Real-World Effectiveness of Golimumab in Ulcerative Colitis: A Pooled Analysis from the Prospective UMBRELLA-IBD Registry in Germany
Abstract
1. Introduction
2. Methods
2.1. Study Design and Data Sources
2.2. Patient Cohort and Homogeneity Analysis
2.3. Baseline Data and Treatment Regimens
2.4. Outcomes and Definitions
2.5. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse event |
| CR | Clinical remission |
| CRP | C-reactive protein |
| DGVS | German Society for Gastroenterology, Digestive and Metabolic Disease |
| ECCO | European Crohn’s and Colitis Organisation |
| HRQoL | Health-related quality of life |
| IBD | Inflammatory Bowel Disease |
| IQR | Interquartile range |
| mITT | Modified intention-to-treat |
| pMayo | Partial Mayo Score |
| RCT | Randomized controlled trial |
| SAE | Severe adverse event |
| SFCR | Steroid-free clinical remission |
| SF-12 | 12-item short form survey |
| UC | Ulcerative Colitis |
| WPAI | Work Productivity and Activity Impairment |
References
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.-F.; Reinisch, W.; et al. Subcutaneous Golimumab Induces Clinical Response and Remission in Patients with Moderate-to-Severe Ulcerative Colitis. Gastroenterology 2014, 146, 85–95, quiz e14–15. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.-F.; Reinisch, W.; et al. Subcutaneous Golimumab Maintains Clinical Response in Patients with Moderate-to-Severe Ulcerative Colitis. Gastroenterology 2014, 146, 96–109.e1. [Google Scholar] [CrossRef] [PubMed]
- Teich, N.; Grümmer, H.; Jörgensen, E.; Liceni, T.; Holtkamp-Endemann, F.; Fischer, T.; Hohenberger, S. Golimumab in Real-World Practice in Patients with Ulcerative Colitis: Twelve-Month Results. World J. Gastroenterol. 2020, 26, 2852–2863. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Park, S.J.; Kim, H.W.; Lim, Y.J.; Park, J.; Cha, J.M.; Ye, B.D.; Kim, T.O.; Kim, H.-S.; Lee, H.S.; et al. Effectiveness and Safety of Golimumab in Patients with Ulcerative Colitis: A Multicenter, Prospective, Postmarketing Surveillance Study. Gut Liver 2022, 16, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Olivera, P.; Danese, S.; Pouillon, L.; Bonovas, S.; Peyrin-Biroulet, L. Effectiveness of Golimumab in Ulcerative Colitis: A Review of the Real World Evidence. Dig. Liver Dis. 2019, 51, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Bokemeyer, B.; Plachta-Danielzik, S.; Wenske, T.; Gilman, E.; Tran, F.; Bokemeyer, A.; Schreiber, S. Real-World Evidence Data Warehouse for Pooled Analyses in Patients with Inflammatory Bowel Diseases in Germany: The “Umbrella-IBD Registry” of the Competence Network IBD. United Eur. Gastroenterol. J. 2023, 11 (Suppl. S8), 1012. [Google Scholar]
- Bokemeyer, B.; Krummenerl, T.; Maaser, C.; Büning, J.; Atreya, R.; Lügering, A.; Helwig, U.; Jessen, P.; Hartmann, P.; Schreiber, S. P386. Anti-TNF Alpha as Induction and Maintenance Therapy in Ulcerative Colitis Patients in the BioColitis Registry in Germany. J. Crohn’s Colitis 2016, 10, S292–S293. [Google Scholar] [CrossRef]
- Bokemeyer, B.; Plachta-Danielzik, S.; Gilman, E.; Howaldt, S.; Mohl, W.; Efken, P.; Ehehalt, R.; Kahl, M.; Krause, T.; Trentmann, L.; et al. Real-World Effectiveness of Ustekinumab versus Anti-TNF or Vedolizumab in Ulcerative Colitis: Induction and 12-Month Maintenance Results from the Prospective, Observational RUN-UC Study. J. Crohn’s Colitis 2025, 19, jjaf052. [Google Scholar] [CrossRef] [PubMed]
- Plachta-Danielzik, S.; Bokemeyer, B.; Tappe, U.; Gilman, E.; Efken, P.; Mohl, W.; Holtkamp-Endemann, F.; von der Ohe, M.; Graßkemper, L.; Salmon, S.F.; et al. TARGET Register: Biologika- und Small Molecules-Register für Patient:innen mit chronisch-entzündlichen Darmerkrankungen in Deutschland—Registerdesign und erste Ergebnisse. In Zeitschrift für Gastroenterologie; Georg Thieme: Teningen, Germany, 2023; Volume 61, p. KV063. [Google Scholar]
- Bokemeyer, B.; Plachta-Danielzik, S.; Di Giuseppe, R.; Efken, P.; Mohl, W.; Krause, T.; Hoffstadt, M.; Ehehalt, R.; Trentmann, L.; Schweitzer, A.; et al. Real-world Effectiveness of Vedolizumab Compared to ANTI-TNF Agents in Biologic-naïve Patients with Ulcerative Colitis: A Two-year Propensity-score-adjusted Analysis from the Prospective, Observational VEDOIBD-study. Aliment. Pharmacol. Ther. 2023, 58, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Kanters, S.; Karim, M.E.; Thorlund, K.; Anis, A.; Bansback, N. When Does the Use of Individual Patient Data in Network Meta-Analysis Make a Difference? A Simulation Study. BMC Med. Res. Methodol. 2021, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, A.; Malara, G.; Pelucchi, C.; Fatiga, F.; Barbera, G.; Franchi, A.; Galeone, C. Effectiveness End Points in Real-World Studies on Biological Therapies in Psoriasis: Systematic Review with Focus on Drug Survival. Dermatology 2018, 234, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Menditto, E.; Cahir, C.; Malo, S.; Aguilar-Palacio, I.; Almada, M.; Costa, E.; Giardini, A.; Gil Peinado, M.; Mesquida, M.M.; Mucherino, S.; et al. Persistence as a Robust Indicator of Medication Adherence-Related Quality and Performance. Int. J. Environ. Res. Public Health 2021, 18, 4872. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Sands, B.E.; Sandborn, W.J.; Germinaro, M.; Vetter, M.; Shao, J.; Sheng, S.; Johanns, J.; Panés, J. VEGA Study Group Guselkumab plus Golimumab Combination Therapy versus Guselkumab or Golimumab Monotherapy in Patients with Ulcerative Colitis (VEGA): A Randomised, Double-Blind, Controlled, Phase 2, Proof-of-Concept Trial. Lancet Gastroenterol. Hepatol. 2023, 8, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Probert, C.S.; Sebastian, S.; Gaya, D.R.; Hamlin, P.J.; Gillespie, G.; Rose, A.; Tate, H.; Wheeler, C.; Irving, P.M. Golimumab Induction and Maintenance for Moderate to Severe Ulcerative Colitis: Results from GO-COLITIS (Golimumab: A Phase 4, UK, Open Label, Single Arm Study on Its Utilization and Impact in Ulcerative Colitis). BMJ Open Gastroenterol. 2018, 5, e000212. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, D.; Privitera, G.; Rogai, F.; Variola, A.; Viola, A.; Laterza, L.; Privitera, A.C.; Allocca, M.; Bossa, F.; Cappello, M.; et al. Two-Year Effectiveness and Safety of Golimumab in Ulcerative Colitis: An IG-IBD Study. United Eur. Gastroenterol. J. 2021, 9, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Iborra, M.; García-Morales, N.; Rubio, S.; Bertoletti, F.; Calvo, M.; Taxonera, C.; Boscá-Watts, M.M.; Sierra, M.; Mancenido, N.; Beltrán, B.; et al. Real-Life Experience with 4 Years of Golimumab Persistence in Ulcerative Colitis Patients. Sci. Rep. 2020, 10, 17774. [Google Scholar] [CrossRef] [PubMed]
- Fumery, M.; Nancey, S.; Filippi, J.; Altwegg, R.; Hébuterne, X.; Boshetti, G.; Barraud, M.; Meynier, J.; Paul, S.; Roblin, X. Effectiveness of Golimumab Intensification in Ulcerative Colitis: A Multicentric Prospective Study. Aliment Pharmacol. Ther. 2023, 57, 1290–1298. [Google Scholar] [CrossRef] [PubMed]

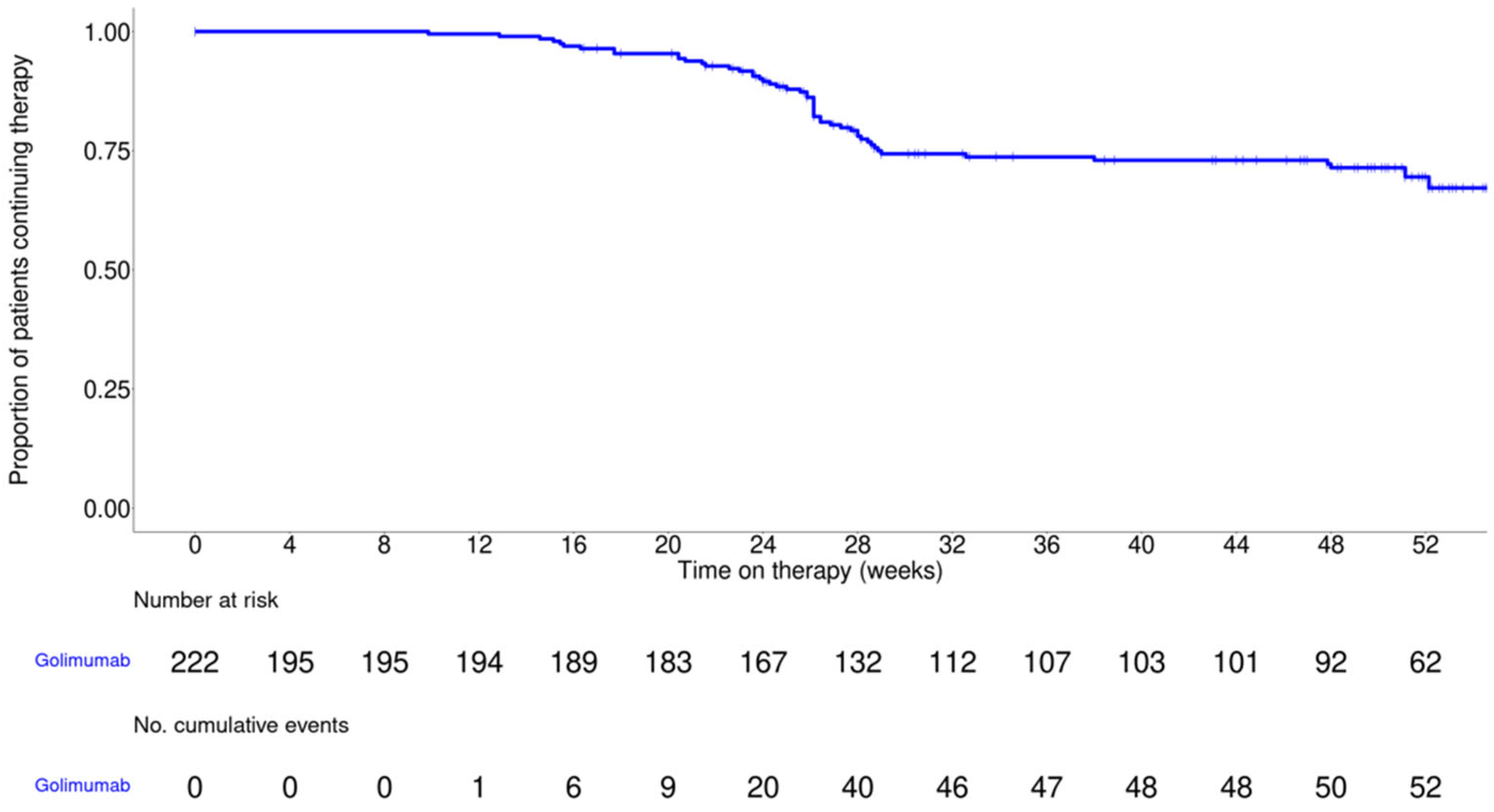
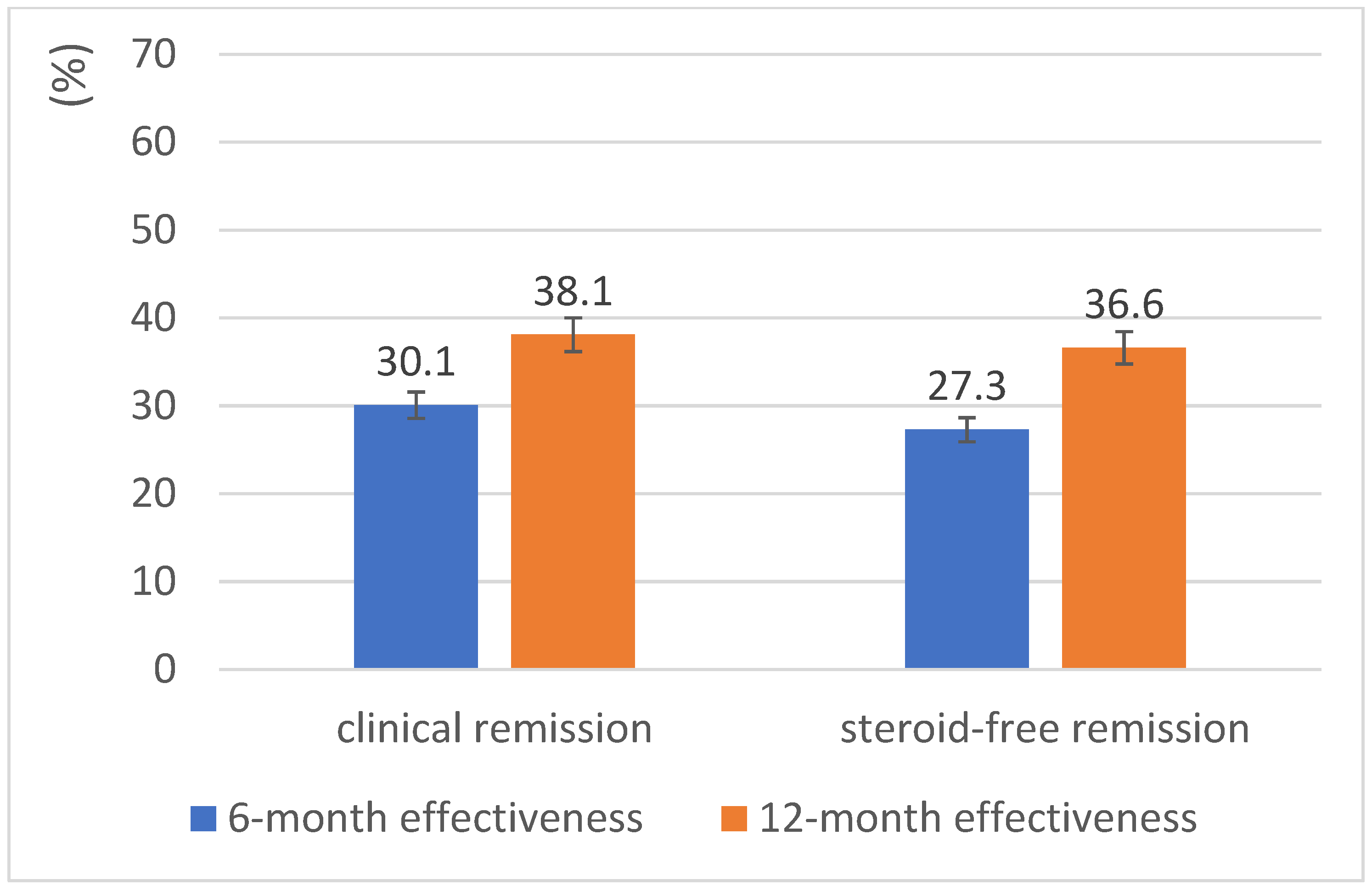
| Variable | Value |
|---|---|
| Number of patients, n | 222 |
| Age, median (IQR) | 39.00 (30.00, 51.40) |
| Female sex, n (%) | 110 (49.5) |
| Time from diagnosis to therapy initiation in years, median (IQR) | 5.85 (2.60, 12.80) |
| Disease extent, n (%) | |
| -E1 (proctitis) | 13 (6.1) |
| -E2 (left-sided colitis) | 89 (41.8) |
| -E3 (extensive colitis) | 111 (52.1) |
| Partial Mayo Score (pMS), median (IQR) | 4.00 (2.00, 6.00) |
| CRP (mg/dL), median (IQR) (data from 157/222 pts.) | 2.55 (0.80, 7.84) |
| Prior exposure to advanced therapies (data from 163/222 pts.) | |
| -no prior advanced therapies (%) | 8.0 |
| -one prior advanced therapy (%) | 11 |
| -≥2 prior advanced therapies (%) | 81 |
| Body weight in kg, median (IQR) | 74.00 (63.00, 86.00) |
| Patients with body weight ≥ 80 kg, n (%) | 86 (38.9) |
| Week 0 | Month 6 | Month 12 | |
|---|---|---|---|
| Golimumab patients, n | 222 | 138 | 101 |
| Clinical remission, n (%) | 33 (15.0) | 55 (39.9) | 51 (50.5) |
| Steroid-free clinical remission, n (%) | 25 (11.4) | 50 (36.2) | 49 (48.5) |
| Week 0 | Weeks 14–16 | Month 6 | Month 12 | |
|---|---|---|---|---|
| <80 kg: n | 20 | 18 | 15 | 15 |
| Clinical remission, n (%) | 1 (5.0) | 6 (33.3) | 5 (33.3) | 4 (26.7) |
| Steroid-free clinical remission, n (%) | 1 (5.0) | 4 (22.2) | 4 (26.7) | 4 (26.7) |
| ≥80 kg: n | 16 | 13 | 17 | 13 |
| Clinical remission, n (%) | 0 (0.0) | 2 (15.4) | 6 (35.3) | 5 (38.5) |
| Steroid-free clinical remission, n (%) | 0 (0.0) | 2 (15.4) | 6 (35.3) | 4 (30.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokemeyer, A.; Wenske, T.; Plachta-Danielzik, S.; Bokemeyer, B. Real-World Effectiveness of Golimumab in Ulcerative Colitis: A Pooled Analysis from the Prospective UMBRELLA-IBD Registry in Germany. J. Clin. Med. 2025, 14, 7347. https://doi.org/10.3390/jcm14207347
Bokemeyer A, Wenske T, Plachta-Danielzik S, Bokemeyer B. Real-World Effectiveness of Golimumab in Ulcerative Colitis: A Pooled Analysis from the Prospective UMBRELLA-IBD Registry in Germany. Journal of Clinical Medicine. 2025; 14(20):7347. https://doi.org/10.3390/jcm14207347
Chicago/Turabian StyleBokemeyer, Arne, Thomas Wenske, Sandra Plachta-Danielzik, and Bernd Bokemeyer. 2025. "Real-World Effectiveness of Golimumab in Ulcerative Colitis: A Pooled Analysis from the Prospective UMBRELLA-IBD Registry in Germany" Journal of Clinical Medicine 14, no. 20: 7347. https://doi.org/10.3390/jcm14207347
APA StyleBokemeyer, A., Wenske, T., Plachta-Danielzik, S., & Bokemeyer, B. (2025). Real-World Effectiveness of Golimumab in Ulcerative Colitis: A Pooled Analysis from the Prospective UMBRELLA-IBD Registry in Germany. Journal of Clinical Medicine, 14(20), 7347. https://doi.org/10.3390/jcm14207347







