Joint Hypermobility: An Under-Recognised Cause of Palpitations, Dizziness, and Syncope in Young Females
Abstract
1. Introduction
2. Methods
2.1. Population and Data Collection
2.2. Diagnostics
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
3.1. Demographics
3.2. Symptoms
3.3. Cardiovascular Investigations
3.3.1. Baseline Physiological Parameters
3.3.2. Electrocardiography and Ambulatory Monitoring
3.3.3. Transthoracic Echocardiography
3.3.4. Tilt-Table Testing
3.4. Response to Management
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCarthy, F.; McMahon, C.; Geary, U.; Plunkett, P.; Kenny, R.; Cunningham, C. Management of syncope in the Emergency Department: A single hospital observational case series based on the application of European Society of Cardiology Guidelines. Europace 2009, 11, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Probst, M.; Mower, W.; Kanzaria, H.; Hoffman, J.; Buch, E.; Sun, B. Analysis of emergency department visits for palpitations (from the National Hospital Ambulatory Medical Care Survey). Am. J. Cardiol. 2014, 113, 1685–1690. [Google Scholar] [CrossRef]
- Fikree, A.; Aziz, Q.; Grahame, R. Joint hypermobility syndrome. Rheum. Dis. Clin. North. Am. 2013, 39, 419–430. [Google Scholar] [CrossRef]
- Russek, L.; Errico, D. Prevalence, injury rate and symptom frequency in generalized joint laxity and joint hypermobility syndrome in a “healthy” college population. Clin. Rheumatol. 2016, 35, 1029–1039. [Google Scholar] [CrossRef]
- Kumar, B.; Lenert, P. Joint Hypermobility Syndrome: Recognizing a Commonly Overlooked Cause of Chronic Pain. Am. J. Med. 2017, 130, 640–647. [Google Scholar] [CrossRef]
- Robinson, S.; Rana, B.; Oxborough, D.; Steeds, R.; Monaghan, M.; Stout, M.; Pearce, K.; Harkness, A.; Ring, L.; Paton, M.; et al. A practical guideline for performing a comprehensive transthoracic echocardiogram in adults: The British Society of Echocardiography minimum datase. Echo Res. Pract. 2020, 7, G59–G93. [Google Scholar] [CrossRef]
- Kenny, R.; O’Shea, D.; Parry, S. The Newcastle protocols for head-up tilt table testing in the diagnosis of vasovagal syncope, carotid sinus hypersensitivity, and related disorders. Heart 2000, 83, 564–569. [Google Scholar] [CrossRef]
- Romeo, D.M.; Moro, M.; Pezone, M.; Venezia, I.; Mirra, F.; De Biase, M.; Polo, A.; Turrini, I.; Lala, M.R.; Velli, C.; et al. Relationship and New Prospectives in Joint Hypermobility in Children with Autism Spectrum Disorder: Preliminary Data. J. Pers. Med. 2023, 13, 1723. [Google Scholar] [CrossRef]
- Owens, A.P.; Mathias, C.J.; Iodice, V. Autonomic Dysfunction in Autism Spectrum Disorder. Front. Integr. Neurosci. 2021, 15, 787037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mathias, C.J.; Owens, A.; Iodice, V.; Hakim, A. Dysautonomia in the Ehlers-Danlos syndromes and hypermobility spectrum disorders-With a focus on the postural tachycardia syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2021, 187, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Peebles, K.C.; Tan, I.; Butlin, M.; Collins, F.; Tofts, L.; Avolio, A.P.; Pacey, V. The prevalence and impact of orthostatic intolerance in young women across the hypermobility spectrum. Am. J. Med. Genet. A 2022, 188, 1761–1776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef]
- Juul-Kristensen, B.; Schmedling, K.; Rombaut, L.; Lund, H.; Engelbert, R. Measurement properties of clinical assessment methods for classifying generalized joint hypermobility-A systematic review. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 116–147. [Google Scholar] [CrossRef]
- Rashed, E.; Ruiz Maya, T.; Black, J.; Fettig, V.; Kadian-Dodov, D.; Olin, J.; Mehta, L.; Gelb, B.D.; Kontorovich, A.R. Cardiovascular manifestations of hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders. Vasc. Med. 2022, 27, 283–289. [Google Scholar] [CrossRef]
- Asher, S.; Chen, R.; Kallish, S. Mitral valve prolapse and aortic root dilation in adults with hypermobile Ehlers-Danlos syndrome and related disorders. Am. J. Med. Genet. Part A 2018, 176, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- Melamed, T.; Badiani, S.; Harlow, S.; Laskar, N.; Treibel, T.; Bhattacharyya, S.; Lloyd, G. Prevalence of mitral valve prolapse: A systematic review and meta-analysis. Heart 2024, 110, 14–16. [Google Scholar]
- Swahn, E.; Lekedal, H.; Engvall, J.; Nyström, F.; Jonasson, L. Prevalence and determinants of dilated ascending aorta in a Swedish population: A case-control study. Eur. Heart J. Open 2023, 3, oead085. [Google Scholar] [CrossRef] [PubMed]
- Alegret, J.; Calvo, N.; Ligero, C.; Palomares, R.; Millá, L.; Martín-Paredero, V.; Montero, M.; Joven, J. Dilated aortic root is related to a global aortic dilating diathesis. J. Vasc. Surg. 2010, 52, 867–871. [Google Scholar] [CrossRef]
- Cappato, R.; Castelvecchio, S.; Ricci, C.; Bianco, E.; Vitali-Serdoz, L.; Gnecchi-Ruscone, T.; Pittalis, M.; De Ambroggi, L.; Baruscotti, M.; Gaeta, M.; et al. Clinical efficacy of ivabradine in patients with inappropriate sinus tachycardia: A prospective, randomized, placebo-controlled, double-blind, crossover evaluation. J. Am. Coll. Cardiol. 2012, 60, 1323–1329. [Google Scholar] [CrossRef]
- Taub, P.; Zadourian, A.; Lo, H.; Ormiston, C.; Golshan, S.; Hsu, J. Randomized Trial of Ivabradine in Patients With Hyperadrenergic Postural Orthostatic Tachycardia Syndrome. J. Am. Coll. Cardiol. 2021, 77, 861–871. [Google Scholar] [CrossRef]
- Rowe, P.; Barron, D.; Calkins, H.; Maumenee, I.; Tong, P.; Geraghty, M. Orthostatic intolerance and chronic fatigue syndrome associated with Ehlers-Danlos syndrome. J. Pediatr. 1999, 135, 494–499. [Google Scholar] [CrossRef]
- Gazit, Y.; Nahir, A.; Grahame, R.; Jacob, G. Dysautonomia in the joint hypermobility syndrome. Am. J. Med. 2003, 115, 33–40. [Google Scholar] [CrossRef]
- Kanjwal, K.; Saeed, B.; Karabin, B.; Kanjwal, Y.; Grubb, B.P. Comparative clinical profile of postural orthostatic tachycardia patients with and without joint hypermobility syndrome. Indian. Pacing Electrophysiol. J. 2010, 10, 173–178. [Google Scholar] [PubMed] [PubMed Central]
- Miller, A.; Stiles, L.; Sheehan, T.; Bascom, R.; Levy, H.; Francomano, C.; Arnold, A.C. Prevalence of hypermobile Ehlers-Danlos syndrome in postural orthostatic tachycardia syndrome. Auton. Neurosci. 2020, 224, 102637. [Google Scholar] [CrossRef] [PubMed]
- Roma, M.; Marden, C.; De Wandele, I.; Francomano, C.; Rowe, P. Postural tachycardia syndrome and other forms of orthostatic intolerance in Ehlers-Danlos syndrome. Auton. Neurosci. 2018, 215, 89–96. [Google Scholar] [CrossRef] [PubMed]
- De Wandele, I.; Rombaut, L.; Leybaert, L.; Van de Borne, P.; De Backer, T.; Malfait, F.; De Paepe, A.; Calders, P. Dysautonomia and its underlying mechanisms in the hypermobility type of Ehlers-Danlos syndrome. Semin. Arthritis Rheum. 2014, 44, 93–100. [Google Scholar] [CrossRef]
- Haensch, C.A.; Tosch, M.; Katona, I.; Weis, J.; Isenmann, S. Small-fiber neuropathy with cardiac denervation in postural tachycardia syndrome. Muscle Nerve 2014, 50, 956–961. [Google Scholar] [CrossRef]
- Streeten, D.H. Pathogenesis of hyperadrenergic orthostatic hypotension. Evidence of disordered venous innervation exclusively in the lower limbs. J. Clin. Invest. 1990, 86, 1582–1588. [Google Scholar] [CrossRef]
- Louisias, M.; Silverman, S.; Maitland, A.L. Prevalence of allergic disorders and mast cell activation syndrome in patients with Ehlers-Danlos syndrome. Ann. Allergy Asthma Immunol. 2013, 111, A12–A13. [Google Scholar]
- Ireland, P.; Mickelsen, D.; Rodenhouse, T.; Bakos, R.; Goldstein, B. Evaluation of the autonomic cardiovascular response in Arnold-Chiari deformities and cough syncope syndrome. Arch. Neurol. 1996, 53, 526–531. [Google Scholar] [CrossRef]
- Milhorat, T.; Bolognese, P.; Nishikawa, M.; McDonnell, N.; Francomano, C. Syndrome of occipito-atlanto-axial hypermobility, cranial settling, and chiari malformation type 1 in patients with hereditary disorders of connective tissue. J. Neurosurg. Spine 2007, 7, 601–609. [Google Scholar] [CrossRef]
- Sun, T.; Hardin, J.; Nieva, H.; Natarajan, K.; Cheng, R.; Ryan, P.; Elhadad, N. Large-scale characterization of gender differences in diagnosis prevalence and time to diagnosis. medRxiv 2023. [Google Scholar] [CrossRef]
- Scott, P.; Unger, E.; Jenkins, M.; Southworth, M.; McDowell, T.; Geller, R.; Elahi, M.; Temple, R.J.; Woodcock, J. Participation of women in clinical trials supporting FDA approval of cardiovascular drugs. J. Am. Coll. Cardiol. 2018, 71, 1960–1969. [Google Scholar] [CrossRef]
- Hiremath, P.; Aversano, T.; Spertus, J.; Lemmon, C.; Naiman, D.; Czarny, M. Sex Differences in Health Status and Clinical Outcomes After Nonprimary Percutaneous Coronary Intervention. Circ. Cardiovasc. Interv. 2022, 15, e011308. [Google Scholar] [CrossRef]
- Maserejian, N.; Link, C.; Lutfey, K.; Marceau, L.; McKinlay, J. Disparities in physicians’ interpretations of heart disease symptoms by patient gender: Results of a video vignette factorial experiment. J. Womens Health 2009, 18, 1661–1667. [Google Scholar] [CrossRef]
- Martinez-Nadal, G.; Miro, O.; Matas, A.; Cepas, P.; Aldea, A.; Izquierdo, M.; Coll-Vinent, B.; Garcia, A.; Carbo, M.; Manuel, O.; et al. An analysis based on sex & gender in the chest pain unit of an emergency department during the last 12 years. Eur. Heart J. Acute Cardiovasc. Care 2021, 10 (Suppl. S1), zuab020.122. [Google Scholar]
- Eccles, J.; Beacher, F.; Gray, M.; Jones, C.; Minati, L.; Harrison, N.; Critchley, H.D. Brain structure and joint hypermobility: Relevance to the expression of psychiatric symptoms. Br. J. Psychiatry 2012, 200, 508–509. [Google Scholar] [CrossRef] [PubMed]

| Initial Orthostatic Hypotension | Decrease in BP > 40 mmhg at Standing with Fast Normalisation so Symptoms Last < 30 s |
|---|---|
| Classical orthostatic hypotension | Decrease in systolic BP ≥ 20 mmHg and diastolic BP ≥ 10 mmHg during the first 3 min after standing. |
| Delayed orthostatic hypotension | Slow and progressive systolic BP decline after the 3rd minute of standing. |
| Type 1 mixed reflex syncope | HR decreases during syncope but does not reach < 40 bpm or reaches < 40 bpm for <10 s. BP decreases before HR falls. |
| Type 2 cardioinhibitory syncope | HR decreases < 40 bpm for >10 s. BP decreases before HR falls. |
| Type 3 vasodepressor syncope | BP falls < 60 mmHg; HR does not fall by more than 10% of the peak value. |
| Postural orthostatic tachycardia syndrome (POTS) | The increase in HR > 30 bpm or HR > 120 bpm after standing is accompanied by symptoms and BP variability. |
| Demographics | n = 218 |
|---|---|
| Age (median, IQR) | 24 (11) |
| Female sex | 208 (95.4) |
| Ethnicity | |
| White British | 143 (65.6) |
| Asian | 7 (3.2) |
| Black | 7 (3.2) |
| Mixed | 5 (2.3) |
| Not reported | 56 (25.7) |
| Body mass index (mean, SD) | 26.5 (6.6) |
| Body mass index category | |
| Underweight | 14 (6.4) |
| Normal | 105 (48.2) |
| Overweight | 36 (16.5) |
| Obese | 60 (27.5) |
| Not reported | 3 (1.4) |
| Mobility status: | |
| No limitation | 129 (59.2) |
| Severe limitation | 18 (8.3) |
| Unable to stand, use of wheelchair | 6 (2.8) |
| Social history | |
| Smoker | 19 (8.7) |
| Alcohol | 63 (28.9) |
| Employment | |
| No work | 64 (29.4) |
| Non-skilled or administrative | 33 (15.1) |
| Skilled or professional | 59 |
| Student | 53 (24.3) |
| Unknown | 9 (4.1) |
| Medication history (pre-clinic) | |
| Ivabradine | 21 (9.6) |
| Beta-blocker | 26 (11.9) |
| Fludrocortisone | 5 (2.3) |
| Other * | 6 (2.8) |
| Main cardiovascular symptom | |
| Dizziness on standing | 168 (77.1) |
| Dizziness (any time) | 5 (2.3) |
| Dizziness (exertion) | 1 (0.5) |
| Syncope | 22 (10.1) |
| Palpitations | 21 (9.6) |
| Other (tachycardia) | 1 (0.5) |
| Non-cardiovascular symptoms | |
| Chronic pain | 75 (34.4) |
| Chronic fatigue | 55 (25.2) |
| Joint pain/dislocation | 61 (28.0) |
| Gastrointestinal symptoms | 88 (40.4) |
| Urinary symptoms | 17 (7.8) |
| Migraine | 76 (34.9) |
| Other neurological symptoms (brain fog and blurred vision) | 5 (2.3) |
| Concomitant disease | |
| Psychiatric | |
| Depression | 50 (22.9) |
| Anxiety | 58 (26.6) |
| Eating disorder | 7 (3.2) |
| ADHD | 17 (7.8) |
| Autism | 16 (7.3) |
| Neurological: | |
| Migraine | 76 (34.9) |
| Chiari malformation | 6 (2.8) |
| Epilepsy | 5 (2.3) |
| Non-epileptiform attack disorder | 6 (2.8) |
| Rheumatological: | |
| Rheumatological diagnosis (SLE, connective tissue disease) | 8 (3.7) |
| Raynaud’s phenomena | 5 (2.3) |
| Mast cell activation/allergy syndrome | 27 (12.4) |
| Fibromyalgia | 15 (6.9) |
| Gynaecological | |
| Endometriosis | 4 (1.8) |
| PCOS | 5 (2.3) |
| Other: | |
| Asthma | 31 (14.2) |
| Autoimmune disease | 6 (2.8) |
| Inflammatory bowel disease | 4 (1.8) |
| Vital Signs | n = 218 |
|---|---|
| Heart rate off rate-limiting medications (mean, SD) | 98.7 (17.1) |
| Heart rate on rate-limiting medications (mean, SD) | 80.6 (13.0) |
| Systolic BP in clinic (mean, SD) | 125.4 (13.9) |
| Diastolic BP in clinic (mean, SD) | 79.8 (8.5) |
| Postural hypotension in clinic | |
| Yes | 23 (10.6) |
| No | 148 (67.9) |
| Not performed | 47 (21.6) |
| ECG | |
| Normal | 166 (76.1) |
| Sinus tachycardia | 27 (12.4) |
| Sinus bradycardia | 2 (0.9) |
| Non-specific ST/T wave changes | 23 (10.6) |
| Long QT | 1 (0.5) |
| RBBB/incomplete RBBB | 3 (1.4) |
| Right axis deviation | 2 (0.9) |
| Echocardiogram | |
| Normal | 183 (83.9) |
| Abnormal | 15 (6.9) |
| Not performed | 20 (9.2) |
| Echocardiogram abnormalities | n = 15 |
| Mild valvular regurgitation | 7 (3.2) |
| Elongated AVML | 5 (2.3) |
| Bileaflet bowing of MV | 3 (1.4) |
| Other incidental finding * | 3 (1.4) |
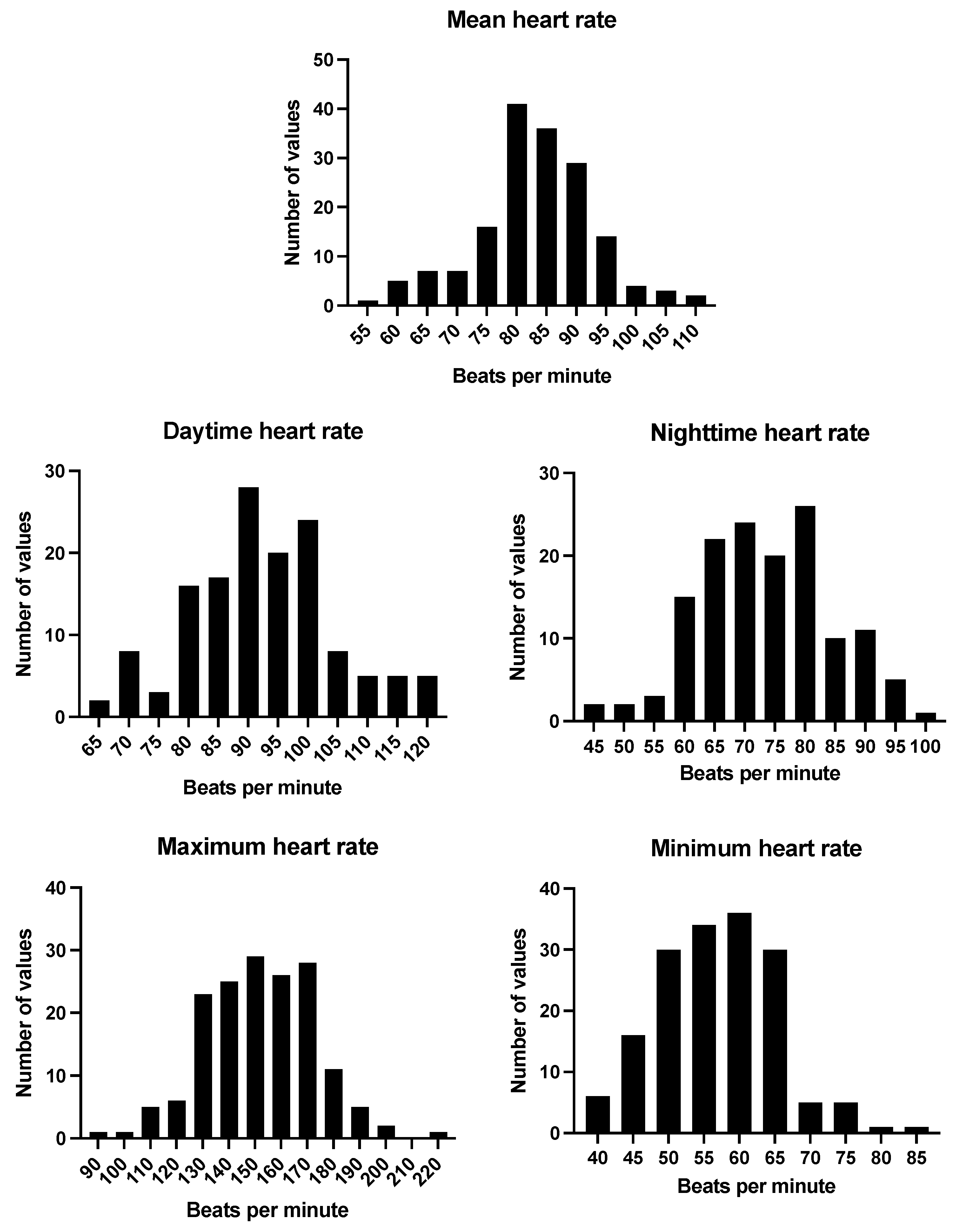
| Ambulatory ECG monitoring (off rate-limiting medications) | n = 165 |
| Mean HR in bpm (mean, SD) | 83 (9.9) |
| Max HR in bpm (mean, SD) | 152 (21.0) |
| Min HR in bpm (mean, SD) | 57 (8.4) |
| Mean daytime HR in bpm (mean, SD) | 92 (12.2) |
| Mean nighttime HR in bpm (mean, SD) | 74 (10.9) |
| Tilt-table test ** | n = 162 |
| Normal | 36 (22.2) |
| Initial OH | 60 (37.0) |
| Classical OH | 9 (5.6) |
| Delayed OH | 6 (3.7) |
| Type 1 mixed reflex syncope | 14 (8.6) |
| Type 3 vasodepressor reflex syncope | 11 (6.8) |
| POTS | 16 (9.9) |
| HR increase > 10 bpm within 10 min | 9 (5.6) |
| HR increase > 20 bpm within 10 min | 8 (4.9) |
| Test not completed | 7 (4.3) |
| n = 218 | |
|---|---|
| Existing management | |
| Already implementing conservative measures | 52 (23.9) |
| Already taking rate limiting medications | 47 (21.6) |
| Conservative management | |
| Response to conservative management | |
| Yes | 127 (58.3) |
| No | 61 (28.0) |
| Not reported or not yet followed up | 30 (13.8) |
| Medical management | |
| New medication initiated | |
| Ivabradine | 63 (28.9) |
| Beta-blocker | 17 (7.8) |
| Fludrocortisone | 39 (17.9) |
| Midodrine | 20 (9.2) |
| Dose of existing medication increased | 14 (6.4) |
| Response to medical management (n = 128) | |
| Complete response | 56 (43.8) |
| Some response | 36 (28.1) |
| No response | 8 (6.3) |
| Not yet followed up or not reported | 28 (21.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Orabi, Z.; Thompson, S.E.; van Vliet, J.; Gee, K.; Roy, A.; Townend, J.N. Joint Hypermobility: An Under-Recognised Cause of Palpitations, Dizziness, and Syncope in Young Females. J. Clin. Med. 2025, 14, 7373. https://doi.org/10.3390/jcm14207373
Abu Orabi Z, Thompson SE, van Vliet J, Gee K, Roy A, Townend JN. Joint Hypermobility: An Under-Recognised Cause of Palpitations, Dizziness, and Syncope in Young Females. Journal of Clinical Medicine. 2025; 14(20):7373. https://doi.org/10.3390/jcm14207373
Chicago/Turabian StyleAbu Orabi, Zeina, Sophie E. Thompson, Jan van Vliet, Kate Gee, Ashwin Roy, and Jonathan N. Townend. 2025. "Joint Hypermobility: An Under-Recognised Cause of Palpitations, Dizziness, and Syncope in Young Females" Journal of Clinical Medicine 14, no. 20: 7373. https://doi.org/10.3390/jcm14207373
APA StyleAbu Orabi, Z., Thompson, S. E., van Vliet, J., Gee, K., Roy, A., & Townend, J. N. (2025). Joint Hypermobility: An Under-Recognised Cause of Palpitations, Dizziness, and Syncope in Young Females. Journal of Clinical Medicine, 14(20), 7373. https://doi.org/10.3390/jcm14207373






