16) | 4.91 (2.37–9.14) | 0.1313 | |
| Total LOS | 11.96 (6.41–20.28) | 10.58 (6.09–18.46) | 0.0515 | |
| Survivors | ||||
| Pre-ICU LOS | 3.9 (1.5–9.1) | 3.2 (1.3–8.4) | 0.1821 | |
| ICU LOS | 5.85 (3.00–11.58) | 5.07 (2.59–9.22) | 0.0565 | |
| Total LOS | 11.95 (6.57–20.40) | 10.50 (6.01–18.17) | 0.0278 |
| Variable | Time | Pre (n) | Post (n) | Pre Mean SD | Post Mean SD | p-Value |
|---|---|---|---|---|---|---|
| Total Population | ||||||
| APACHE IV | Day | 363 | 249 | 89.15 ± 32.06 | 80.34 ± 29.58 | 0.0006 |
| Night | 293 | 215 | 91.08 ± 34.40 | 88.15 ± 30.50 | 0.3218 | |
| APS | Day | 363 | 249 | 72.99 ± 29.34 | 64.47 ± 27.90 | 0.0003 |
| Night | 293 | 215 | 74.64 ± 32.04 | 71.56 ± 28.58 | 0.2634 | |
| Pre Median [IQR] | Post Median [IQR] | |||||
| ICU LOS | Day | 363 | 249 | 5.31 [2.68–10.93] | 4.93 [2.32–9.00] | 0.2029 |
| Night | 293 | 215 | 5.73 [2.67–11.31] | 4.71 [2.60–9.78] | 0.3785 | |
| Pre-ICU LOS | Day | 363 | 249 | 4.10 [1.60–9.70] | 4.80 [1.70–10.20] | 0.8169 |
| Night | 293 | 215 | 4.20 [1.40–9.80] | 2.90 [1.20–7.60] | 0.0410 | |
| Total LOS | Day | 363 | 249 | 11.86 [6.74–19.14] | 11.36 [6.23–18.82] | 0.3856 |
| Night | 293 | 215 | 12.12 [6.30–21.01] | 10.24 [6.01–17.90] | 0.0559 | |
| Survivors | ||||||
| Time | Pre (n) | Post (n) | Pre Median [IQR] | Post Median [IQR] | p-value | |
| APACHE IV | Day | 323 | 229 | 85.33 ± 29.19 | 78.34 ± 28.66 | 0.0369 |
| Night | 257 | 196 | 87.67 ± 31.21 | 85.15 ± 28.70 | 0.3793 | |
| APS | Day | 323 | 229 | 69.40 ± 26.31 | 62.76 ± 27.22 | 0.0041 |
| Night | 257 | 196 | 71.46 ± 28.95 | 68.73 ± 26.69 | 0.3038 | |
| ICU LOS | Day | 323 | 229 | 5.45 [3.01–11.28] | 5.09 [2.36–9.09] | |
| Night | 257 | 196 | 6.20 [3.23–11.19] | 5.24 [2.53–9.60] | 0.1467 | |
| Pre-ICU LOS | Day | 323 | 229 | 3.68 [1.09–8.48] | 3.80 [1.32–8.39] | 0.1956 |
| Night | 257 | 196 | 4.00 [1.12–8.67] | 2.54 [0.87–7.14] | 0.8183 | |
| Total LOS | Day | 323 | 229 | 11.89 [7.50–19.90] | 11.07 [6.62–18.63] | 0.0384 |
| Night | 257 | 196 | 11.82 [6.99–20.23] | 10.00 [5.78–17.16] | 0.2979 |
| Variable | ICU Mortality OR (95% CI)—Univariable | p-Value | ICU Mortality OR (95% CI)—Multivariable | p-Value | APACHE IV β (95% CI)—Univariable | p-Value | APACHE IV β (95% CI)—Multivariable | p-Value |
|---|---|---|---|---|---|---|---|---|
| Pre vs. Post | 1.43 (0.95–2.14) | 0.085 | 1.18 (0.77–1.81) | 0.458 | 6.05 (2.25 to 9.85) | 0.002 | 5.71 (2.09 to 9.33) | 0.002 |
| APACHE IV Score | 1.03 (1.02–1.03) | <0.001 | 1.03 (1.02–1.03) | <0.001 | — | — | — | — |
| Age | 1.01 (1.00–1.03) | 0.061 | 1.00 (0.98–1.01) | 0.578 | 0.70 (0.58 to 0.82) | <0.001 | 0.70 (0.58 to 0.82) | <0.001 |
| Gender (Male) | 1.03 (0.70–1.52) | 0.862 | 1.08 (0.71–1.62) | 0.725 | −1.08 (−4.85 to 2.70) | 0.576 | −0.79 (–4.36 to 2.78) | 0.664 |
| Race | ||||||||
| Black vs. White | 1.15 (0.74–1.78) | 0.538 | 1.15 (0.72–1.84) | 0.548 | 1.87 (−2.45 to 6.18) | 0.396 | 2.11 (−1.96 to 6.19) | 0.309 |
| Other vs. White | 1.50 (0.81–2.78) | 0.198 | 1.31 (0.68–2.55) | 0.418 | 5.16 (−1.51 to 11.82) | 0.129 | 6.32 (0.02 to 12.62) | 0.049 |
| ICU Mortality | Estimate (95% CI) | Adjusted for Age (95% CI) |
|---|---|---|
| Metric | Estimate (95% CI) | Adjusted for Age (95% CI) |
| Pre-intervention mortality | 11.59% (9.28–13.90) | 11.55% (9.11–13.99) |
| Post-intervention mortality | 8.41% (5.89–10.93) | 8.44% (5.91–10.97) |
| Absolute Risk Reduction (ARR) | 3.18% (−0.52–6.87) | 3.12% (−0.40–6.63) |
| Relative Risk (RR) | 0.73 (0.48–1.10) | 0.731 (0.530–1.010) |
| Relative Risk Reduction (RRR) | 27% (−10–52) | 26.9% |
| Odds Ratio (OR) | 0.70 (0.45–1.07) | 0.705 (0.469–1.058) |
| Number Needed to Treat (NNT) | 32 | 32 |
| p-value | 0.0841 | 0.082 |
| Estimated deaths prevented | 25 | 21 |
| Author(s) | Design/Setting | RPM Modality | CERT/RRT Timing Reported | Key Outcomes |
|---|---|---|---|---|
| Brown et al., (2014) [12] | Before-and-after study in a medical–surgical unit | Continuous vital sign monitoring | - | ↓ ICU days reduced (120.1 → 63.5/1000 pts; p = 0.04) ↓ Code-blue events (6.3 → 0.9/1000; p = 0.02) ↓ Hospital LOS (4.0 → 3.6 days; p < 0.05) ICU transfers unchanged |
| Fernando et al., 2018 [13] | Multicenter RRT study (nighttime activations) | Not RPM, but related to RRT activation timing | Nighttime vs. daytime RRT outcomes | ↑ Mortality with nighttime RRT activation |
| Downey et al., 2018 [15] | Pilot cluster RCT, surgical wards | Wireless chest patch, 2 min interval | - | ↓ Time to antibiotics (p = 0.04) ↓ LOS, ↓ 30-day readmissions (non-significant due to THE small sample size) |
| Kadar et al., 2019 [16] | ED telemonitoring of critically ill patients awaiting ICU transfer | Telemonitoring via remote intensivist oversight (eICU) | Indirect—surrogate for early CERT/RRT activation | ↓ In-hospital mortality (5.4% vs. 20.0%); adjusted OR 0.20 |
| Eddahchouri et al., 2022 [17] | Before-and-after ward study | Wireless vital sign monitors | Yes: RRT and ICU transfer rates reported | ↓ ICU transfers (3.4% → 2.3%); ↓ RRT activations |
| Balshi et al., 2022 [18] | Before-and-after with Tele-RRT | Patient safety network with remote alerts | Tele-RRT activation tracked | ↓ CPR events; increased RRT activations. ↓ Hospital mortality |
| Watanabe et al., 2023 [19] | Retrospective study of tele-ICU implementation across Japanese ICUs for ED patients awaiting ICU transfer | Tele-ICU monitoring with remote intensivist consultation | - | ↓ ICU mortality (8.5% → 3.8%) ↓ Hospital mortality (12.4% → 7.7%) ↓ LOS ↓ Physician frequency to EMR ↓ Predicted mortality (based on severity, measured by APACHE-IV scores) found reductions, especially significant in medium in higher-risk patients |
| Rowland 2025 et al., [20] | Propensity-matched ward monitoring study | Continuous vs. intermittent monitoring | - | Intermittent monitoring associated with significantly higher odds of ICU transfer or death compared to continuous monitoring (OR 2.79; 95% CI 1.89–4.25; p < 0.001). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narcisse, V.; Ishaq, F.; Gomez, M.; Homer, S.; Griffin, L.; Pletcher, S.; Nguyen, N.-A. Implementation of Remote Patient Monitoring and Earlier CERT Activation: Effects on ICU Transfer and Mortality. J. Clin. Med. 2025, 14, 7434. https://doi.org/10.3390/jcm14207434
Narcisse V, Ishaq F, Gomez M, Homer S, Griffin L, Pletcher S, Nguyen N-A. Implementation of Remote Patient Monitoring and Earlier CERT Activation: Effects on ICU Transfer and Mortality. Journal of Clinical Medicine. 2025; 14(20):7434. https://doi.org/10.3390/jcm14207434
Chicago/Turabian StyleNarcisse, Victor, Farhan Ishaq, Melissa Gomez, Sarah Homer, Laura Griffin, Sarah Pletcher, and Ngoc-Anh Nguyen. 2025. "Implementation of Remote Patient Monitoring and Earlier CERT Activation: Effects on ICU Transfer and Mortality" Journal of Clinical Medicine 14, no. 20: 7434. https://doi.org/10.3390/jcm14207434
APA StyleNarcisse, V., Ishaq, F., Gomez, M., Homer, S., Griffin, L., Pletcher, S., & Nguyen, N.-A. (2025). Implementation of Remote Patient Monitoring and Earlier CERT Activation: Effects on ICU Transfer and Mortality. Journal of Clinical Medicine, 14(20), 7434. https://doi.org/10.3390/jcm14207434





