Immunohistochemical Comparison of Dopamine-2 Receptor Expression in Resistant and Non-Resistant Prolactinomas
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Study Design—Patient and Tumor Characteristics
2.1.2. Study Design—IHC
2.2. Statistical Analysis
3. Results
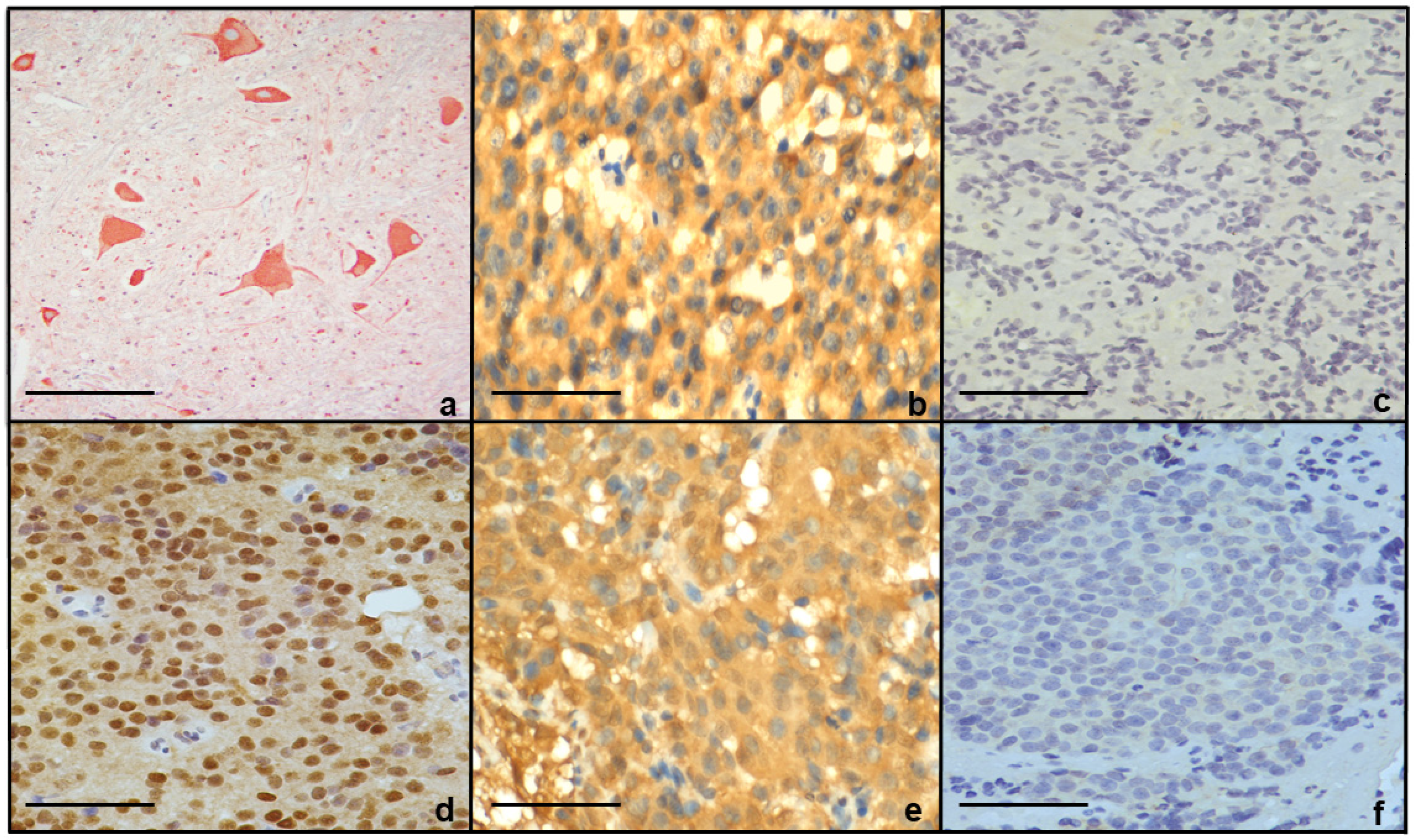
Immunohistochemical Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Auriemma, R.S.; Pirchio, R.; De Alcubierre, D.; Pivonello, R.; Colao, A. Dopamine Agonists: From the 1970s to Today. Neuroendocrinology 2019, 109, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.; Shaikh, S.; Tamagno, G. Prolactinoma. In Pituitary Adenomas; Springer Nature: Cham, Switzerland, 2022; pp. 173–193. [Google Scholar]
- Gillam, M.P.; Molitch, M.E.; Lombardi, G.; Colao, A. Advances in the Treatment of Prolactinomas. Endocr. Rev. 2006, 27, 485–534. [Google Scholar] [CrossRef]
- Melmed, S.; Casanueva, F.F.; Hoffman, A.R.; Kleinberg, D.L.; Montori, V.M.; Schlechte, J.A.; Wass, J.A.H. Diagnosis and Treatment of Hyperprolactinemia: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Petersenn, S.; Fleseriu, M.; Casanueva, F.F.; Giustina, A.; Biermasz, N.; Biller, B.M.K.; Bronstein, M.; Chanson, P.; Fukuoka, H.; Gadelha, M.; et al. Diagnosis and management of prolactin-secreting pituitary adenomas: A Pituitary Society international Consensus Statement. Nat. Rev. Endocrinol. 2023, 19, 722–740. [Google Scholar] [CrossRef]
- Shimon, I. Prolactinomas Resistant to Dopamine Agonists: Pathophysiology and Treatment. Arch. Med. Res. 2023, 54, 102883. [Google Scholar] [CrossRef] [PubMed]
- Zanettini, R.; Antonini, A.; Gatto, G.; Gentile, R.; Tesei, S.; Pezzoli, G. Valvular Heart Disease and the Use of Dopamine Agonists for Parkinson’s Disease. N. Engl. J. Med. 2007, 356, 39–46. [Google Scholar] [CrossRef]
- Wildemberg, L.E.; Fialho, C.; Gadelha, M.R. Prolactinomas. La Presse Médicale 2021, 50, 104080. [Google Scholar] [CrossRef]
- Schade, R.; Andersohn, F.; Suissa, S.; Haverkamp, W.; Garbe, E. Dopamine Agonists and the Risk of Cardiac-Valve Regurgitation. N. Engl. J. Med. 2007, 356, 29–38. [Google Scholar] [CrossRef]
- Ilie, M.-D.; Tabarin, A.; Vasiljevic, A.; Bonneville, J.-F.; Moreau-Grangé, L.; Schillo, F.; Delemer, B.; Barlier, A.; Figarella-Branger, D.; Bisot-Locard, S.; et al. Predictive Factors of Somatostatin Receptor Ligand Response in Acromegaly—A Prospective Study. J. Clin. Endocrinol. Metab. 2022, 107, 2982–2991. [Google Scholar] [CrossRef]
- Pivonello, C.; Patalano, R.; Negri, M.; Pirchio, R.; Colao, A.; Pivonello, R.; Auriemma, R.S. Resistance to Dopamine Agonists in Pituitary Tumors: Molecular Mechanisms. Front. Endocrinol. 2022, 12, 791633. [Google Scholar] [CrossRef]
- Urwyler, S.A.; Karavitaki, N. Refractory lactotroph adenomas. Pituitary 2023, 26, 273–277. [Google Scholar] [CrossRef]
- Radl, D.B.; Zárate, S.; Jaita, G.; Ferraris, J.; Zaldivar, V.; Eijo, G.; Seilicovich, A.; Pisera, D. Apoptosis of Lactotrophs Induced by D2 Receptor Activation Is Estrogen Dependent. Neuroendocrinology 2008, 88, 43–52. [Google Scholar] [CrossRef]
- Inoshita, N.; Nishioka, H. The 2017 WHO classification of pituitary adenoma: Overview and comments. Brain Tumor Pathol. 2018, 35, 51–56. [Google Scholar] [CrossRef]
- Passos, V.Q.; Fortes, M.A.H.; Giannella-Neto, D.; Bronstein, M.D. Genes Differentially Expressed in Prolactinomas Responsive and Resistant to Dopamine Agonists. Neuroendocrinology 2008, 89, 163–170. [Google Scholar] [CrossRef]
- Caccavelli, L.; Feron, F.; Morange, I.; Rouer, E.; Benarous, R.; Dewailly, D.; Jaquet, P.; Kordon, C.; Enjalbert, A. Decreased Expression of the Two D2 Dopamine Receptor Isoforms in Bromocriptine-Resistant Prolactinomas. Neuroendocrinology 1994, 60, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Peverelli, E.; Treppiedi, D.; Mangili, F.; Catalano, R.; Spada, A.; Mantovani, G. Drug resistance in pituitary tumours: From cell membrane to intracellular signalling. Nat. Rev. Endocrinol. 2021, 17, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, I.; Rasolonjanahary, R.; Gunz, G.; Bertrand, P.; Delivet, S.; Jedynak, C.P.; Kordon, C.; Peillon, F.; Jaquet, P.; Enjalbert, A. Resistance to Bromocriptine in Prolactinomas. J. Clin. Endocrinol. Metab. 1989, 69, 500–509. [Google Scholar] [CrossRef]
- Tang, H.; Cheng, Y.; Lou, X.; Yao, H.; Xie, J.; Gu, W.; Huang, X.; Liu, Y.; Lin, S.; Dai, Y.; et al. DRD2 expression based on 18F-fallypride PET/MR predicts the dopamine agonist resistance of prolactinomas: A pilot study. Endocrine 2023, 80, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yin, H.; Pei, X.; Zhang, Y.; Wang, C.; Zheng, X.; Liang, H.; Yang, H.; Li, S. Cholesterol-activated stress granules reduce the membrane localization of DRD2 and promote prolactinoma dopamine agonists resistance. Acta Neuropathol. Commun. 2025, 13, 84. [Google Scholar] [CrossRef]
- Trouillas, J.; Delgrange, E.; Wierinckx, A.; Vasiljevic, A.; Jouanneau, E.; Burman, P.; Raverot, G. Clinical, Pathological, and Molecular Factors of Aggressiveness in Lactotroph Tumours. Neuroendocrinology 2019, 109, 70–76. [Google Scholar] [CrossRef]
- Rajan, R.R.; Shyamasunder, H.; Chacko, G.; S, D.; Jeyachandran, R.; Chacko, A.G.; Prabu, K.; Rajaratnam, S.; Kapoor, N.; Thomas, N.; et al. Dopamine receptor expression predicts cabergoline response in persistent somatotroph adenoma. Pituitary 2025, 28, 87. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Sarkar, D.K. Estrogen inhibits D2S receptor-regulated Gi3 and Gs protein interactions to stimulate prolactin production and cell proliferation in lactotropic cells. J. Endocrinol. 2012, 214, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.B.; Zheng, W.M.; Su, Z.P.; Chen, Y.; Wu, J.S.; De Wang, C.; Lin, C.; Zeng, Y.J.; Zhuge, Q.C. Expression of D2RmRNA isoforms and ERmRNA isoforms in prolactinomas: Correlation with the response to bromocriptine and with tumor biological behavior. J. Neuro-Oncol. 2010, 99, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Cristina, C.; Díaz-Torga, G.S.; Goya, R.G.; Kakar, S.S.; Perez-Millán, M.I.; Passos, V.Q.; Giannella-Neto, D.; Bronstein, M.D.; Becu-Villalobos, D. PTTG expression in different experimental and human prolactinomas in relation to dopaminergic control of lactotropes. Mol. Cancer 2007, 6, 4. [Google Scholar] [CrossRef]
| Peptide/Protein | Antibody Manufacturer | Catalog Number | Species Raised in (Mono- or Polyclonal) | Dilution | RRID Number |
|---|---|---|---|---|---|
| D2R | Novus Bio | NLS1403 | Rabbit; polyclonal | 1:1000 | AB_523304 |
| Prolactin | BioGenex | AM978-5M | Mouse; monoclonal | Ready to use | AB_3065212 |
| ERα | Dako | M7047 | Mouse; monoclonal | 1:100 | AB_2101946 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramer Bass, I.; Ferreira de Carvalho, J.; Umphlett, M.; Shuman, W.; Kirschenbaum, A.; Milgrim, E.; Milgrim, L.; Bederson, J.; Post, K.; Shrivastava, R.; et al. Immunohistochemical Comparison of Dopamine-2 Receptor Expression in Resistant and Non-Resistant Prolactinomas. J. Clin. Med. 2025, 14, 7344. https://doi.org/10.3390/jcm14207344
Ramer Bass I, Ferreira de Carvalho J, Umphlett M, Shuman W, Kirschenbaum A, Milgrim E, Milgrim L, Bederson J, Post K, Shrivastava R, et al. Immunohistochemical Comparison of Dopamine-2 Receptor Expression in Resistant and Non-Resistant Prolactinomas. Journal of Clinical Medicine. 2025; 14(20):7344. https://doi.org/10.3390/jcm14207344
Chicago/Turabian StyleRamer Bass, Ilana, Julia Ferreira de Carvalho, Melissa Umphlett, William Shuman, Alexander Kirschenbaum, Emily Milgrim, Lucas Milgrim, Joshua Bederson, Kalmon Post, Raj Shrivastava, and et al. 2025. "Immunohistochemical Comparison of Dopamine-2 Receptor Expression in Resistant and Non-Resistant Prolactinomas" Journal of Clinical Medicine 14, no. 20: 7344. https://doi.org/10.3390/jcm14207344
APA StyleRamer Bass, I., Ferreira de Carvalho, J., Umphlett, M., Shuman, W., Kirschenbaum, A., Milgrim, E., Milgrim, L., Bederson, J., Post, K., Shrivastava, R., & Levine, A. C. (2025). Immunohistochemical Comparison of Dopamine-2 Receptor Expression in Resistant and Non-Resistant Prolactinomas. Journal of Clinical Medicine, 14(20), 7344. https://doi.org/10.3390/jcm14207344





