Pulmonary Hypertension Drives Prognosis in Idiopathic Pulmonary Fibrosis: Insights from the European IPF Registry
Abstract
1. Introduction
2. Study Objectives
- 1.
- To perform cluster analysis on an IPF cohort using comorbidities, alongside variables such as lung function, age, sex, smoking status, antifibrotic use, and to evaluate the effectiveness of this analysis in predicting patient outcomes.
- 2.
- To identify high-risk variables through recursive partitioning (RP) analysis and evaluate their role in predicting survival and disease progression in IPF.
3. Materials and Methods
Statistics
4. Results
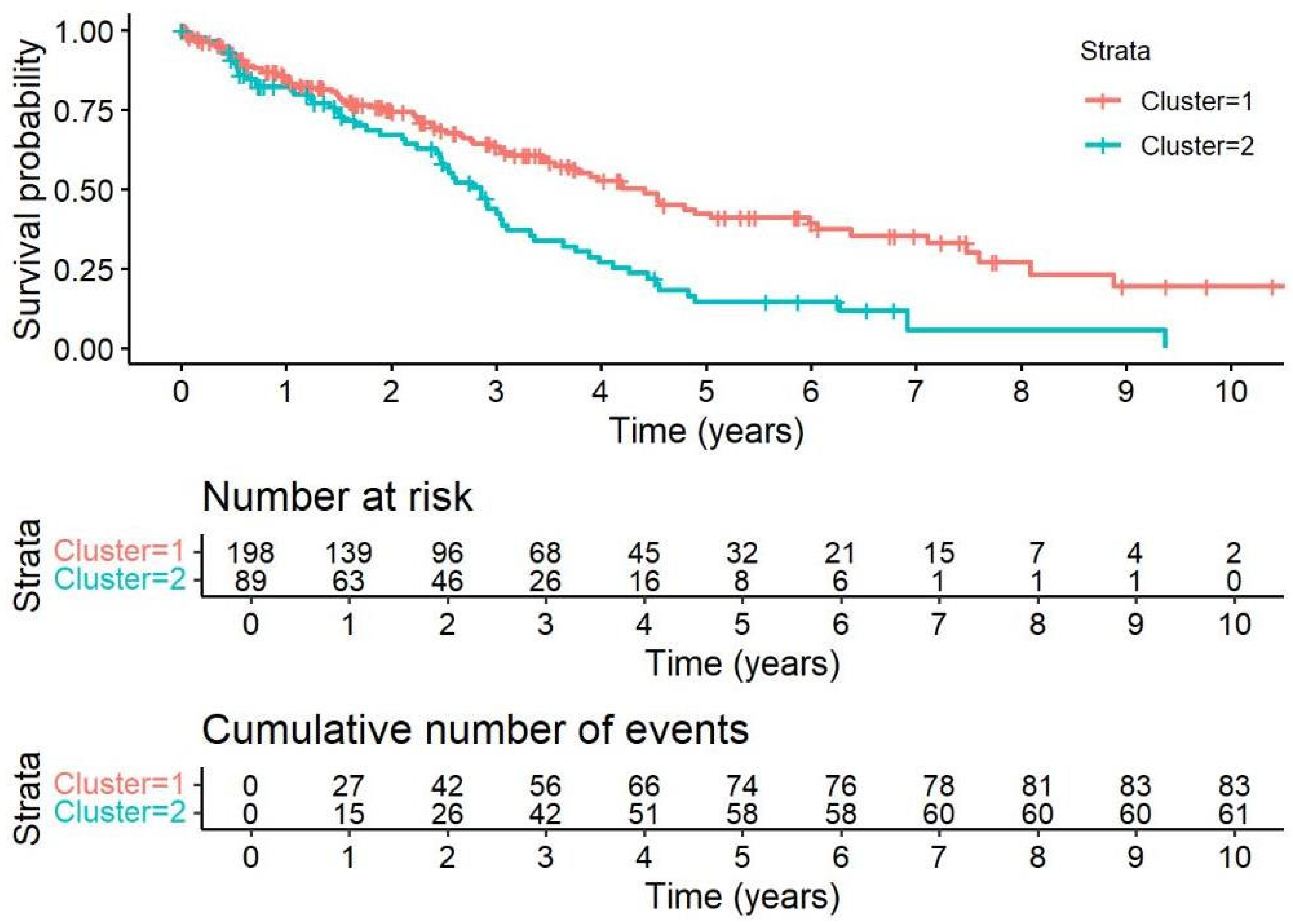
4.1. Cluster Analysis of Comorbidities
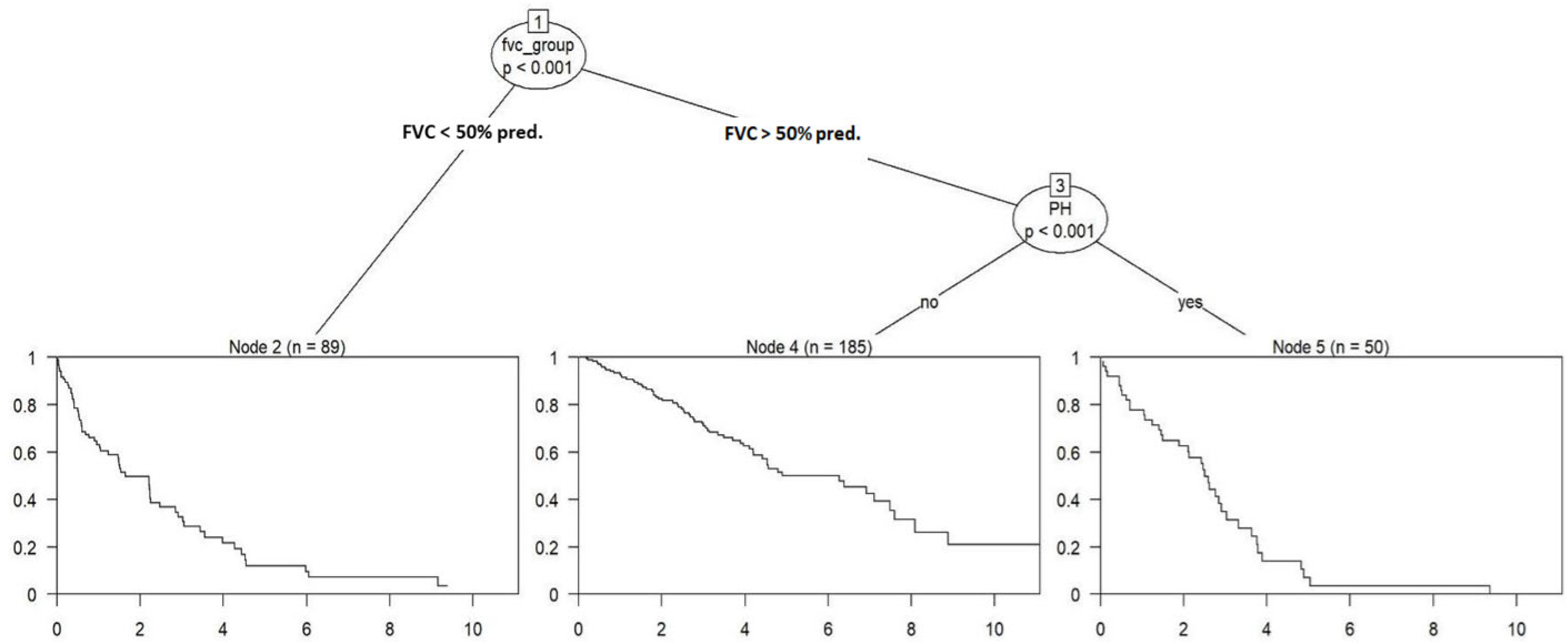
4.2. Recursive Partitioning-Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stewart, D.; Gibson-Smith, K.; MacLure, K.; Mair, A.; Alonso, A.; Codina, C.; Cittadini, A.; Fernandez-Llimos, F.; Fleming, G.; Gennimata, D.; et al. A modified Delphi study to determine the level of consensus across the European Union on the structures, processes and desired outcomes of the management of polypharmacy in older people. PLoS ONE 2017, 12, e0188348. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Koudstaal, T.; Wijsenbeek, M.S. Idiopathic Pulmonary Fibrosis. Presse Med. 2023, 52, 104166. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Rochwerg, B.; Zhang, Y.; Garcia, C.A.C.; Azuma, A.; Behr, J.; Brozek, J.L.; Collard, H.R.; Cunningham, W.; Homma, S.; et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2015, 192, e3–e19. [Google Scholar] [CrossRef] [PubMed]
- Millan-Billi, P.; Serra, C.; Alonso Leon, A.; Castillo, D. Comorbidities, Complications and Non-Pharmacologic Treatment in Idiopathic Pulmonary Fibrosis. Med. Sci. 2018, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kondoh, Y. The clinical impact of major comorbidities on idiopathic pulmonary fibrosis. Respir. Investig. 2017, 55, 94–103. [Google Scholar] [CrossRef]
- Raghu, G.; Amatto, V.C.; Behr, J.; Stowasser, S. Comorbidities in idiopathic pulmonary fibrosis patients: A systematic literature review. Eur. Respir. J. 2015, 46, 1113–1130. [Google Scholar] [CrossRef]
- van Cleemput, J.; Sonaglioni, A.; Wuyts, W.A.; Bengus, M.; Stauffer, J.L.; Harari, S. Idiopathic Pulmonary Fibrosis for Cardiologists: Differential Diagnosis, Cardiovascular Comorbidities, and Patient Management. Adv. Ther. 2019, 36, 298–317. [Google Scholar] [CrossRef]
- Mortimer, K.; Hartmann, N.; Chan, C.; Norman, H.; Wallace, L.; Enger, C. Characterizing idiopathic pulmonary fibrosis patients using US Medicare-advantage health plan claims data. BMC Pulm. Med. 2019, 19, 11. [Google Scholar] [CrossRef]
- Wang, D.; Ma, Y.; Tong, X.; Zhang, Y.; Fan, H. Diabetes Mellitus Contributes to Idiopathic Pulmonary Fibrosis: A Review from Clinical Appearance to Possible Pathogenesis. Front. Public Health 2020, 8, 196. [Google Scholar] [CrossRef]
- Raghu, G.; Freudenberger, T.D.; Yang, S.; Curtis, J.R.; Spada, C.; Hayes, J.; Sillery, J.K.; Pope, C.E.; Pellegrini, C.A. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur. Respir. J. 2006, 27, 136–142. [Google Scholar] [CrossRef]
- Lee, J.S.; Ryu, J.H.; Elicker, B.M.; Lydell, C.P.; Jones, K.D.; Wolters, P.J.; King, T.E.; Collard, H.R. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2011, 184, 1390–1394. [Google Scholar] [CrossRef]
- Kreuter, M.; Raghu, G. Gastro-oesophageal reflux and idiopathic pulmonary fibrosis: The heart burn in patients with IPF can no longer be silent. Eur. Respir. J. 2018, 51, 1800921. [Google Scholar] [CrossRef]
- Johannson, K.A.; Strâmbu, I.; Ravaglia, C.; Grutters, J.C.; Valenzuela, C.; Mogulkoc, N.; Luppi, F.; Richeldi, L.; Wells, A.U.; Vancheri, C.; et al. Antacid therapy in idiopathic pulmonary fibrosis: More questions than answers? Lancet Respir. Med. 2017, 5, 591–598. [Google Scholar] [CrossRef]
- de Boer, K.; Lee, J.S. Under-recognised co-morbidities in idiopathic pulmonary fibrosis: A review. Respirology 2016, 21, 995–1004. [Google Scholar] [CrossRef]
- Lancaster, L.H.; Mason, W.R.; Parnell, J.A.; Rice, T.W.; Loyd, J.E.; Milstone, A.P.; Collard, H.R.; Malow, B.A. Obstructive Sleep Apnea Is Common in Idiopathic Pulmonary Fibrosis. Chest 2009, 136, 772–778. [Google Scholar] [CrossRef]
- Holland, A.E.; Fiore, J.F.; Bell, E.C.; Goh, N.; Westall, G.; Symons, K.; Dowman, L.; Glaspole, I. Dyspnoea and comorbidity contribute to anxiety and depression in interstitial lung disease. Respirology 2014, 19, 1215–1221. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Nikkho, S.M.; Richter, M.J.; Shen, E.; Abman, S.H.; Antoniou, K.; Chung, J.; Fernandes, P.; Hassoun, P.; Lazarus, H.M.; Olschewski, H.; et al. Clinical significance of pulmonary hypertension in interstitial lung disease: A consensus statement from the Pulmonary Vascular Research Institute’s innovative drug development initiative-Group 3 pulmonary hypertension. Pulm. Circ. 2022, 12, e12127. [Google Scholar] [CrossRef]
- Nathan, S.D.; Barbera, J.A.; Gaine, S.P.; Harari, S.; Martinez, F.J.; Olschewski, H.; Olsson, K.M.; Peacock, A.J.; Pepke-Zaba, J.; Provencher, S.; et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur. Respir. J. 2019, 53. [Google Scholar] [CrossRef]
- Torrisi, S.E.; Ley, B.; Kreuter, M.; Wijsenbeek, M.; Vittinghoff, E.; Collard, H.R.; Vancheri, C. The added value of comorbidities in predicting survival in idiopathic pulmonary fibrosis: A multicenter observational study. Eur. Respir. J. 2018, 53, 1801587. [Google Scholar] [CrossRef]
- Nathan, S.D.; Waxman, A.; Rajagopal, S.; Case, A.; Johri, S.; DuBrock, H.; de La Zerda, D.J.; Sahay, S.; King, C.; Melendres-Groves, L.; et al. Inhaled treprostinil and forced vital capacity in patients with interstitial lung disease and associated pulmonary hypertension: A post-hoc analysis of the INCREASE study. Lancet Respir. Med. 2021, 9, 1266–1274. [Google Scholar] [CrossRef]
- Cottin, V.; Cordier, J.-F. Combined pulmonary fibrosis and emphysema: An experimental and clinically relevant phenotype. Am. J. Respir. Crit. Care Med. 2005, 172, 1605. [Google Scholar] [CrossRef]
- Hage, R.; Gautschi, F.; Steinack, C.; Schuurmans, M.M. Combined Pulmonary Fibrosis and Emphysema (CPFE) Clinical Features and Management. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 167–177. [Google Scholar] [CrossRef]
- Guenther, A.; Krauss, E.; Tello, S.; Wagner, J.; Paul, B.; Kuhn, S.; Maurer, O.; Heinemann, S.; Costabel, U.; Barbero, M.A.N.; et al. The European IPF registry (eurIPFreg): Baseline characteristics and survival of patients with idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 141. [Google Scholar] [CrossRef]
- Krauss, E.; Tello, S.; Naumann, J.; Wobisch, S.; Ruppert, C.; Kuhn, S.; Mahavadi, P.; Majeed, R.W.; Bonniaud, P.; Molina-Molina, M.; et al. Protocol and research program of the European registry and biobank for interstitial lung diseases (eurILDreg). BMC Pulm. Med. 2024, 24, 572. [Google Scholar] [CrossRef]
- Kimura, M.; Taniguchi, H.; Kondoh, Y.; Kimura, T.; Kataoka, K.; Nishiyama, O.; Aso, H.; Sakamoto, K.; Hasegawa, Y. Pulmonary hypertension as a prognostic indicator at the initial evaluation in idiopathic pulmonary fibrosis. Respiration 2013, 85, 456–463. [Google Scholar] [CrossRef]
- Lettieri, C.J.; Nathan, S.D.; Barnett, S.D.; Ahmad, S.; Shorr, A.F. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest 2006, 129, 746–752. [Google Scholar] [CrossRef]
- Kim, H.J.; Snyder, L.D.; Neely, M.L.; Hellkamp, A.S.; Hotchkin, D.L.; Morrison, L.D.; Bender, S.; Leonard, T.B.; Culver, D.A. Clinical Outcomes of Patients with Combined Idiopathic Pulmonary Fibrosis and Emphysema in the IPF-PRO Registry. Lung 2022, 200, 21–29. [Google Scholar] [CrossRef]
- Kreuter, M.; Ehlers-Tenenbaum, S.; Palmowski, K.; Bruhwyler, J.; Oltmanns, U.; Muley, T.; Heussel, C.P.; Warth, A.; Kolb, M.; Herth, F.J.F. Impact of Comorbidities on Mortality in Patients with Idiopathic Pulmonary Fibrosis. PLoS ONE 2016, 11, e0151425. [Google Scholar] [CrossRef]
- Hyldgaard, C.; Hilberg, O.; Bendstrup, E. How does comorbidity influence survival in idiopathic pulmonary fibrosis? Respir. Med. 2014, 108, 647–653. [Google Scholar] [CrossRef]
- Bordas-Martínez, J.; Gavaldà, R.; Shull, J.G.; Vicens-Zygmunt, V.; Planas-Cerezales, L.; Bermudo-Peloche, G.; Santos, S.; Salord, N.; Monasterio, C.; Molina-Molina, M.; et al. Idiopathic pulmonary fibrosis cluster analysis highlights diagnostic delay and cardiovascular comorbidity association with outcome. ERJ Open Res. 2021, 7, 00897-2020. [Google Scholar] [CrossRef]
- Kam, M.L.W.; Tiew, P.Y.; Chai, H.Z.; Low, S.Y. Cluster phenotypes in a non-idiopathic pulmonary fibrosis fibrotic interstitial lung diseases cohort in Singapore. J. Thorac. Dis. 2022, 14, 2481–2492. [Google Scholar] [CrossRef]
- Aoshima, Y.; Karayama, M.; Horiike, Y.; Mori, K.; Yasui, H.; Hozumi, H.; Suzuki, Y.; Furuhashi, K.; Fujisawa, T.; Enomoto, N.; et al. Cluster analysis-based clinical phenotypes of idiopathic interstitial pneumonias: Associations with acute exacerbation and overall survival. BMC Pulm. Med. 2021, 21, 63. [Google Scholar] [CrossRef]
- Adegunsoye, A.; Oldham, J.M.; Chung, J.H.; Montner, S.M.; Lee, C.; Witt, L.J.; Stahlbaum, D.; Bermea, R.S.; Chen, L.W.; Hsu, S.; et al. Phenotypic Clusters Predict Outcomes in a Longitudinal Interstitial Lung Disease Cohort. Chest 2018, 153, 349–360. [Google Scholar] [CrossRef]
- Prior, T.S.; Hoyer, N.; Hilberg, O.; Shaker, S.B.; Davidsen, J.R.; Rasmussen, F.; Bendstrup, E. Clusters of comorbidities in idiopathic pulmonary fibrosis. Respir. Med. 2021, 185, 106490. [Google Scholar] [CrossRef]
- Du Bois, R.M.; Weycker, D.; Albera, C.; Bradford, W.Z.; Costabel, U.; Kartashov, A.; King, T.E.; Lancaster, L.; Noble, P.W.; Sahn, S.A.; et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: Test properties and minimal clinically important difference. Am. J. Respir. Crit. Care Med. 2011, 184, 1382–1389. [Google Scholar] [CrossRef]
- Nathan, S.D.; Fernandes, P.; Psotka, M.; Vitulo, P.; Piccari, L.; Antoniou, K.; Nikkho, S.M.; Stockbridge, N. Pulmonary hypertension in interstitial lung disease: Clinical trial design and endpoints: A consensus statement from the Pulmonary Vascular Research Institute’s Innovative Drug Development Initiative-Group 3 Pulmonary Hypertension. Pulm. Circ. 2022, 12, e12178. [Google Scholar] [CrossRef]
- John, T.; Avian, A.; John, N.; Eger, A.; Foris, V.; Zeder, K.; Olschewski, H.; Richter, M.; Tello, K.; Kovacs, G.; et al. Prognostic Relevance of Tricuspid Annular Plane Systolic Excursion to Systolic Pulmonary Arterial Pressure Ratio and Its Association With Exercise Hemodynamics in Patients With Normal or Mildly Elevated Resting Pulmonary Arterial Pressure. Chest 2024, 167, 573–584. [Google Scholar] [CrossRef]
- Todor, S.B.; Ichim, C.; Boicean, A.; Mihaila, R.G. Cardiovascular Risk in Philadelphia-Negative Myeloproliferative Neoplasms: Mechanisms and Implications-A Narrative Review. Curr. Issues Mol. Biol. 2024, 46, 8407–8423. [Google Scholar] [CrossRef]
- Nathan, S.D.; Behr, J.; Cottin, V.; Lancaster, L.; Smith, P.; Deng, C.Q.; Pearce, N.; Bell, H.; Peterson, L.; Flaherty, K.R. Study design and rationale for the TETON phase 3, randomised, controlled clinical trials of inhaled treprostinil in the treatment of idiopathic pulmonary fibrosis. BMJ Open Respir. Res. 2022, 9, e001310. [Google Scholar] [CrossRef] [PubMed]



| Variables | The Data Presented as % of the Study Cohort |
|---|---|
| Age (groups) | (n = 324) |
| 18–59 | 20.37 |
| 60 + 75 | 58.95 |
| ≥ 76 | 20.68 |
| Men | 78.40 |
| Women | 21.60 |
| Antifibrotics (pirfenidone, nintedanib) | 80.56 |
| BMI (groups) | (n = 319) |
| ≤18.4 | 0.32 |
| 18.5–29.9 | 68.65 |
| ≥30 | 31.03 |
| Smoking status (groups) | (n = 298) |
| Active smoker | 3.02 |
| Ex-smoker | 72.48 |
| Never smoked | 24.50 |
| Pack years (mean value ± SD) | 27.4 ± 19.0 |
| Long-term oxygen therapy | 43.94 |
| 6MWD (groups) | (n = 192) |
| ≥350 m | 65.10 |
| <350 m | 34.90 |
| NYHA (groups) | (n = 254) |
| NYHA I | 19.69 |
| NYHA II–III | 75.59 |
| NYHA IV | 4.72 |
| Familial IPF | 19.77 |
| GAP stage (groups) | (n = 227) |
| I | 48.46 |
| II | 42.73 |
| III | 8.81 |
| Death | 44.14 |
| Lung transplant | 5.86 |
| Variable | Cluster 1 (n = 198) | Cluster 2 (n = 89) | p-Value |
|---|---|---|---|
| AH | 24.8% (49/198) | 87.6% (78/89) | <0.0011 |
| DM | 15.7% (31/198) | 38.2% (34/89) | <0.0011 |
| PH | 10.1% (20/198) | 61.8% (55/89) | <0.0011 |
| Dyslipidemia | 11.1% (22/198) | 30.3% (27/89) | <0.0011 |
| CVD | 18.2% (36/198) | 77.5% (69/89) | <0.0011 |
| Age (mean ± SD) | 66.35 ± 9.70 | 70.15 ± 8.72 | 0.0032 |
| Arrhythmia | 11.1% (22/198) | 21.4% (19/89) | 0.0221 |
| Sex_group (male) | 75.8% (150/198) | 86.5% (77/89) | 0.0381 |
| CPFE | 3.54% (7/198) | 7.87% (7/89) | 0.1151 |
| FVC_group | 0.1741 | ||
| 1: 27.8% (55/198) | 1: 27.0% (24/89) | |
| 2: 45.0% (89/198) | 2: 55.1% (49/89) | |
| 3: 27.3% (54/198) | 3: 18.0% (16/89) | |
| Familial_IPF | 18.2% (36/198) | 12.4% (11/89) | 0.2181 |
| GERD | 8.59% (17/198) | 5.62% (5/89) | 0.3821 |
| Lung_malignancy | 4.55% (9/198) | 6.74% (6/89) | 0.4391 |
| Smoking_group | 0.5061 | ||
| 1: 3.54% (7/198) | 1: 1.12% (1/89) | |
| 2: 71.21% (141/198) | 2: 74.16% (66/89) | |
| 3: 25.25% (50/198) | 3: 24.72% (22/89) | |
| Sleep_apnea | 13.1% (26/198) | 15.7% (14/89) | 0.5571 |
| Antifibrotics | 80.3% (159/198) | 83.2% (74/89) | 0.5691 |
| Depression_anxiety | 6.06% (12/198) | 7.87% (7/89) | 0.571 |
| BMI (mean ± SD) | 28.0 ± 4.29 | 27.6 ± 4.49 | 0.622 |
| Other_malignancies | 13.1% (26/198) | 11.2% (10/89) | 0.6541 |
| Thyroid_disease | 20.2% (40/198) | 18.0% (16/89) | 0.661 |
| Neurological_ischemic | 7.07% (14/198) | 7.87% (7/89) | 0.8111 |
| PE | 5.56% (11/198) | 5.62% (5/89) | 0.9831 |
| Variable | HR (Univariate) | HR (Multivariate) |
|---|---|---|
| PH | 2.80 (2.04–3.86, p < 0.001) | 2.03 (1.42–2.90, p < 0.001) |
| FVC Group | ||
| <50% pred. | - | - |
| 50–75% pred. | 0.44 (0.31–0.62, p < 0.001) | 0.51 (0.35–0.73, p < 0.001) |
| >75% pred. | 0.19 (0.12–0.32, p < 0.001) | 0.23 (0.14–0.40, p < 0.001) |
| Age | 1.02 (1.00–1.03, p = 0.030) | 1.01 (0.99–1.03, p = 0.235) |
| BMI | 0.96 (0.93–1.00, p = 0.045) | 0.98 (0.94–1.02, p = 0.244) |
| AH | 1.36 (1.00–1.85, p = 0.053) | 1.21 (0.84–1.75, p = 0.305) |
| CVD | 1.35 (0.99–1.85, p = 0.062) | 0.89 (0.61–1.31, p = 0.565) |
| Sex (male) | 0.67 (0.45–1.01, p = 0.053) | 0.66 (0.42–1.03, p = 0.069) |
| PE | 1.32 (0.71–2.43, p = 0.380) | - |
| DM | 1.20 (0.85–1.70, p = 0.302) | - |
| GERD | 0.92 (0.51–1.65, p = 0.773) | - |
| CPFE | 1.57 (0.91–2.72, p = 0.108) | - |
| Sleep apnea | 1.08 (0.72–1.63, p = 0.704) | - |
| Arrhythmia | 1.27 (0.85–1.88, p = 0.245) | - |
| Lung malignancy | 1.09 (0.51–2.34, p = 0.820) | - |
| Other malignancies | 0.82 (0.51–1.32, p = 0.422) | - |
| Depression or Anxiety | 0.67 (0.35–1.29, p = 0.226) | - |
| Neurological ischemic disorders | 1.27 (0.74–2.17, p = 0.379) | - |
| Thyroid disease | 1.07 (0.73–1.57, p = 0.722) | - |
| Dyslipidemia | 1.04 (0.70–1.54, p = 0.849) | - |
Smoking group
| - | - |
| 1.88 (0.69–5.11, p = 0.215) | - |
| 2.07 (0.74–5.82, p = 0.167) | - |
| Familial IPF | 0.91 (0.57–1.46, p = 0.708) | - |
| Antifibrotic treatment | 0.68 (0.44–1.06, p = 0.090) | - |
| Node | Definition | n (%) |
|---|---|---|
| 2 | Patients with FVC < 50% predicted | 89 (27.5) |
| 4 | Patients with FVC ≥ 50% predicted and no PH | 185 (57.1) |
| 5 | Patients with FVC ≥ 50% predicted and PH present | 50 (15.4) |
| Node | n (%) | HR (Univariable) | HR (Multivariable) |
|---|---|---|---|
| 2 | 89 (27.5) | - | - |
| 4 | 185 (57.1) | 0.29 (0.20–0.41, p < 0.001) | 0.29 (0.20–0.41, p < 0.001) |
| 5 | 50 (15.4) | 0.88 (0.59–1.33, p = 0.557) | 0.88 (0.59–1.33, p = 0.557) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guenther, A.; Tello, S.; Schoppe, M.C.; Pons-Kuehnemann, J.; Seeger, W.; Stiben, J.; Tello, K.; Molina Molina, M.; Vancheri, C.; Crestani, B.; et al. Pulmonary Hypertension Drives Prognosis in Idiopathic Pulmonary Fibrosis: Insights from the European IPF Registry. J. Clin. Med. 2025, 14, 7352. https://doi.org/10.3390/jcm14207352
Guenther A, Tello S, Schoppe MC, Pons-Kuehnemann J, Seeger W, Stiben J, Tello K, Molina Molina M, Vancheri C, Crestani B, et al. Pulmonary Hypertension Drives Prognosis in Idiopathic Pulmonary Fibrosis: Insights from the European IPF Registry. Journal of Clinical Medicine. 2025; 14(20):7352. https://doi.org/10.3390/jcm14207352
Chicago/Turabian StyleGuenther, Andreas, Silke Tello, Marc Carre Schoppe, Joern Pons-Kuehnemann, Werner Seeger, Johannes Stiben, Khodr Tello, Maria Molina Molina, Carlo Vancheri, Bruno Crestani, and et al. 2025. "Pulmonary Hypertension Drives Prognosis in Idiopathic Pulmonary Fibrosis: Insights from the European IPF Registry" Journal of Clinical Medicine 14, no. 20: 7352. https://doi.org/10.3390/jcm14207352
APA StyleGuenther, A., Tello, S., Schoppe, M. C., Pons-Kuehnemann, J., Seeger, W., Stiben, J., Tello, K., Molina Molina, M., Vancheri, C., Crestani, B., & Krauss, E. (2025). Pulmonary Hypertension Drives Prognosis in Idiopathic Pulmonary Fibrosis: Insights from the European IPF Registry. Journal of Clinical Medicine, 14(20), 7352. https://doi.org/10.3390/jcm14207352





