Extended ECG Monitoring in Patients with Hypertrophic Cardiomyopathy: The Tempo-HCM Study
Highlights
- Thromboembolic events and SCD are two well-established complications of HCM linked, respectively, to atrial and ventricular arrhythmias.
- Clinical practice guidelines recommend monitoring using periodic 24–48 h Holter to aid clinicians in decisions regarding oral anticoagulation to prevent thromboembolic events and ICD placement for SCD prevention.
- Extended electrocardiographic monitoring well beyond 24–48 h has proven valuable in different clinical scenarios but has not been studied in patients with HCM.
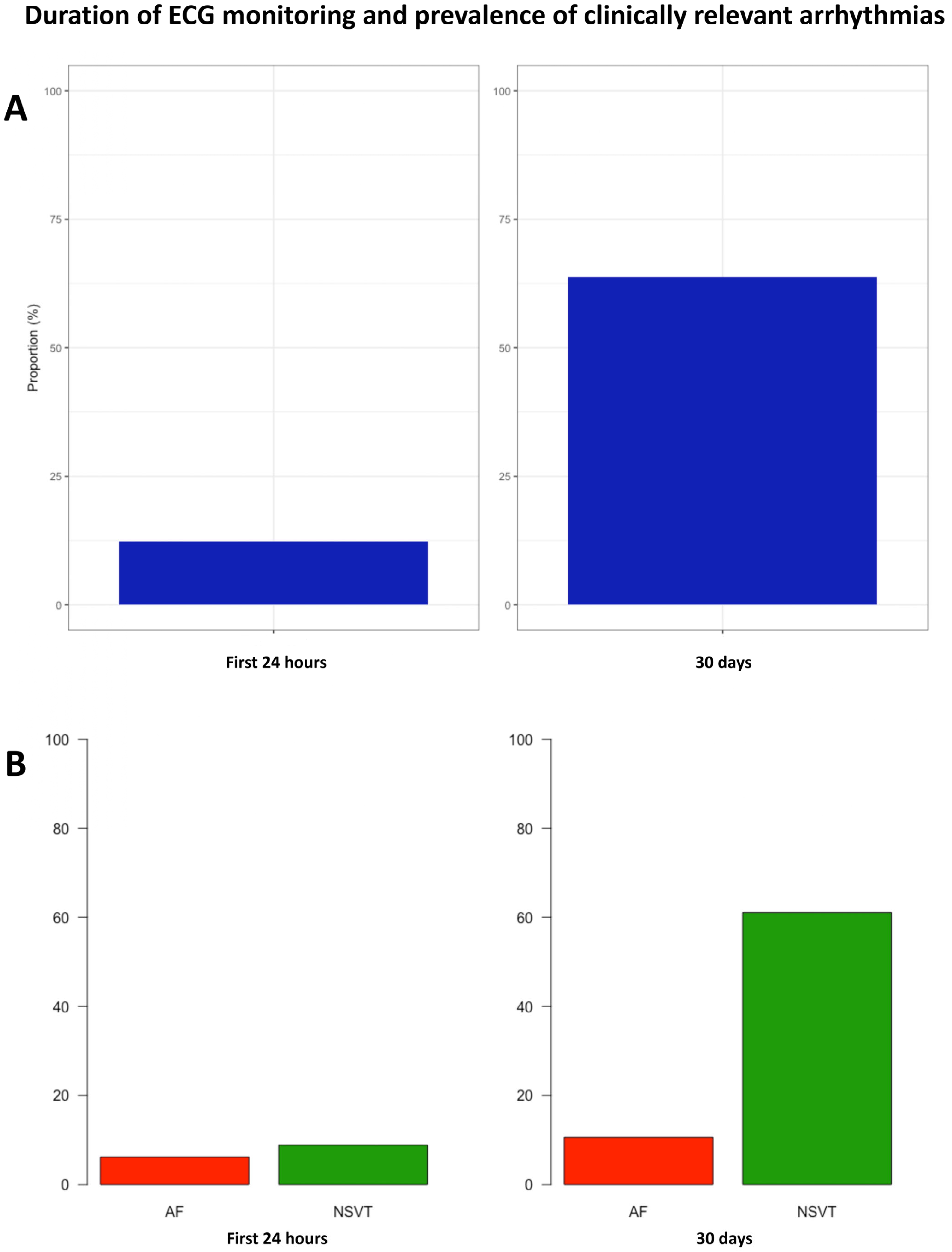
- Extended ECG monitoring significantly enhances the detection of relevant arrhythmias in patients with HCM.
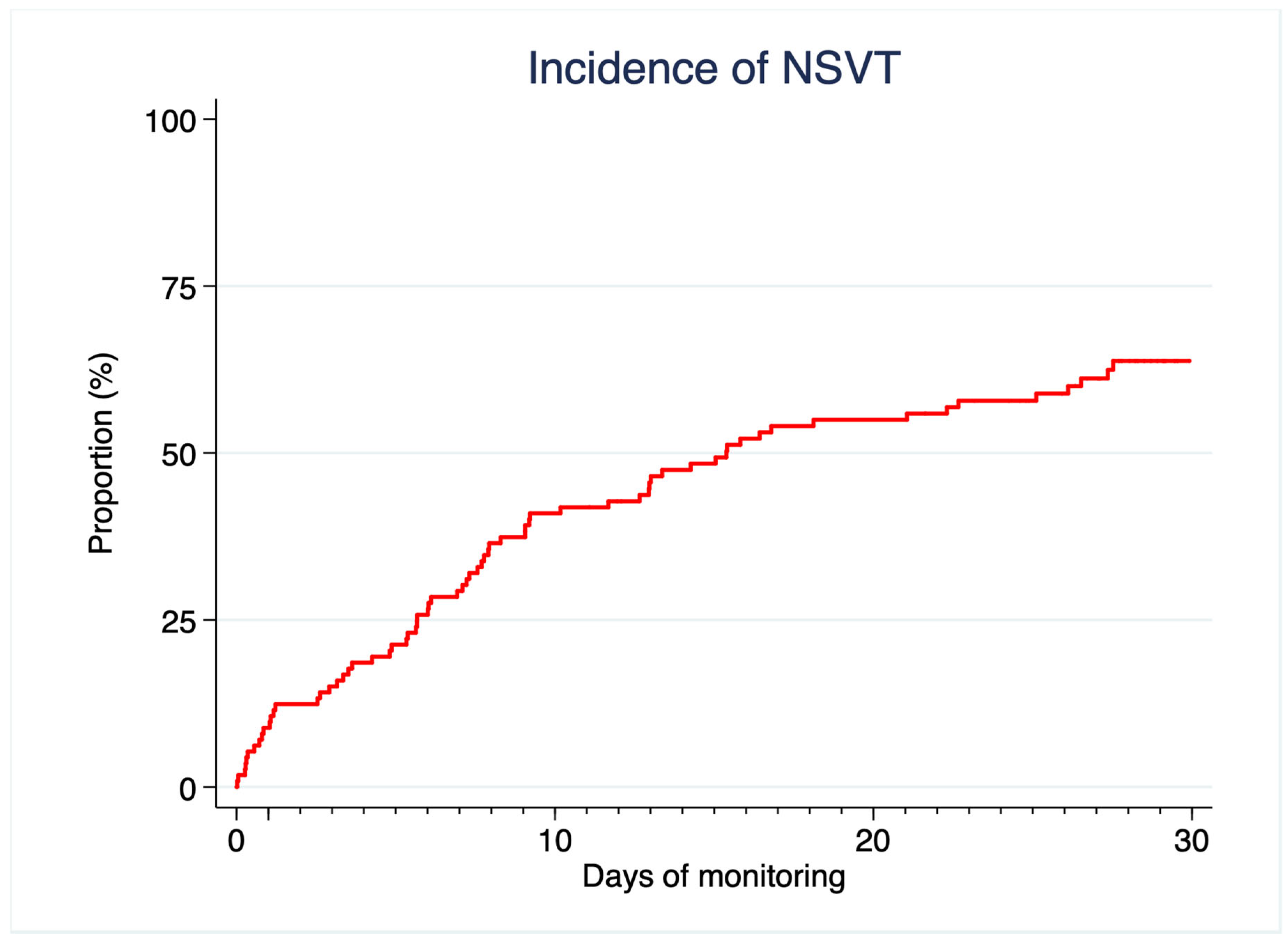
- NSVT are detected in most low-risk HCM patients using this technique.
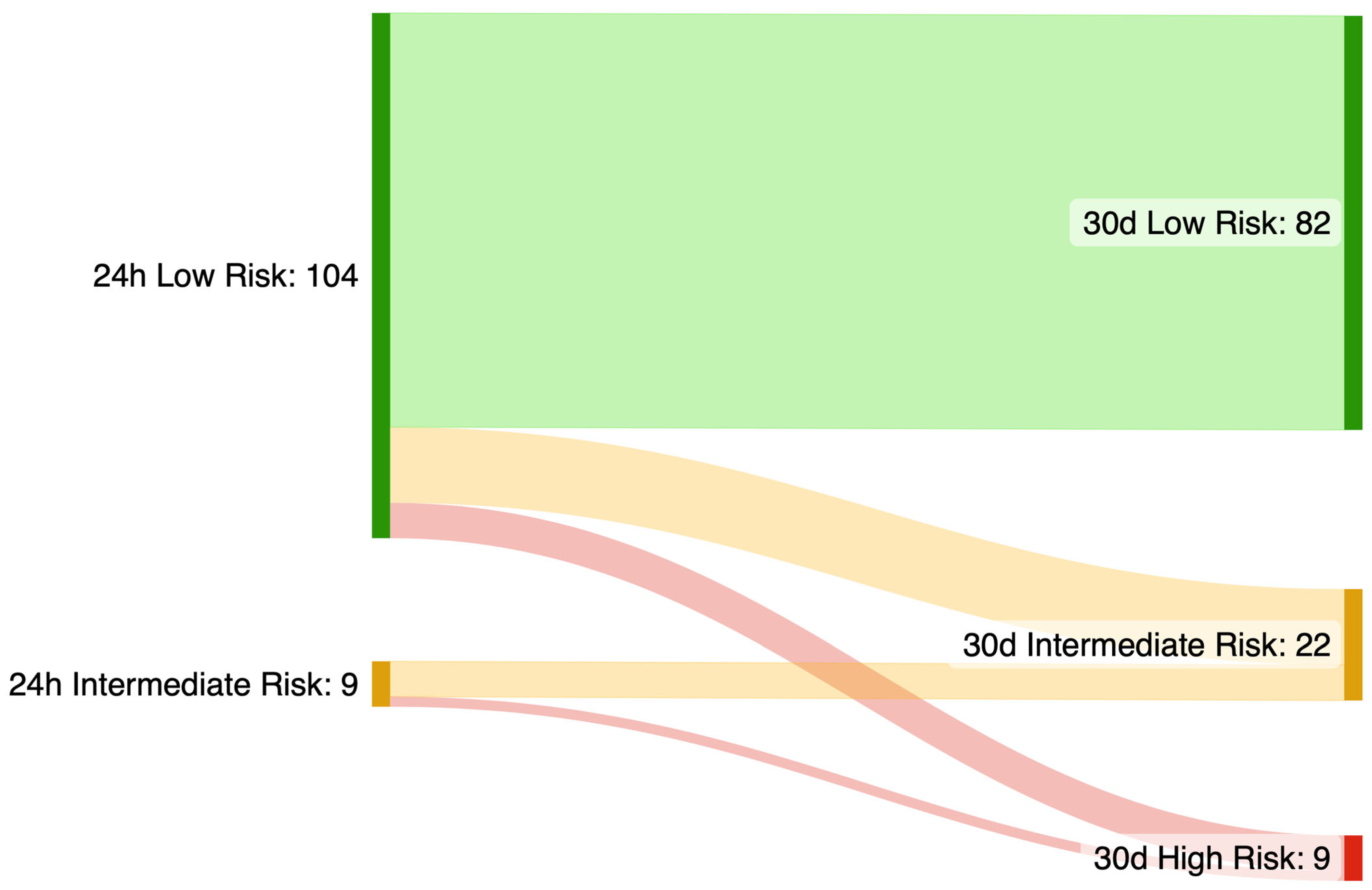
- Further research is needed before routinely including findings from extended monitoring in the risk stratification process for SCD, as this strategy may compromise HCM-SCD calculator specificity.
- Extended monitoring might improve AF screening in HCM.
Abstract
1. Background
2. Methods
2.1. Study Design
2.2. Patients
2.3. ECG Monitoring
2.4. Outcomes
2.5. Additional Testing and Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Patients Demographics and Characteristics
3.2. Monitoring Results
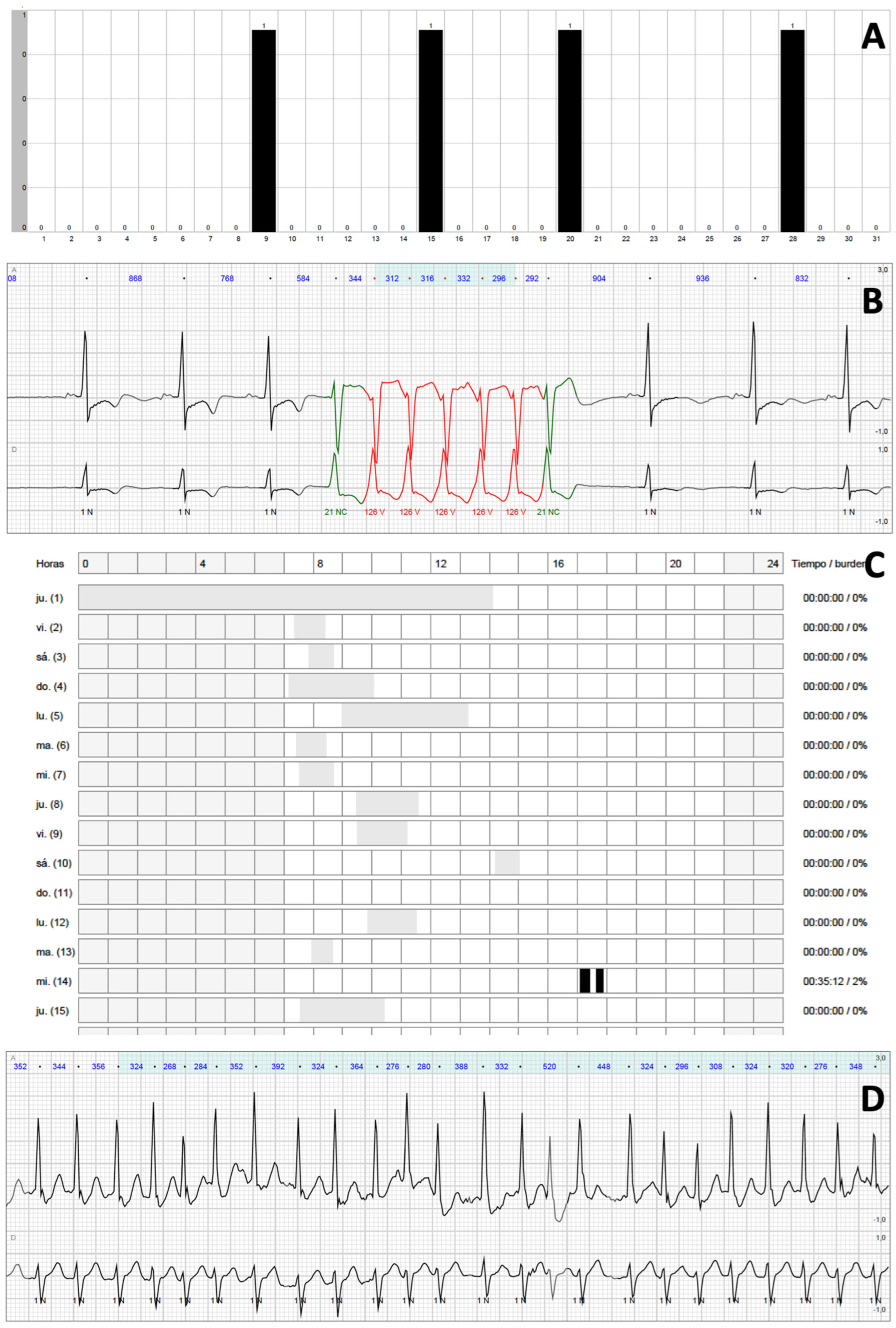
3.3. NSVT
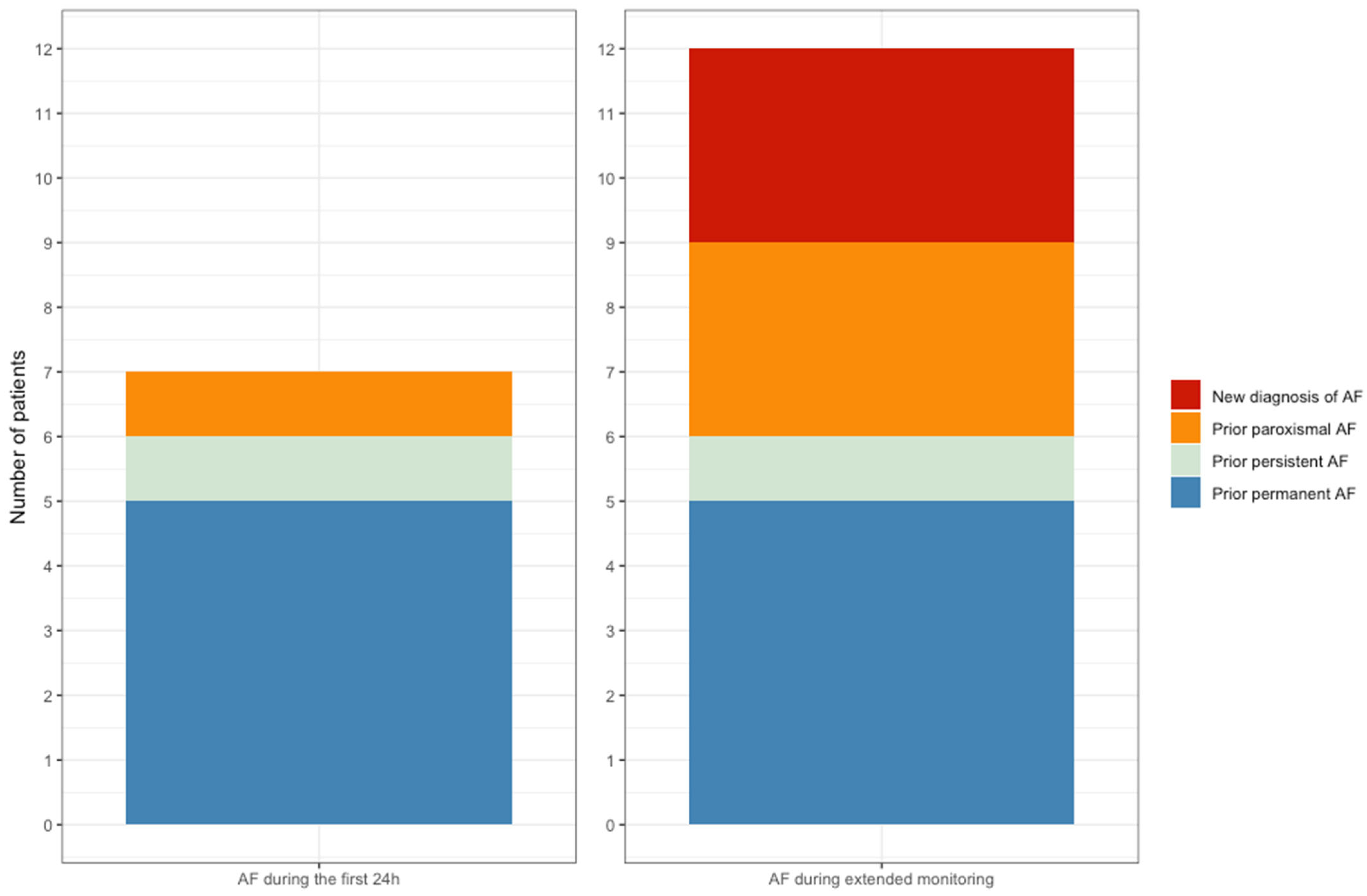
3.4. Atrial Fibrillation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| HCM | Hypertrophic cardiomyopathy |
| ICD | Implantable cardioverter defibrillator |
| NSVT | Non-sustained ventricular tachycardia |
| SCD | Sudden cardiac death |
References
- Maron, B.J.; Maron, M.S.; Rowin, E.J. Perspectives on the Overall Risks of Living with Hypertrophic Cardiomyopathy. Circulation 2017, 135, 2317–2319. [Google Scholar] [CrossRef] [PubMed]
- Semsarian, C.; Ingles, J.; Maron, M.S.; Maron, B.J. New perspectives on the prevalence of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Guttmann, O.P.; Rahman, M.S.; O’Mahony, C.; Anastasakis, A.; Elliott, P.M. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: Systematic review. Heart 2014, 100, 465–472. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, C.; Elliott, P.; McKenna, W. Sudden Cardiac Death in Hypertrophic Cardiomyopathy. Circ. Arrhythmia Electrophysiol. 2013, 6, 443–451. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology. Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients with Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020, 142, e533–e557. [Google Scholar]
- Sanna, T.; Diener, H.-C.; Passman, R.S.; Di Lazzaro, V.; Bernstein, R.A.; Morillo, C.A.; Rymer, M.M.; Thijs, V.; Rogers, T.; Beckers, F.; et al. Cryptogenic Stroke and Underlying Atrial Fibrillation. N. Engl. J. Med. 2014, 370, 2478–2486. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Spring, M.; Dorian, P.; Panzov, V.; Thorpe, K.E.; Hall, J.; Vaid, H.; O’Donnell, M.; Laupacis, A.; Côté, R.; et al. Atrial Fibrillation in Patients with Cryptogenic Stroke. N. Engl. J. Med. 2014, 370, 2467–2477. [Google Scholar] [CrossRef]
- Hindricks, G.; Piorkowski, C.; Tanner, H.; Kobza, R.; Gerds-Li, J.H.; Carbucicchio, C.; Kottkamp, H. Perception of Atrial Fibrillation Before and After Radiofrequency Catheter Ablation: Relevance of Asymptomatic Arrhythmia Recurrence. Circulation 2005, 112, 307–313. [Google Scholar] [CrossRef]
- Boriani, G.; Auricchio, A.; Botto, G.L.; Joseph, J.M.; Roberts, G.J.; Grammatico, A.; Nabutovsky, Y.; Piccini, J.P. Insertable cardiac monitoring results in higher rates of atrial fibrillation diagnosis and oral anticoagulation prescription after ischaemic stroke. Europace 2023, 25, euad212. [Google Scholar] [CrossRef]
- Van Velzen, H.G.; Theuns, D.A.M.J.; Yap, S.-C.; Michels, M.; Schinkel, A.F. Incidence of Device-Detected Atrial Fibrillation and Long-Term Outcomes in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2017, 119, 100–105. [Google Scholar] [CrossRef]
- Wilke, I.; Witzel, K.; Münch, J.; Pecha, S.; Blankenberg, S.; Reichenspurner, H.; Willems, S.; Patten, M.; Aydin, A. High Incidence of De Novo and Subclinical Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy and Cardiac Rhythm Management Device. Cardiovasc. Electrophysiol. 2016, 27, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, C.; Jichi, F.; Pavlou, M.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; McKenna, W.J.; et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM Risk-SCD). Eur. Heart J. 2014, 35, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, C.; Jichi, F.; Ommen, S.R.; Christiaans, I.; Arbustini, E.; Garcia-Pavia, P.; Cecchi, F.; Olivotto, I.; Kitaoka, H.; Gotsman, I.; et al. International External Validation Study of the 2014 European Society of Cardiology Guidelines on Sudden Cardiac Death Prevention in Hypertrophic Cardiomyopathy (EVIDENCE-HCM). Circulation 2018, 137, 1015–1023. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Olmos, C.; Franco, E.; Suárez Barrientos, A.; Fortuny, E.; Martín-García, A.; Viliani, D.; Macaya, C.; de Isla, L.P. Wearable wireless remote monitoring system: An alternative for prolonged electrocardiographic monitoring. Int. J. Cardiol. 2014, 172, e43–e44. [Google Scholar] [CrossRef]
- Pagola, J.; Juega, J.; Francisco-Pascual, J.; Moya, A.; Sanchis, M.; Bustamante, A.; Penalba, A.; Usero, M.; Cortijo, E.; Arenillas, J.F.; et al. Yield of atrial fibrillation detection with Textile Wearable Holter from the acute phase of stroke: Pilot study of Crypto-AF registry. Int. J. Cardiol. 2018, 251, 45–50. [Google Scholar] [CrossRef]
- Carrick, R.T.; Maron, M.S.; Adler, A.; Wessler, B.; Hoss, S.; Chan, R.H.; Sridharan, A.; Huang, D.; Cooper, C.; Drummond, J.; et al. Development and Validation of a Clinical Predictive Model for Identifying Hypertrophic Cardiomyopathy Patients at Risk for Atrial Fibrillation: The HCM-AF Score. Circ. Arrhythmia Electrophysiol. 2021, 14, e009796. [Google Scholar] [CrossRef]
- Monserrat, L.; Elliott, P.M.; Gimeno, J.R.; Sharma, S.; Penas-Lado, M.; McKenna, W.J. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2003, 42, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Rowin, E.J.; Hausvater, A.; Link, M.S.; Abt, P.; Gionfriddo, W.; Wang, W.; Rastegar, H.; Estes, N.M.; Maron, M.S.; Maron, B.J. Clinical Profile and Consequences of Atrial Fibrillation in Hypertrophic Cardio-myopathy. Circulation 2017, 136, 2420–2436. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lian, Z.; Rowin, E.J.; Maron, B.J.; Maron, M.S.; Link, M.S. Prognostic Implications of Nonsustained Ventricular Tachycardia in High-Risk Patients with Hypertrophic Cardiomyopathy. Circ. Arrhythmia Electrophysiol. 2017, 10, e004604. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Savage, D.D.; Wolfson, J.K.; Epstein, S.E. Prognostic significance of 24 hour ambulatory electrocardiographic monitoring in patients with hypertrophic cardiomyopathy: A prospective study. Am. J. Cardiol. 1981, 48, 252–257. [Google Scholar] [CrossRef]
- Gimeno, J.R.; Tomé-Esteban, M.; Lofiego, C.; Hurtado, J.; Pantazis, A.; Mist, B.; Lambiase, P.; McKenna, W.J.; Elliott, P.M. Exercise-induced ventricular arrhythmias and risk of sudden cardiac death in patients with hypertrophic cardiomyopathy. Eur. Heart J. 2009, 30, 2599–2605. [Google Scholar] [CrossRef]
- Christiaans, I.; Van Engelen, K.; Van Langen, I.M.; Birnie, E.; Bonsel, G.J.; Elliott, P.M.; Wilde, A.A. Risk stratification for sudden cardiac death in hypertrophic cardio-myopathy: Systematic review of clinical risk markers. Europace 2010, 12, 313–321. [Google Scholar] [CrossRef]
- Tfelt-Hansen, J.; Garcia, R.; Albert, C.; Merino, J.; Krahn, A.; Marijon, E.; Basso, C.; Wilde, A.A.M.; Haugaa, K.H. Risk stratification of sudden cardiac death: A review. Europace 2023, 25, euad203. [Google Scholar] [CrossRef]
- Goldstein, S.A.; Kennedy, K.F.; Friedman, D.J.; Al-Khatib, S.M.; Wang, A. Utilization and Outcomes of Primary Prevention Implantable Cardioverter-Defibrillators in Patients with Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2023, 12, e029293. [Google Scholar] [CrossRef]
- Weissler-Snir, A.; Chan, R.H.; Adler, A.; Care, M.; Chauhan, V.; Gollob, M.H.; Ziv-Baran, T.; Fourey, D.; Hindieh, W.; Rakowski, H.; et al. Usefulness of 14-Day Holter for Detection of Nonsustained Ventricular Tachycardia in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2016, 118, 1258–1263. [Google Scholar] [CrossRef]
- Fumagalli, C.; Bonanni, F.; Beltrami, M.; Ruggieri, R.; Zocchi, C.; Tassetti, L.; Maurizi, N.; Berteotti, M.; Zampieri, M.; Argirò, A.; et al. Incidence of stroke in patients with hypertrophic cardiomyopathy in stable sinus rhythm during long-term monitoring. Int. J. Cardiol. 2023, 381, 70–75. [Google Scholar] [CrossRef]
- Guttmann, O.P.; Pavlou, M.; O’Mahony, C.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; Garcia-Pavia, P.; et al. Prediction of thrombo-embolic risk in patients with hypertrophic cardio-myopathy (HCM Risk-CVA). Eur. J. Heart Fail. 2015, 17, 837–845. [Google Scholar] [CrossRef]
- Burczak, D.R.; Julakanti, R.R.; Balla, A.K.; Scott, C.G.; Geske, J.B.; Ommen, S.R.; Nkomo, V.T.; Gersh, B.J.; Noseworthy, P.A.; Siontis, K.C. Risk of Left Atrial Thrombus in Patients with Hypertrophic Cardiomyopathy and Atrial Fibrillation. J. Am. Coll. Cardiol. 2023, 82, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, S.; Ma, X.; Zhao, K.; Yang, K.; Yu, S.; Yin, G.; Dong, Z.; Song, Y.; Cui, C.; et al. Assessment of late gadolinium enhancement in hypertrophic cardiomyopathy improves risk stratification based on current guidelines. Eur. Heart J. 2023, 44, 4781–4792. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, J.; Cheng, W.; Wan, K.; Li, W.; Pu, L.; Xu, Y.; Sun, J.; Han, Y.; Chen, Y. Incremental significance of myocardial oedema for prognosis in hypertrophic cardiomyopathy. Eur. Heart J.-Cardiovasc. Imaging 2023, 24, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z.; Li, Y.; Xu, Y.; Wan, K.; Chen, Y. Variable and Limited Predictive Value of the European Society of Cardiology Hypertrophic Cardiomyopathy Sudden-Death Risk Model: A Meta-analysis. Can. J. Cardiol. 2019, 35, 1791–1799. [Google Scholar] [CrossRef]
- Maron, B.J.; Rowin, E.J.; Maron, M.S. Evolution of risk stratification and sudden death prevention in hypertrophic cardiomyopathy: Twenty years with the implantable cardioverter-defibrillator. Heart Rhythm. 2021, 18, 1012–1023. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Wachter, R.; Schmalstieg-Bahr, K.; Quinn, F.R.; Hummers, E.; Ivers, N.; Marsden, T.; Thornton, A.; Djuric, A.; Suerbaum, J.; et al. Screening for Atrial Fibrillation in the Older Population: A Randomized Clinical Trial. JAMA Cardiol. 2021, 6, 558–567. [Google Scholar] [CrossRef]
- Jones, N.R.; Taylor, C.J.; Hobbs, F.D.R.; Bowman, L.; Casadei, B. Screening for atrial fibrillation: A call for evidence. Eur. Heart J. 2020, 41, 1075–1085. [Google Scholar] [CrossRef]
- Healey, J.S.; Lopes, R.D.; Granger, C.B.; Alings, M.; Rivard, L.; McIntyre, W.F.; Atar, D.; Birnie, D.H.; Boriani, G.; Camm, A.J.; et al. Apixaban for Stroke Prevention in Subclinical Atrial Fibrillation. N. Engl. J. Med. 2024, 390, 107–117. [Google Scholar] [CrossRef]
- McIntyre, W.F.; Benz, A.P.; Becher, N.; Healey, J.S.; Granger, C.B.; Rivard, L.; Camm, A.J.; Goette, A.; Zapf, A.; Alings, M.; et al. Direct Oral Anticoagulants for Stroke Prevention in Patients with Device-Detected Atrial Fibrillation: A Study-Level Meta-Analysis of the NOAH-AFNET 6 and ARTESiA Trials. Circulation 2024, 149, 981–988. [Google Scholar] [CrossRef]
- 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [CrossRef]
| Variable | All Patients (n = 113) |
|---|---|
| Male sex (%) | 88 (77.9) |
| Age (years) | 57.9 (48.0–67.1) |
| Hypertension (%) | 54 (47.8) |
| Diabetes (%) | 13 (11.5) |
| Dyslipidemia (%) | 57 (50.4) |
| Cerebrovascular disease (%) | 6 (5.3) |
| PAD (%) | 2 (1.8) |
| Chronic kidney disease (%) | 3 (2.7) |
| COPD (%) | 5 (4.4) |
| Coronary artery disease (%) | 6 (5.3) |
| Valvular heart disease (%) | 12 (10.6) |
| ACEi/ARB (%) | 42 (37.2) |
| Beta blockers (%) | 65 (57.5) |
| Dysopiramide (%) | 5 (4.4) |
| Other antiarrhythmics (%) | 3 (2.7) |
| Antiplatelets (%) | 16 (14.2) |
| Anticoagulation (%) | 21 (18.6) |
| Age at diagnosis | 51.2 (40.5–60.6) |
| Family history of SCD (%) | 16 (14.2) |
| P/LP variant carrier (%) | 36 (36.4) |
| Proband status (%) | 85 (75.2) |
| NYHA class (%) | |
| I | 77 (68.1) |
| II | 33 (29.2) |
| III | 3 (2.7) |
| Prior HF decompensations (%) | 10 (8.9) |
| Prior syncope (%) | 6 (5.3) |
| Palpitations (%) | 31 (27.4) |
| Prior atrial fibrillation (%) | 18 (15.9) |
| Type of AF (%) | |
| Paroxysmal | 10 (55.5) |
| Persistent | 3 (16.7) |
| Permanent | 5 (27.8) |
| Prior AF ablation (%) | 7 (38.9) |
| History of NSVT (%) | 16 (14.2) |
| Baseline 5-year risk SCD (%) | 1.89 (1.36–2.60) |
| ASA (%) | 4 (3.5) |
| Septal myectomy (%) | 3 (2.7) |
| Variable | All Patients (n = 113) | CR Arrhythmia (n = 72) | No CR Arrhythmia (n = 41) | p Value |
|---|---|---|---|---|
| Male sex (%) | 88 (77.9) | 59 (81.8) | 29 (70.7) | 0.167 |
| Age (years) | 57.9 (48.0–67.1) | 60.6 (51.1–71.3) | 53.3 (41.6–61.0) | 0.004 |
| Hypertension (%) | 54 (47.8) | 34 (47.2) | 20 (48.8) | 0.873 |
| Diabetes (%) | 13 (11.5) | 9 (12.5) | 4 (9.8) | 0.766 |
| Dyslipidemia (%) | 57 (50.4) | 33 (45.8) | 24 (58.5) | 0.194 |
| Cerebrovascular disease (%) | 6 (5.3) | 5 (6.9) | 1 (2.4) | 0.414 |
| PAD (%) | 2 (1.8) | 1 (1.4) | 1 (2.4) | 1.000 |
| Chronic kidney disease (%) | 3 (2.7) | 2 (2.8) | 1 (2.4) | 1.000 |
| COPD (%) | 5 (4.4) | 3 (4.2) | 2 (4.9) | 1.000 |
| Coronary artery disease (%) | 6 (5.3) | 5 (6.9) | 1 (2.4) | 0.414 |
| Valvular heart disease (%) | 12 (10.6) | 9 (12.5) | 3 (7.3) | 0.531 |
| ACEi/ARB (%) | 42 (37.2) | 29 (40.3) | 13 (31.7) | 0.365 |
| Beta blockers (%) | 65 (57.5) | 44 (61.1) | 21 (51.2) | 0.306 |
| Dysopiramide (%) | 5 (4.4) | 4 (5.6) | 1 (2.4) | 0.651 |
| Other antiarrhythmics (%) | 3 (2.7) | 3 (4.7) | 0 (0.0) | 0.552 |
| Antiplatelets (%) | 16 (14.2) | 9 (12.5) | 7 (17.1) | 0.503 |
| Anticoagulation (%) | 21 (18.6) | 15 (20.8) | 6 (14.6) | 0.415 |
| Age at diagnosis | 51.2 (40.5–60.6) | |||
| Family history of SCD (%) | 16 (14.2) | 10 (13.9) | 6 (14.6) | 0.913 |
| P/LP variant carrier (%) | 36 (36.4) | 26 (39.4) | 10 (30.3) | 0.375 |
| Proband status (%) | 85 (75.2) | 56 (77.8) | 29 (70.7) | 0.404 |
| NYHA class (%) | ||||
| I | 77 (68.1) | 50 (69.4) | 27 (65.9) | |
| II | 33 (29.2) | 20 (27.8) | 13 (31.7) | 0.853 |
| III | 3 (2.7) | 2 (2.8) | 1 (2.4) | |
| Prior HF decompensations (%) | 10 (8.9) | 7 (9.7) | 3 (7.3) | 0.745 |
| Prior syncope (%) | 6 (5.3) | 4 (5.6) | 2 (4.9) | 1.000 |
| Palpitations (%) | 31 (27.4) | 20 (27.8) | 11 (26.8) | 0.913 |
| Prior atrial fibrillation (%) | 18 (15.9) | 14 (19.4) | 4 (9.8) | 0.284 |
| Type of AF (%) | ||||
| Paroxysmal | 10 (55.5) | 7 (50.0) | 3 (75.0) | |
| Persistent | 3 (16.7) | 2 (14.3) | 1 (25.0) | 0.436 |
| Permanent | 5 (27.8) | 5 (35.7) | 0 (0.0) | |
| Prior AF ablation (%) | 7 (38.9) | 6 (42.9) | 1 (25.0) | 1.000 |
| History of NSVT (%) | 16 (14.2) | 15 (20.8) | 1 (2.4) | 0.009 |
| Baseline 5-year risk SCD (%) | 1.89 (1.36–2.60) | 1.90 (1.38–2.91) | 1.82 (1.32–2.42) | 0.335 |
| ASA (%) | 4 (3.5) | 1 (1.4) | 3 (7.3) | 0.135 |
| Septal myectomy (%) | 3 (2.7) | 2 (2.8) | 1 (2.4) | 1.000 |
| Imaging studies | ||||
| PLAX LA diameter (mm) | 41.5 ± 7.5 | 42.9 ± 7.5 | 38.9 ± 6.9 | 0.007 |
| Maximum LV thickness (mm) | 17 (15–20) | 18 (16–20) | 17 (14.5–19) | 0.039 |
| LVEF (%) | 66.3 ± 7.0 | 65.6 ± 7.6 | 67.5 ± 5.6 | 0.180 |
| Significant LVOT obstruction (%) | 22 (19.5) | 13 (18.1) | 9 (22.0) | 0.615 |
| Apical aneurysm (%) | 5 (4.4) | 4 (5.6) | 1 (2.4) | 0.652 |
| Late gadolinium enhancement (%) | 58 (67.4) | 46 (85.2) | 12 (37.5) | <0.001 |
| 24 h Holter monitoring (n = 96) | ||||
| NSVT (%) | 17 (17.7) | 16 (24.6) | 1 (3.2) | 0.010 |
| AF (%) | 5 (5.2) | 5 (7.7) | 0 (0.0) | 0.171 |
| Premature atrial complexes (n) | 47 (13–220) | 87 (15–225) | 37 (8–82) | 0.255 |
| Premature ventricular complexes (n) | 32 (3–181) | 36 (7–178) | 16 (2–196) | 0.344 |
| NSVT Characteristics | All Patients with NSVT (n = 69) | NSVT 0–24 h (n = 10) | NSVT 24 h-30 d (n = 59) | p Value |
|---|---|---|---|---|
| Number of NSVT per patient | 3 (1–6) | 10 (9–19) | 2 (1–4) | <0.001 |
| Heart rate (bpm) | 153 (135–172) | 170 (160–175) | 148 (132–169) | 0.034 |
| Duration (beats) | 8 (4–12) | 12 (8–16) | 8 (4–11) | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caro-Codón, J.; Castrejón, S.; Cadenas, R.; Casanova, C.; Vélez, A.; Basurte, M.; Lacuey, G.; Climent, V.; Salvador, Ó.; Severo-Sánchez, A.; et al. Extended ECG Monitoring in Patients with Hypertrophic Cardiomyopathy: The Tempo-HCM Study. J. Clin. Med. 2025, 14, 7432. https://doi.org/10.3390/jcm14207432
Caro-Codón J, Castrejón S, Cadenas R, Casanova C, Vélez A, Basurte M, Lacuey G, Climent V, Salvador Ó, Severo-Sánchez A, et al. Extended ECG Monitoring in Patients with Hypertrophic Cardiomyopathy: The Tempo-HCM Study. Journal of Clinical Medicine. 2025; 14(20):7432. https://doi.org/10.3390/jcm14207432
Chicago/Turabian StyleCaro-Codón, Juan, Sergio Castrejón, Rosalía Cadenas, Carlos Casanova, Andrea Vélez, Mayte Basurte, Gemma Lacuey, Vicente Climent, Óscar Salvador, Andrea Severo-Sánchez, and et al. 2025. "Extended ECG Monitoring in Patients with Hypertrophic Cardiomyopathy: The Tempo-HCM Study" Journal of Clinical Medicine 14, no. 20: 7432. https://doi.org/10.3390/jcm14207432
APA StyleCaro-Codón, J., Castrejón, S., Cadenas, R., Casanova, C., Vélez, A., Basurte, M., Lacuey, G., Climent, V., Salvador, Ó., Severo-Sánchez, A., Fernández, L., Pérez-David, E., Peinado, R., Valbuena-López, S., Guzmán, G., Jiménez-Mas, Á., Moreno, R., & Merino, J. L. (2025). Extended ECG Monitoring in Patients with Hypertrophic Cardiomyopathy: The Tempo-HCM Study. Journal of Clinical Medicine, 14(20), 7432. https://doi.org/10.3390/jcm14207432





