Body Mass Index and Spinopelvic Alignment as Predictors of Incident Knee Osteoarthritis: An 8-Year Longitudinal Study from the TOEI Cohort of Older Japanese Women
Abstract
1. Introduction
2. Materials and Methods
- Physical Function Evaluation
- Questionnaires
- Radiographic Evaluation
- Sagittal vertical axis (SVA): The horizontal distance between the vertical plumb line dropped from the center of the C7 vertebral body and the posterosuperior corner of the sacrum.
- Thoracic kyphosis (TK): The angle between the superior endplate of T1 and the inferior endplate of T12.
- Lumbar lordosis (LL): The angle between the superior endplate of L1 and the superior endplate of S1.
- Sacral slope (SS): The angle between the superior endplate of S1 and a horizontal reference line.
- Pelvic tilt (PT): The angle between the line connecting the midpoint of the S1 superior endplate to the femoral head center and a vertical reference line
- Pelvic incidence (PI): The angle between a line perpendicular to the S1 superior endplate at its midpoint and the line connecting this midpoint to the femoral head center. By definition, PI = SS + PT.
- PI–LL mismatch: Difference between PI and LL (i.e., PI minus LL).
- Femoral inclination (FI): The angle between the femoral axis and the vertical reference line. Mean values from both sides were used.
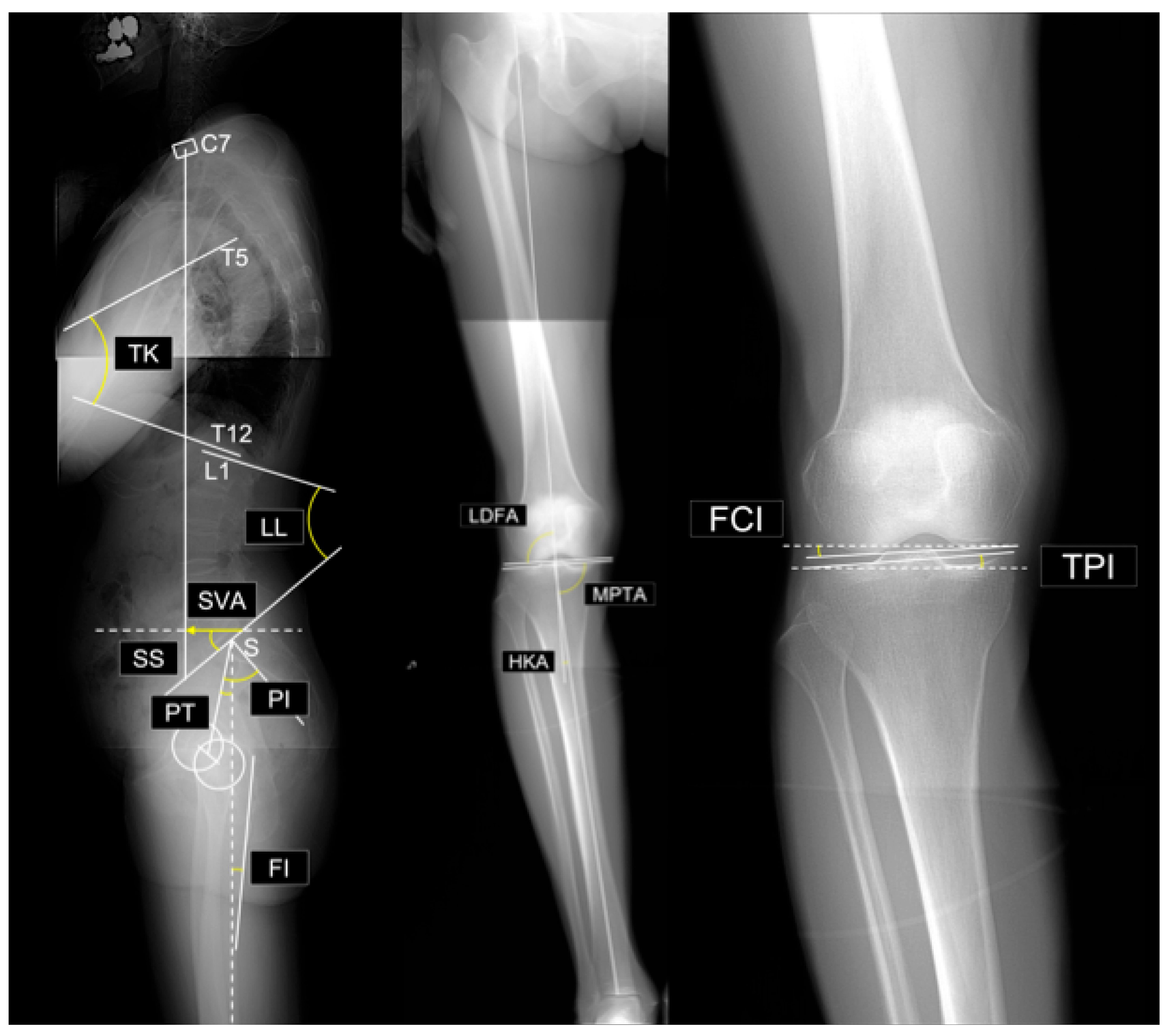
- Lateral distal femoral angle (LDFA): The lateral angle between the femoral mechanical axis and distal joint line.
- Medial proximal tibial angle (MPTA): The medial angle between the tibial mechanical axis and proximal joint line.
- Arithmetic hip–knee–ankle angle (aHKA): Defined as MPTA minus LDFA, representing overall lower-limb alignment. Negative values indicate varus alignment.
- Joint line obliquity (JLO): Calculated as the sum of the MPTA and LDFA, reflecting the inclination of the joint line relative to the floor in a bipedal standing position. Values less than 180° indicate the apex distal configuration.
- Hip-knee-ankle angle (HKA): The angle between the mechanical axes of the femur and tibia. A value of 0° represents neutral alignment, and positive values indicate varus alignment.
- Joint line convergence angle (JLCA): The angle formed by the intersection of the distal femoral and proximal tibial joint lines. Positive values indicate a lateral opening.
- Femoral condyle inclination (FCI): The inclination of the femoral joint surface relative to the horizontal plane. Negative values indicate lateral distal inclination.
- Tibial plateau inclination (TPI): inclination of the tibial joint surface relative to the horizontal plane. Negative values indicate medial-distal inclination.
- Group classification and analysis
- Statistical Analysis
- Internal validation and performance
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| FRT | Functional reach test |
| GLFS | Geriatric Locomotive Function Scale |
| EQ-5D | EuroQol 5-dimension |
| SVA | Sagittal vertical axis |
| TK | Thoracic kyphosis |
| LL | Lumbar lordosis |
| SS | Sacral slope |
| PT | Pelvic tilt |
| PI | Pelvic incidence |
| PI–LL | Pelvic incidence minus lumbar lordosis |
| FI | Femoral inclination |
| LDFA | Lateral distal femoral angle |
| MPTA | Medial proximal tibial angle |
| aHKA | Arithmetic hip–knee–ankle angle |
| JLO | Joint line obliquity |
| HKA | Hip–knee–ankle angle |
| JLCA | Joint line convergence angle |
| FCI | Femoral condyle inclination |
| TPI | Tibial plateau inclination |
| Β | Regression coefficient |
| CI | Confidence interval |
| OR | Odds ratio |
| AUC | Area under the curve |
| VIF | Variance inflation factor |
| KOA | Knee osteoarthritis |
| ROC | Receiver operating characteristics |
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Li, E.; Tan, J.; Xu, K.; Pan, Y.; Xu, P. Global burden and socioeconomic impact of knee osteoarthritis: A comprehensive analysis. Front. Med. 2024, 11, 1323091. [Google Scholar] [CrossRef]
- Summanen, M.; Ukkola-Vuoti, L.; Kurki, S.; Tuominen, S.; Madanat, R. The burden of hip and knee osteoarthritis in Finnish occupational healthcare. BMC Musculoskelet. Disord. 2021, 22, 501. [Google Scholar] [CrossRef]
- GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Wallace, I.J.; Worthington, S.; Felson, D.T.; Jurmain, R.D.; Wren, K.T.; Maijanen, H.; Woods, R.J.; Lieberman, D.E. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl. Acad. Sci. USA 2017, 114, 9332–9336. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, Y.; Zhou, J.; Zhou, Q.; Wei, H. Evidence on risk factors for knee osteoarthritis in middle-older aged: A systematic review and meta analysis. J. Orthop. Surg. Res. 2023, 18, 634. [Google Scholar] [CrossRef]
- Duong, V.; Shaheed, C.A.; Ferreira, M.L.; Narayan, S.W.; Venkatesha, V.; Hunter, D.J.; Zhu, J.; Atukorala, I.; Kobayashi, S.; Goh, S.L.; et al. Risk factors for the development of knee osteoarthritis across the lifespan: A systematic review and meta-analysis. Osteoarthr. Cart. 2025, 31, 1162–1179. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Hasegawa, M.; Kato, K.; Yamada, T.; Uchida, A.; Sudo, A. Risk factors for the incidence and progression of radiographic knee osteoarthritis of the knee among Japanese. Int. Orthop. 2011, 35, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Hou, H.; Han, Q.; Cai, K. Prevalence and risk factors of knee osteoarthritis: A cross-sectional survey in Nanjing, China. Front. Public Health 2024, 12, 1441408. [Google Scholar] [CrossRef]
- Yoshimura, N.; Muraki, S.; Oka, H.; Tanaka, S.; Kawaguchi, H.; Nakamura, K.; Akune, T. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: A 3-year follow-up of the ROAD study. Osteoarthr. Cart. 2012, 20, 1217–1226. [Google Scholar] [CrossRef]
- Amarasinghe, P.; Wadugodapitiya, S.; Weerasekara, I. Biomechanical and clinical relationships between lower back pain and knee osteoarthritis: A systematic review. Syst. Rev. 2023, 12, 28. [Google Scholar] [CrossRef]
- Obeid, I.; Hauger, O.; Aunoble, S.; Bourghli, A.; Pellet, N.; Vital, J.M. Global analysis of sagittal spinal alignment in major deformities: Correlation between lack of lumbar lordosis and flexion of the knee. Eur. Spine J. 2011, 20 (Suppl. 5), 681–685. [Google Scholar] [CrossRef]
- Aksoy, O.T.; Bütün, B. Relationship of spinopelvic alignment on knee osteoarthritis in female patients: A cross-sectional study. J. Back Musculoskelet. Rehabil. 2025, 38, 10538127251321767. [Google Scholar] [CrossRef]
- Fu, P.; Xu, W.; Xu, P.; Huang, J.; Guo, J.J. Relationship between spinal imbalance and knee osteoarthritis by using full-body EOS. BMC Musculoskelet. Disord. 2023, 24, 402. [Google Scholar] [CrossRef]
- Shimizu, M.; Kobayashi, T.; Chiba, H.; Senoo, I.; Abe, S.; Matsukura, K.; Ito, H. Examination of the changes in lower extremities related to progression of adult spinal deformity: A longitudinal study of over 22 years. Sci. Rep. 2020, 10, 11605. [Google Scholar] [CrossRef]
- Wang, J.; Ushirozako, H.; Yamato, Y.; Hasegawa, T.; Yoshida, G.; Banno, T.; Arima, H.; Oe, S.; Yamada, T.; Ide, K.; et al. Why does knee flexion in the standing position occur? Spinal deformity or knee osteoarthritis. J. Orthop. Surg. 2023, 31, 10225536231169575. [Google Scholar] [CrossRef]
- Higano, Y.; Hayami, T.; Omori, G.; Koga, Y.; Endo, K.; Endo, N. The varus alignment and morphologic alterations of the proximal tibia affect the onset of medial knee osteoarthritis in rural Japanese women: A case–control study from the longitudinal evaluation of Matsudai Knee Osteoarthritis Survey. J. Orthop. Sci. 2016, 21, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Hanada, M.; Hoshino, H.; Koyama, H.; Matsuyama, Y. Relationship between severity of knee osteoarthritis and radiography findings of lower limbs: A cross-sectional study from the TOEI survey. J. Orthop. 2017, 14, 484–488. [Google Scholar] [CrossRef]
- Nomoto, K.; Hanada, M.; Hotta, K.; Matsuyama, Y. Distribution of coronal plane alignment of the knee classification does not change as knee osteoarthritis progresses: A longitudinal study from the TOEI study. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 5507–5513. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Togawa, D.; Hasegawa, T.; Yamato, Y.; Kobayashi, S.; Yoshida, G.; Banno, T.; Arima, H.; Oe, S.; Hoshino, H.; et al. Relationship between knee osteoarthritis and spinopelvic sagittal alignment in volunteers over 50 years of age. Asian Spine J. 2020, 14, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Ide, K.; Yamato, Y.; Hasegawa, T.; Yoshida, G.; Yasuda, T.; Banno, T.; Arima, H.; Oe, S.; Mihara, Y.; Ushirozako, H.; et al. Prospective nursing care certification using the 25-question Geriatric Locomotive Function Scale. Geriatr. Gerontol. Int. 2021, 21, 492–497. [Google Scholar] [CrossRef]
- Seichi, A.; Hoshino, Y.; Doi, T.; Akai, M.; Tobimatsu, Y.; Iwaya, T. Development of a screening tool for risk of locomotive syndrome in the elderly: The 25-question Geriatric Locomotive Function Scale. J. Orthop. Sci. 2012, 17, 163–172. [Google Scholar] [CrossRef]
- EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Sasaki, E.; Ota, S.; Chiba, D.; Kimura, Y.; Sasaki, S.; Yamamoto, Y.; Tsuda, E.; Nakaji, S.; Ishibashi, Y. Early knee osteoarthritis prevalence is highest among middle-aged adult females with obesity based on new set of diagnostic criteria from a large sample cohort study in the Japanese general population. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 984–994. [Google Scholar] [CrossRef]
- Briggs, A.M.; Cross, M.J.; Hoy, D.G.; Sànchez-Riera, L.; Blyth, F.M.; Woolf, A.D.; March, L. Musculoskeletal health conditions represent a global threat to healthy aging: A report for the 2015 world health organization world report on ageing and health. Gerontologist 2016, 56 (Suppl. 2), S243–S255. [Google Scholar] [CrossRef]
- Messier, S.P.; Mihalko, S.L.; Legault, C.; Miller, G.D.; Nicklas, B.J.; DeVita, P.; Beavers, D.P.; Hunter, D.J.; Lyles, M.F.; Eckstein, F.; et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: The IDEA randomized clinical trial. JAMA 2013, 310, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Törnblom, M.; Bremander, A.; Aili, K.; Andersson, M.L.E.; Nilsdotter, A.; Haglund, E. Development of radiographic knee osteoarthritis and the associations to radiographic changes and baseline variables in individuals with knee pain: A 2-year longitudinal study. BMJ Open 2024, 14, e081999. [Google Scholar] [CrossRef]
- Anuurad, E.; Shiwaku, K.; Nogi, A.; Kitajima, K.; Enkhmaa, B.; Shimono, K.; Yamane, Y. The new BMI criteria for Asians by the regional office for the western pacific region of WHO are suitable for screening of overweight to prevent metabolic syndrome in elder Japanese workers. J. Occup. Health 2003, 45, 335–343. [Google Scholar] [CrossRef]
- Muraki, S.; Oka, H.; Akune, T.; En-Yo, Y.; Yoshida, M.; Nakamura, K.; Kawaguchi, H.; Yoshimura, N. Association of occupational activity with joint space narrowing and osteophytosis in the medial compartment of the knee: The ROAD study (OAC5914R2). Osteoarthr. Cart. 2011, 19, 840–846. [Google Scholar] [CrossRef]
- Wanezaki, Y.; Suzuki, A.; Takakubo, Y.; Nakajima, T.; Toyono, S.; Toyoshima, S.; Fukushima, S.; Yamamoto, T.; Ito, T.; Takagi, M. Lower limb alignment in healthy Japanese adults. J. Orthop. Sci. 2023, 28, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Jalai, C.M.; Diebo, B.G.; Cruz, D.L.; Poorman, G.W.; Vira, S.; Buckland, A.J.; Lafage, R.; Bess, S.; Errico, T.J.; Lafage, V.; et al. The impact of obesity on compensatory mechanisms in response to progressive sagittal malalignment. Spine J. 2017, 17, 681–688. [Google Scholar] [CrossRef]
- Ito, K.; Ando, K.; Kobayashi, K.; Nakashima, H.; Hasegawa, Y.; Imagama, S. A longitudinal study of lumbar sagittal change in middle-aged healthy volunteers. Spine Surg. Relat. Res. 2021, 5, 160–164. [Google Scholar] [CrossRef]
- Ide, K.; Yamato, Y.; Hasegawa, T.; Yoshida, G.; Banno, T.; Arima, H.; Oe, S.; Mihara, Y.; Ushirozako, H.; Yamada, T.; et al. Sex differences between the relationship of trunk muscle mass and whole body sagittal plane alignment in older adults. J. Orthop. Sci. 2023, 28, 315–320. [Google Scholar] [CrossRef]
- Marcos-Pardo, P.J.; Abelleira-Lamela, T.; Vaquero-Cristóbal, R.; Espeso-García, A.; Esparza-Ros, F.; González-Gálvez, N. Improving spinal alignment through innovative resistance training with outdoor fitness equipment in middle-aged and older adults: A randomized controlled trial. Sci. Rep. 2025, 15, 14499. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.S.; Jones, L.D.; Monk, A.P.; Nevitt, M.; Lynch, J.; Beard, D.J.; Javaid, M.K.; Price, A.J. Varus alignment of the proximal tibia is associated with structural progression in early to moderate varus osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 3279–3286. [Google Scholar] [CrossRef] [PubMed]

| Variable | Overall | Non-Incident KOA | Incident KOA | p-Value |
|---|---|---|---|---|
| Age | 69.3 ± 6.1 | 69.1 ± 6.0 | 69.5 ± 6.3 | 0.691 |
| Height | 150.1 ± 5.8 | 150.2 ± 5.9 | 149.6 ± 5.6 | 0.521 |
| Body weight | 49.4 ± 7.4 | 47.6 ± 5.9 | 53.4 ± 8.8 | <0.001 |
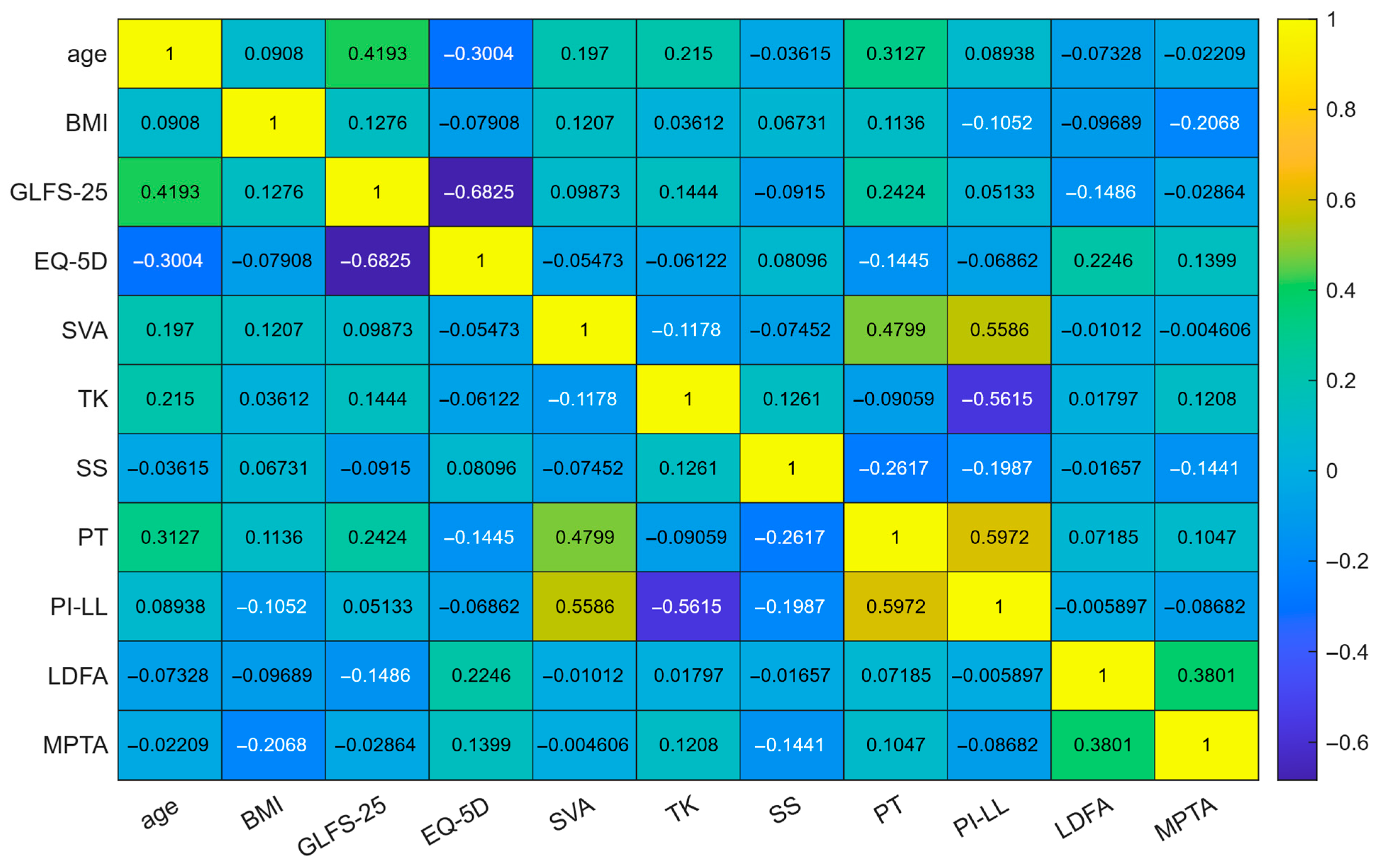
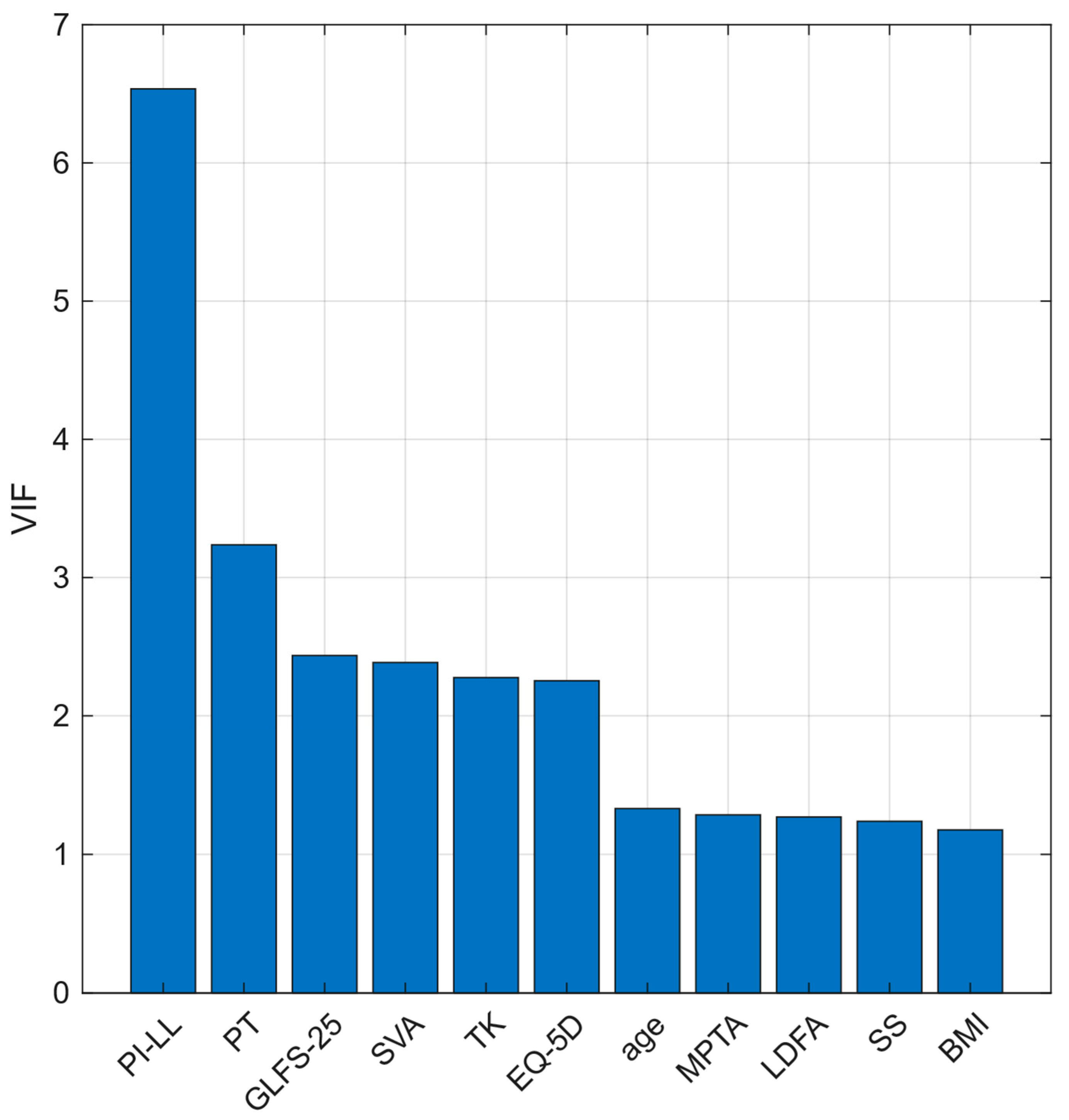
| BMI | 21.9 ± 3.0 | 21.1 ± 2.5 | 23.8 ± 3.1 | <0.001 |
| One-leg standing time | 46.0 ± 19.9 | 47.2 ± 19.3 | 43.3 ± 20.8 | 0.226 |
| FRT | 36.5 ± 6.5 | 36.4 ± 6.7 | 36.8 ± 5.9 | 0.694 |
| GLFS-25 | 7.6 ± 9.6 | 6.2 ± 6.9 | 10.7 ± 13.6 | 0.022 |
| EQ-5D | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.2 | 0.02 |
| SVA | 31.6 ± 39.6 | 29.4 ± 37.9 | 36.7 ± 42.6 | 0.267 |
| TK | 34.8 ± 14.1 | 36.0 ± 13.9 | 31.9 ± 14.2 | 0.073 |
| LL | 42.5 ± 15.8 | 44.0 ± 14.5 | 39.0 ± 17.9 | 0.064 |
| SS | 30.6 ± 9.2 | 31.1 ± 8.2 | 29.3 ± 11.0 | 0.254 |
| PT | 18.8 ± 8.2 | 17.9 ± 8.1 | 20.8 ± 8.0 | 0.022 |
| PI | 49.7 ± 9.9 | 49.3 ± 10.0 | 50.5 ± 9.6 | 0.462 |
| PI–LL | 7.2 ± 16.0 | 5.3 ± 14.5 | 11.5 ± 18.3 | 0.026 |
| FI | 3.3 ± 4.4 | 2.9 ± 4.2 | 4.1 ± 4.6 | 0.08 |
| LDFA | 89.1 ± 2.6 | 89.3 ± 2.7 | 88.7 ± 2.3 | 0.131 |
| MPTA | 85.6 ± 2.1 | 85.8 ± 2.1 | 85.1 ± 2.1 | 0.032 |
| aHKA | −3.5 ± 2.6 | −3.5 ± 2.5 | −3.6 ± 3.0 | 0.763 |
| JLO | 174.8 ± 3.9 | 175.1 ± 4.1 | 173.8 ± 3.3 | 0.022 |
| HKA | 2.6 ± 2.6 | 2.3 ± 2.2 | 3.1 ± 3.2 | 0.097 |
| JLCA | −1.0 ± 1.8 | −1.2 ± 1.8 | −0.7 ± 1.8 | 0.083 |
| FCI | −2.1 ± 2.8 | −2.2 ± 3.0 | −1.8 ± 2.5 | 0.311 |
| TPI | 1.1 ± 2.6 | 1.1 ± 2.7 | 1.1 ± 2.3 | 0.887 |
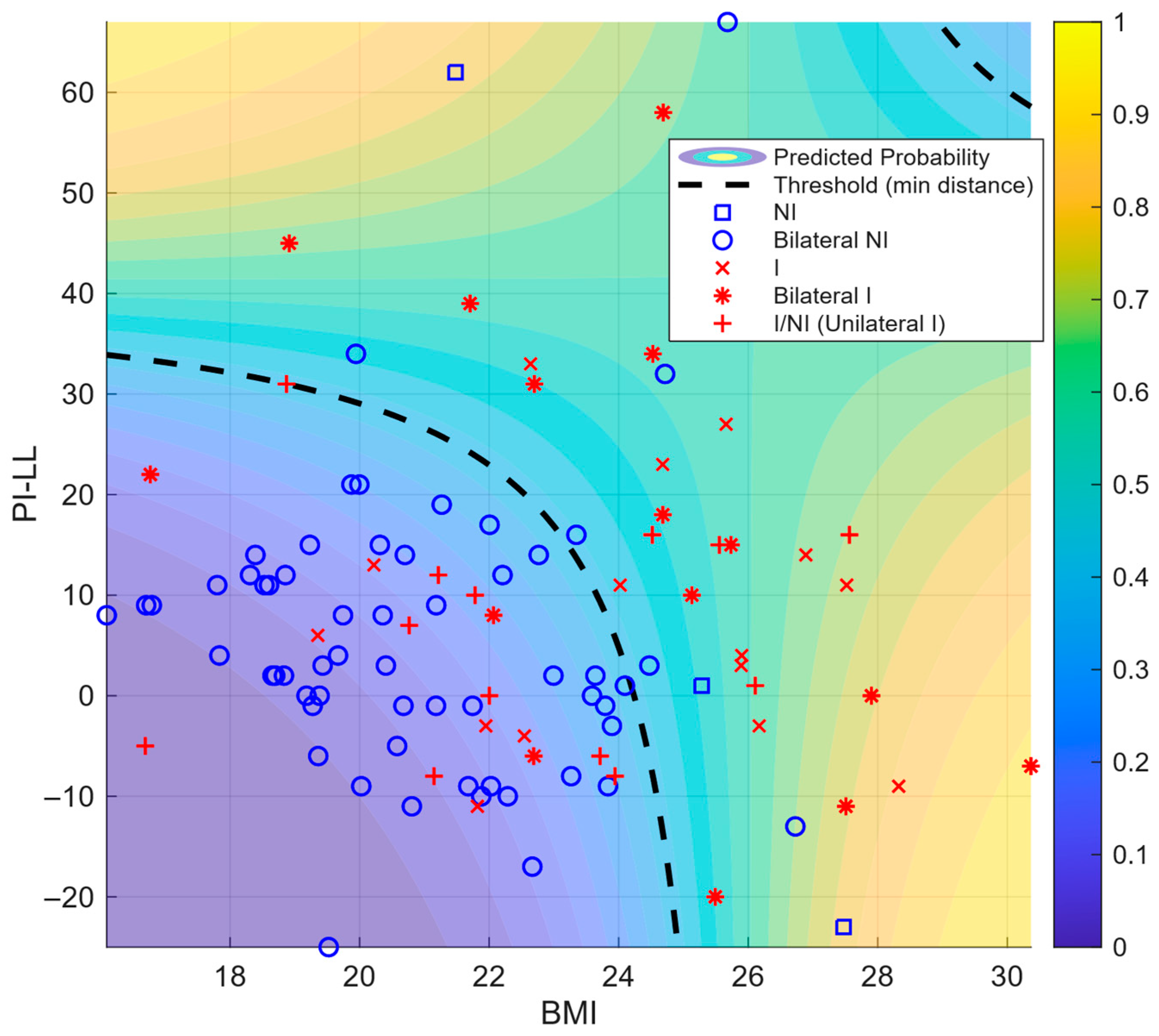
| Variable | β | 95% CI (β) Lower | 95% CI (β) Upper | OR | 95% CI (OR) Lower | 95% CI (OR) Upper | p-Value |
|---|---|---|---|---|---|---|---|
| (Intercept) | −11.445 | −15.293 | −7.597 | 0.00001 | 2.283 | 0.0005 | <0.001 |
| BMI | 0.455 | 0.291 | 0.619 | 1.577 | 1.338 | 1.858 | <0.001 |
| PI–LL | 0.286 | 0.087 | 0.486 | 1.332 | 1.091 | 1.625 | 0.005 |
| BMI × PI–LL | −0.011 | −0.019 | −0.003 | 0.989 | 0.981 | 0.997 | 0.010 |
| Logistic Model (Variables) | AUC | Cut-Off Probability | Cut-Point Value | TPR | FPR |
|---|---|---|---|---|---|
| β0 + β1·BMI | 0.7529 | 0.2791 | 22.0667 | 0.7069 | 0.3233 |
| β0 + β1·PI–LL | 0.5956 | 0.3126 | 10.0000 | 0.5345 | 0.3383 |
| β0 + β1·BMI + β2·PI–LL + β3·BMI·PI–LL | 0.8027 | 0.3997 | N/A | 0.6724 | 0.1504 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, Y.; Hanada, M.; Nomoto, K.; Hotta, K.; Yamagishi, Y.; Matsuyama, Y. Body Mass Index and Spinopelvic Alignment as Predictors of Incident Knee Osteoarthritis: An 8-Year Longitudinal Study from the TOEI Cohort of Older Japanese Women. J. Clin. Med. 2025, 14, 7343. https://doi.org/10.3390/jcm14207343
Murakami Y, Hanada M, Nomoto K, Hotta K, Yamagishi Y, Matsuyama Y. Body Mass Index and Spinopelvic Alignment as Predictors of Incident Knee Osteoarthritis: An 8-Year Longitudinal Study from the TOEI Cohort of Older Japanese Women. Journal of Clinical Medicine. 2025; 14(20):7343. https://doi.org/10.3390/jcm14207343
Chicago/Turabian StyleMurakami, Yuki, Mitsuru Hanada, Kazuki Nomoto, Kensuke Hotta, Yuki Yamagishi, and Yukihiro Matsuyama. 2025. "Body Mass Index and Spinopelvic Alignment as Predictors of Incident Knee Osteoarthritis: An 8-Year Longitudinal Study from the TOEI Cohort of Older Japanese Women" Journal of Clinical Medicine 14, no. 20: 7343. https://doi.org/10.3390/jcm14207343
APA StyleMurakami, Y., Hanada, M., Nomoto, K., Hotta, K., Yamagishi, Y., & Matsuyama, Y. (2025). Body Mass Index and Spinopelvic Alignment as Predictors of Incident Knee Osteoarthritis: An 8-Year Longitudinal Study from the TOEI Cohort of Older Japanese Women. Journal of Clinical Medicine, 14(20), 7343. https://doi.org/10.3390/jcm14207343





