Cardiac Magnetic Resonance in Adults: An Updated Review of the Diagnostic Approach to Major Heart Diseases
Abstract
1. Introduction
2. Myocardial Scarring in Ischemic and Non-Ischemic Cardiomyopathies
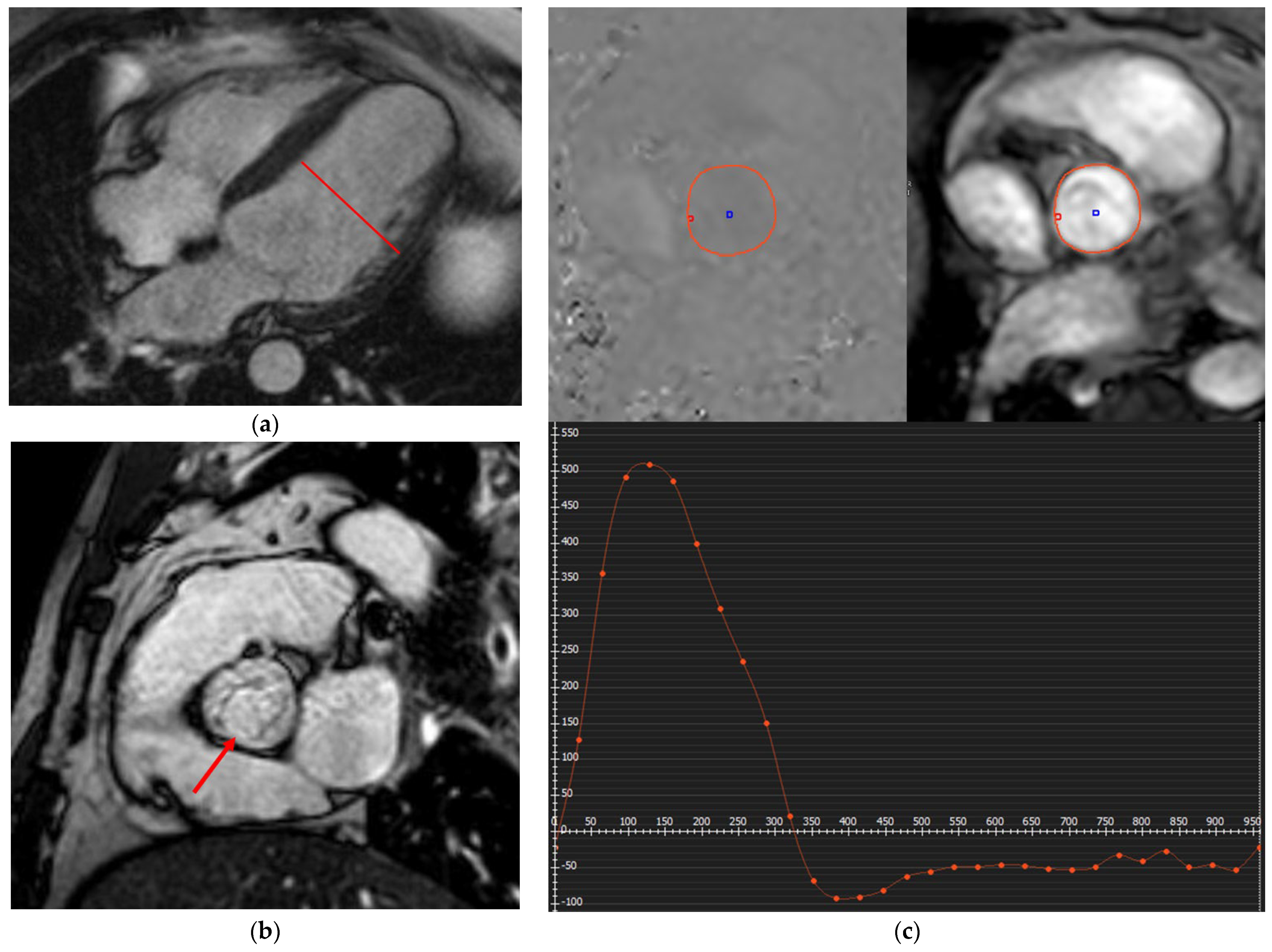
2.1. Ischemic Myocardial Scarring
2.1.1. Acute Coronary Syndrome (ACS)
2.1.2. Chronic Coronary Syndrome (CCS)
2.1.3. MINOCA
2.2. Non-Ischemic Myocardial Scarring
2.2.1. Myocarditis and Pericarditis
2.2.2. Cardiomyopathies
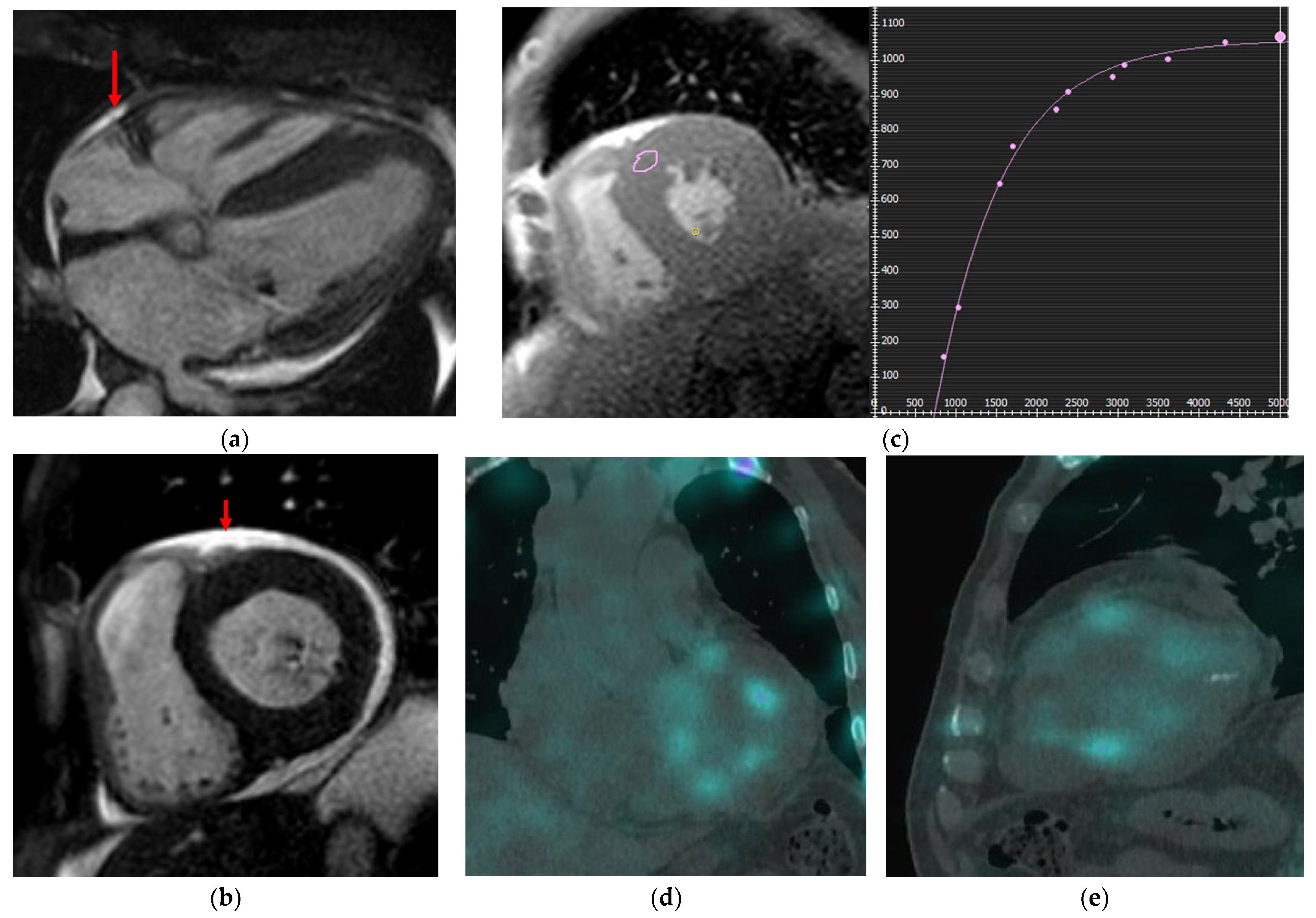
Hypertrophic Cardiomyopathy (HCM)
Dilated Cardiomyopathy (DCM)
Non-Dilated Left Ventricular Myocardiopathy (NDLVC)
Restrictive Cardiomyopathy (RCM)
Arrhythmogenic Cardiomyopathy (ACM)
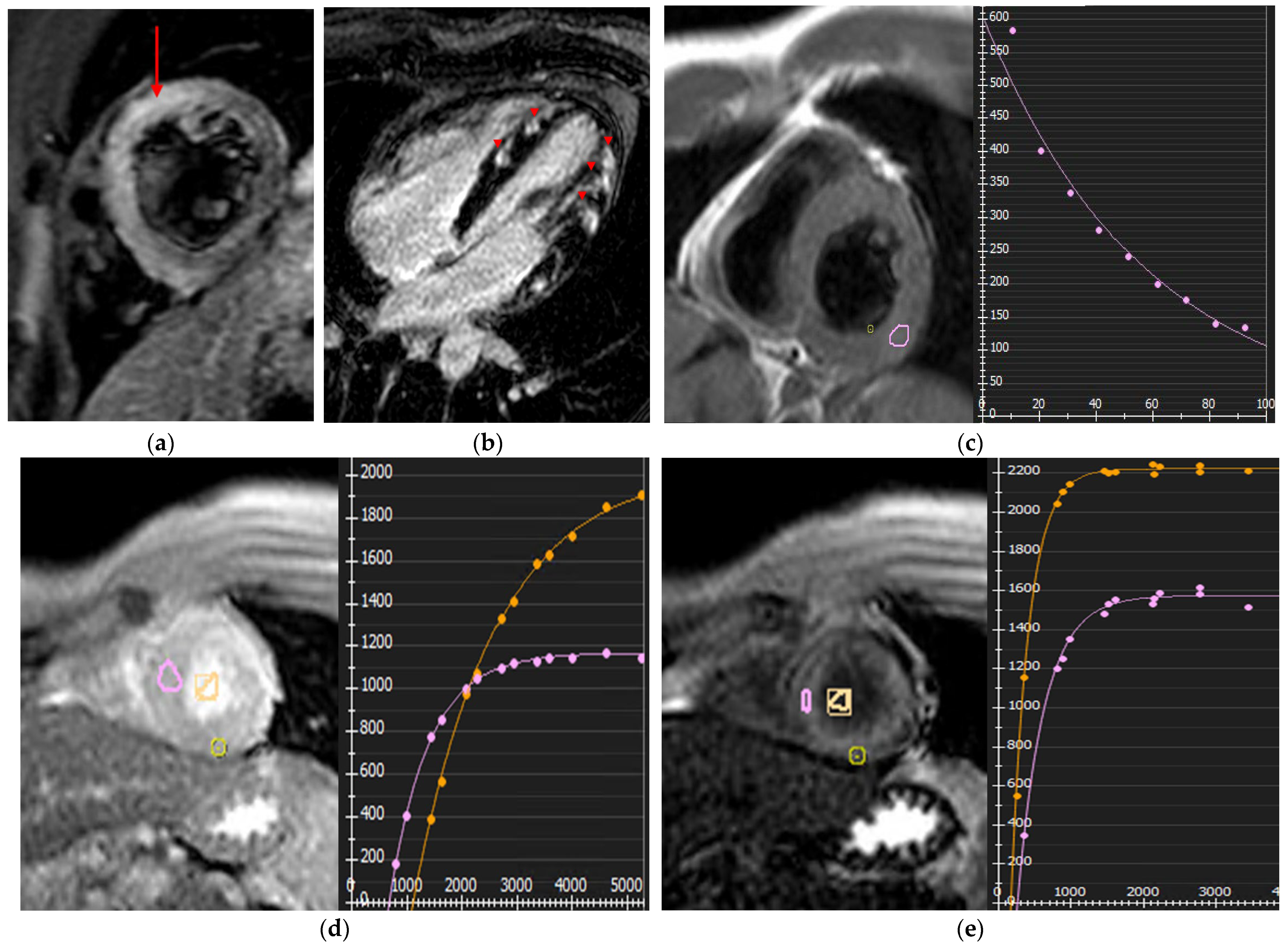
3. Infiltrative Myocardial Diseases
3.1. Cardiac Amyloidosis (CA)
3.2. Anderson-Fabry Disease
3.3. Cardiac Sarcoidosis
3.4. Iron Overload Cardiomyopathy
4. Valvular Heart Disease
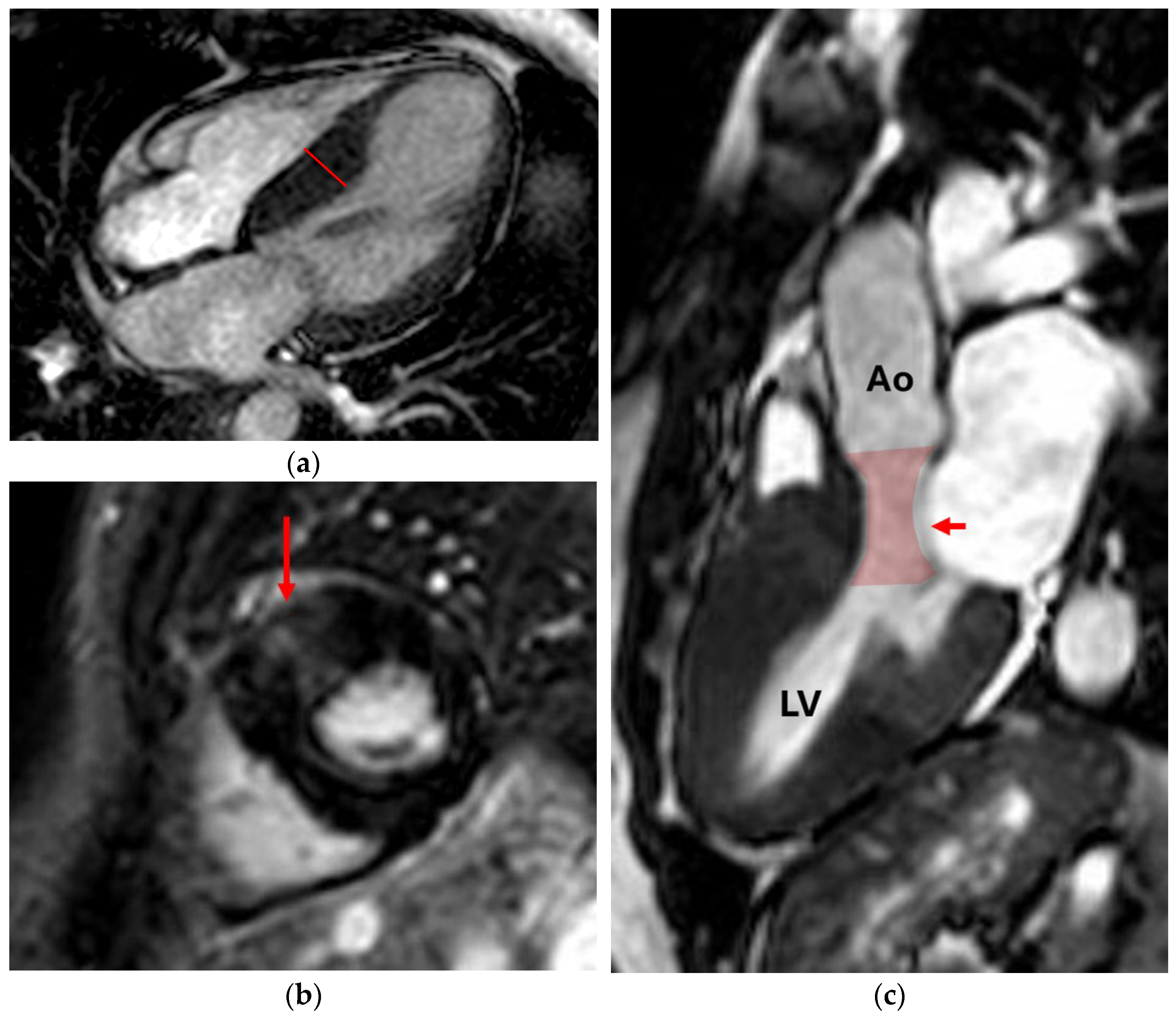
4.1. Regurgitation
4.1.1. Aortic Regurgitation (AR)
4.1.2. Mitral Regurgitation (MR)
4.1.3. Tricuspid Regurgitation (TR)
4.1.4. Pulmonary Regurgitation (PR)
4.2. Stenosis
4.2.1. Aortic Stenosis (AS)
4.2.2. Mitral Stenosis (MS)
4.2.3. Tricuspid Stenosis (TS)
4.2.4. Pulmonary Stenosis (PS)
5. Adult Congenital Heart Disease
6. Pulmonary Hypertension and RV Morpho-Functional Evaluation
6.1. Pulmonary Hypertension
6.2. RV Morpho-Functional Evaluation
7. CMR in Cardio-Oncology
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACHD | Adult congenital heart disease |
| ACS | Acute Coronary Syndrome |
| ACM | Arrhythmogenic Cardiomyopathy |
| AR | Aortic Regurgitation |
| ARVC | Arrhythmogenic Right Ventricular Cardiomyopathy |
| AS | Aortic Stenosis |
| CAD | Coronary Artery Disease |
| CCS | Chronic Coronary Syndrome |
| CMR | Cardiac Magnetic Resonance |
| DCM | Dilated Cardiomyopathy |
| ESC | European Society of Cardiology |
| HCM | Hypertrophic Cardiomyopathy |
| LGE | Late Gadolinium Enhancement |
| LoE | Level of Evidence |
| LV | Left Ventricle |
| MINOCA | Myocardial Infarction with Non-Obstructive Coronary Arteries |
| MR | Mitral Regurgitation |
| MS | Mitral Stenosis |
| NDLVC | Non-Dilated Left Ventricular Cardiomyopathy |
| PH | Pulmonary Hypertension |
| PR | Pulmonary Regurgitation |
| PS | Pulmonary Stenosis |
| RCM | Restrictive Cardiomyopathy |
| RV | Right Ventricle |
| TR | Tricuspid Regurgitation |
| TS | Tricuspid Stenosis |
| TTE | Transthoracic Echocardiography |
References
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the management of heart failure. Circulation 2022, 145, 895–1032. [Google Scholar]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease. Circulation 2021, 143, e72–e227. [Google Scholar]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the evaluation and diagnosis of chest pain. Circulation 2021, 144, e368–e454. [Google Scholar]
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the management of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2024, 83, 2324–2405. [Google Scholar] [CrossRef]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; De Backer, J.; Gatzoulis, M.; Geva, T.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e698–e800. [Google Scholar] [CrossRef]
- Leiner, T.; Bogaert, J.; Friedrich, M.G.; Mohiaddin, R.; Muthurangu, V.; Myerson, S.; Powell, A.J.; Raman, S.V.; Pennell, D.J. SCMR Position Paper (2020) on clinical indications for cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2020, 22, 76. [Google Scholar] [CrossRef]
- Sirajuddin, A.; Mirmomen, S.M.; Henry, T.S.; Kandathil, A.; Kelly, A.M.; King, C.S.; Kuzniewski, C.T.; Lai, A.R.; Lee, E.; Martin, M.D.; et al. ACR Appropriateness Criteria® suspected pulmonary hypertension: 2022 update. J. Am. Coll. Radiol. 2022, 19, S502–S512. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Mickley, H.; Crea, F.; Van de Werf, F.; et al. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- El-Saadi, W.; Engvall, J.E.; Alfredsson, J.; Karlsson, J.E.; Martins, M.; Sederholm, S.; Zaman, S.F.; Ebbers, T.; Kihlberg, J. A head-to-head comparison of myocardial strain by fast-strain encoding and feature tracking imaging in acute myocardial infarction. Front. Cardiovasc. Med. 2022, 9, 1028821. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; Dharmakumar, R.; Arai, A.E.; Berry, C.; Hausenloy, D.J. Cardiovascular magnetic resonance in acute myocardial infarction. Circulation 2018, 137, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Holtackers, R.J.; Emrich, T.; Botnar, R.M.; Kooi, M.E.; Wildberger, J.E.; Kreitner, K.F. Late gadolinium enhancement cardiac magnetic resonance imaging: From basic concepts to emerging methods. RoFo 2022, 194, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Eisenblätter, M.; Gielen, S. Myocardial late gadolinium enhancement (LGE) in cardiac magnetic resonance imaging (CMR)—An important risk marker for cardiac disease. J. Cardiovasc. Dev. Dis. 2024, 11, 40. [Google Scholar] [CrossRef]
- Matusik, P.S.; Bryll, A.; Matusik, P.T.; Popiela, T.J. Ischemic and non-ischemic patterns of late gadolinium enhancement in heart failure with reduced ejection fraction. Cardiol. J. 2021, 28, 67–76. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Moroni, F.; Montone, R.A.; Azzalini, L.; Sanna, T.; Abbate, A. Ischemic cardiomyopathy and heart failure after acute myocardial infarction. Curr. Cardiol. Rep. 2022, 24, 1505–1515. [Google Scholar] [CrossRef]
- Bakhshi, H.; Gibson, C.M. MINOCA: Myocardial infarction with non-obstructive coronary artery disease. Am. Heart J. Plus Cardiol. Res. Pract. 2023, 33, 100312. [Google Scholar] [CrossRef]
- Tornvall, P.; Gerbaud, E.; Behaghel, A.; Chopard, R.; Collste, O.; Laraudogoitia, E.; Leurent, G.; Meneveau, N.; Montaudon, M.; Perez-David, E.; et al. Myocarditis or “true” infarction by cardiac magnetic resonance in patients with a clinical diagnosis of myocardial infarction without obstructive coronary disease: A meta-analysis of individual patient data. Atherosclerosis 2015, 241, 87–91. [Google Scholar] [CrossRef]
- Niccoli, G.; Camici, P.G. Myocardial infarction with non-obstructive coronary arteries: What is the prognosis? Eur. Heart J. Suppl. 2020, 22, E40–E45. [Google Scholar] [CrossRef]
- Drazner, M.H.; Bozkurt, B.; Cooper, L.T.; Aggarwal, N.R.; Basso, C.; Bhave, N.M.; Caforio, A.L.P.; Ferreira, V.M.; Heidecker, B.; Kontorovich, A.R.; et al. 2024 ACC Expert Consensus Decision Pathway on strategies and criteria for the diagnosis and management of myocarditis. J. Am. Coll. Cardiol. 2025, 85, 391–431. [Google Scholar] [CrossRef]
- Luetkens, J.A.; Faron, A.; Isaak, A.; Dabir, D.; Kuetting, D.; Feisst, A.; Schmeel, F.C.; Sprinkart, A.M.; Thomas, D. Comparison of original and 2018 Lake Louise criteria for diagnosis of acute myocarditis: Results of a validation cohort. Radiol. Cardiothorac. Imaging 2019, 1, e190010. [Google Scholar] [CrossRef]
- Schulz-Menger, J.; Collini, V.; Gröschel, J.; Adler, Y.; Brucato, A.; Christian, V.; Ferreira, V.M.; Gandjbakhch, E.; Heidecker, B.; Kerneis, M. 2025 ESC Guidelines for the management of myocarditis and pericarditis: Developed by the Task Force for the Management of Myocarditis and Pericarditis of the European Society of Cardiology (ESC); Endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2025, 1, ehaf192. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kuhl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Hindieh, W.; Weissler-Snir, A.; Hammer, H.; Adler, A.; Rakowski, H.; Chan, R.H. Discrepant measurements of maximal left ventricular wall thickness between cardiac magnetic resonance imaging and echocardiography in patients with hypertrophic cardiomyopathy. Circ. Cardiovasc. Imaging 2017, 10, e006309. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic value of quantitative contrast-enhanced CMR for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef]
- O’Hanlon, R.; Grasso, A.; Roughton, M.; Moon, J.C.; Clark, S.; Wage, R.; Webb, J.; Kulkarni, M.; Dawson, D.; Sulaibeekh, L.; et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 867–874. [Google Scholar] [CrossRef]
- Baxi, A.J.; Restrepo, C.S.; Vargas, D.; Marmol-Velez, A.; Ocazionez, D.; Murillo, H. Hypertrophic cardiomyopathy from A to Z: Genetics, pathophysiology, imaging, and management. Radiographics 2016, 36, 335–354. [Google Scholar] [CrossRef]
- Fujita, N.; Duerinekx, A.J.H.C. Variation in left ventricular regional wall stress with cine magnetic resonance imaging. Am. Heart J. 1993, 125, 1337–1345. [Google Scholar] [CrossRef]
- Crean, A.M.; Maredia, N.; Ballard, G.; Menezes, R.; Wharton, G.; Forster, J.; Greenwood, J.P.; Thomson, J.D. 3D echocardiography systematically underestimates right ventricular volumes compared to cardiovascular magnetic resonance in adult congenital heart disease patients with moderate or severe RV dilatation. J. Cardiovasc. Magn. Reson. 2011, 13, 78. [Google Scholar] [CrossRef]
- Assomull, R.G.; Prasad, S.K.; Lyne, J.; Smith, G.; Burman, E.D.; Khan, M.; Sheppard, M.N.; Poole-Wilson, P.A.; Pennell, D.J. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J. Am. Coll. Cardiol. 2006, 48, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, B.; Li, S.; Zhang, H.; Sun, Z.; Wang, H.; Xu, L. Cardiac magnetic resonance comparison of non-dilated and dilated cardiomyopathy: Imaging features and prognostic predictors in non-dilated left ventricular cardiomyopathy. Open Heart 2025, 12, e003476. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Aimo, A.; Barison, A.; Emdin, M.; Porcari, A.; Linhart, A.; Keren, A.; Merlo, M.; Sinagra, G. A Restrictive cardiomyopathy: Definition and diagnosis. Eur. Heart J. 2022, 43, 4679–4693. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Basso, C.; Judge, D.P. Arrhythmogenic cardiomyopathy. Circ. Res. 2017, 121, 785–802. [Google Scholar] [CrossRef]
- Corrado, D.; Perazzolo Marra, M.; Zorzi, A.; Beffagna, G.; Cipriani, A.; De Lazzari, M.; Migliore, F.; Pilichou, K.; Rampazzo, A.; Rigato, I.; et al. Diagnosis of arrhythmogenic cardiomyopathy: The Padua criteria. Int. J. Cardiol. 2020, 319, 106–114. [Google Scholar] [CrossRef]
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.; Daubert, J.P.; et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Eur. Heart J. 2010, 31, 806–814. [Google Scholar] [CrossRef]
- Kottam, A.; Hanneman, K.; Schenone, A.; Daubert, M.A.; Sidhu, G.D.; Gropler, R.J.; Garcia, M.J. State-of-the-art imaging of infiltrative cardiomyopathies: A scientific statement from the American Heart Association. Circ. Cardiovasc. Imaging 2023, 16, e000081. [Google Scholar] [CrossRef]
- Oda, S.; Kidoh, M.; Nagayama, Y.; Takashio, S.; Usuku, H.; Ueda, M.; Kido, T.; Kawakami, H.; Nakaura, T.; Yamashita, Y. Trends in diagnostic imaging of cardiac amyloidosis: Emerging knowledge and concepts. Radiographics 2020, 40, 961–981. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Zimmerman, S.L. Grading cardiac risk in Fabry disease: Is MRI the answer? Radiology 2020, 294, 50–51. [Google Scholar] [CrossRef]
- Jeudy, J.; Burke, A.P.; White, C.S.; Kramer, G.B.G.; Frazier, A.A. Cardiac sarcoidosis: The challenge of radiologic–pathologic correlation. Radiographics 2015, 35, 657–679. [Google Scholar] [CrossRef]
- Meloni, A.; Martini, N.; Positano, V.; De Luca, A.; Pistoia, L.; Sbragi, S.; Spasiano, A.; Casini, T.; Bitti, P.P.; Allò, M.; et al. Myocardial iron overload by cardiovascular magnetic resonance native segmental T1 mapping: A sensitive approach that correlates with cardiac complications. J. Cardiovasc. Magn. Reson. 2021, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Praz, F.; Borger, M.A.; Lanz, J.; Marin-Cuartas, M.; Abreu, A.; Adamo, M.; Ajmone Marsan, N.; Barili, F.; Bonaros, N.; Cosyns, B.; et al. 2025 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2025, ehaf194. [Google Scholar] [CrossRef] [PubMed]
- Glockner, J.F.; Johnston, D.L.; McGee, K.P. Evaluation of cardiac valvular disease with MR imaging: Qualitative and quantitative techniques. Radiographics 2003, 23, e9. [Google Scholar] [CrossRef]
- Herzog, B.A.; Greenwood, J.P.; Plein, S.; Garg, P.; Haaf, P.; Onciul, S. Aortic Regurgitation. In Cardiovascular Magnetic Resonance Pocket Guide, 1st ed.; Society for Cardiovascular Magnetic Resonance: Minneapolis, MN, USA, 2017; pp. 78–82. [Google Scholar]
- Myerson, S.G.; D’Arcy, J.; Mohiaddin, R.; Greenwood, J.P.; Karamitsos, T.D.; Francis, J.M.; Banning, A.P.; Christiansen, J.P.; Neubauer, S. Aortic regurgitation quantification using cardiovascular magnetic resonance: Association with clinical outcome. Circulation 2012, 126, 1452–1460. [Google Scholar] [CrossRef]
- De Rubeis, G.; Galea, N.; Ceravolo, I.; Dacquino, G.M.; Carbone, I.; Catalano, C.; Francone, M. Aortic valvular imaging with cardiovascular magnetic resonance: Seeking comprehensiveness. Br. J. Radiol. 2019, 92, 20190109. [Google Scholar] [CrossRef]
- Garg, P.; Pavon, A.G.; Penicka, M.; Uretsky, S. Cardiovascular magnetic resonance imaging in mitral valve disease. Eur. Heart J. 2025, 46, 606–619. [Google Scholar] [CrossRef]
- Penicka, M.; Vecera, J.; Mirica, D.C.; Kotrc, M.; Kockova, R.; Van Camp, G. Prognostic implications of magnetic resonance-derived quantification in asymptomatic patients with organic mitral regurgitation: Comparison with Doppler echocardiography. Circulation 2018, 137, 1349–1360. [Google Scholar] [CrossRef]
- Myerson, S.G. CMR in evaluating valvular heart disease: Diagnosis, severity, and outcomes. JACC Cardiovasc. Imaging 2021, 14, 2020–2032. [Google Scholar] [CrossRef]
- D’Arcy, J.L.; Coffey, S.; Loudon, M.A.; Kennedy, A.; Pearson-Stuttard, J.; Birks, J.; Frangou, E.; Farmer, A.J.; Mant, D.; Wilson, J.; et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: The OxVALVE Population Cohort Study. Eur. Heart J. 2016, 37, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Yadgir, S.; Johnson, C.O.; Aboyans, V.; Adebayo, O.M.; Adedoyin, R.A.; Afarideh, M.; Alahdab, F.; Alashi, A.; Alipour, V.; Arabloo, J.; et al. Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990–2017. Circulation 2020, 141, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Iung, B.; Delgado, V.; Rosenhek, R.; Price, S.; Prendergast, B.; Wendler, O.; Bonis, M.D.; Tribouilloy, C.; Evangelista, A.; Bogachev-Prokophiev, A.; et al. Contemporary presentation and management of valvular heart disease: The EURObservational Research Programme Valvular Heart Disease II Survey. Circulation 2019, 140, 1156–1169. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease: The Task Force for the management of adult congenital heart disease of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Lewis, R.A.; Johns, C.S.; Cogliano, M.; Capener, D.; Tubman, E.; Elliot, C.A.; Charalampopoulos, A.; Sabroe, I.; Thompson, A.A.R.; Billings, C.G.; et al. Identification of CMR thresholds for risk stratification in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2020, 201, 458–466. [Google Scholar] [CrossRef]
- Swift, A.J.; Capener, D.; Johns, C.; Hamilton, N.; Rothman, A.; Elliot, C.; Condliffe, R.; Charalampopoulos, A.; Rajaram, S.; Lawrie, A.; et al. Magnetic resonance imaging in the prognostic evaluation of patients with pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2017, 196, 228–239. [Google Scholar] [CrossRef]
- Zhao, X.; Leng, S.; Tan, R.S.; Chai, P.; Yeo, T.J.; Bryant, J.A.; Teo, L.L.S.; Fortier, M.V.; Ruan, W.; Low, T.T.; et al. Right ventricular energetic biomarkers from 4D flow CMR are associated with exertional capacity in pulmonary arterial hypertension. J. Cardiovasc. Magn. Reson. 2022, 24, 61. [Google Scholar] [CrossRef]
- Alabed, S.; Shahin, Y.; Garg, P.; Alandejani, F.; Johns, C.S.; Lewis, R.A.; Condliffe, R.; Wild, J.M.; Kiely, D.G.; Swift, A.J. Cardiac MRI predicts clinical worsening and mortality in pulmonary arterial hypertension: A systematic review and meta-analysis. JACC Cardiovasc. Imaging 2021, 14, 931–942. [Google Scholar] [CrossRef]
- Borhani, A.; Porter, K.K.; Umair, M.; Chu, L.C.; Mathai, S.C.; Kolb, T.M.; Damico, R.L.; Hassoun, P.M.; Kamel, I.R.; Zimmerman, S.L. Quantifying 4D flow cardiovascular magnetic resonance vortices in patients with pulmonary hypertension: A pilot study. Pulm. Circ. 2023, 13, e12236. [Google Scholar] [CrossRef] [PubMed]
- Banjade, P.; Subedi, A.; Acharya, S.; Itani, A.; Sharma, M.; Kassam, N.; Ghamande, S.; Surani, S. The role of cardiac MRI in pulmonary hypertension—Is it still an underutilized tool? Open Respir. Med. J. 2024, 18, e18743064288565. [Google Scholar] [CrossRef] [PubMed]
- Cain, M.T.; Schäfer, M.; Ross, L.K.; Ivy, D.D.; Mitchell, M.B.; Fenster, B.E.; Bull, T.M.; Barker, A.J.; Vargas, D.; Hoffman, J.R.H. 4D-flow MRI intracardiac flow analysis considering different subtypes of pulmonary hypertension. Pulm. Circ. 2023, 13, e12247. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the ASE and the EACVI. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Herzog, B.A.; Greenwood, J.P.; Ballard, G. CMR Congenital Heart Disease Pocket Guide; Society for Cardiovascular Magnetic Resonance: Minneapolis, MN, USA, 2014; Available online: https://www.escardio.org/static_file/Escardio/Subspecialty/EACVI/CMR-guide-CHD-2014.pdf (accessed on 10 July 2025).
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar]
- Eyre, K.; Rafiee, M.J.; Leo, M.; Ma, J.; Hillier, E.; Amini, N.; Pressacco, J.; Janich, M.A.; Zhu, X.; Friedrich, M.G. Clinical utility of a rapid two-dimensional balanced steady-state free precession sequence with deep learning reconstruction. J. Cardiovasc. Magn. Reson. 2024, 26, 101069. [Google Scholar] [CrossRef]
- Zhang, Q.; Fotaki, A.; Ghadimi, S.; Wang, Y.; Doneva, M.; Wetzl, J.; Delfino, J.G.; O’Regan, D.P.; Prieto, C.; Epstein, F.H. Improving the efficiency and accuracy of cardiovascular magnetic resonance with artificial intelligence-review of evidence and proposition of a roadmap to clinical translation. J. Cardiovasc. Magn. Reson. 2024, 26, 101051. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Wu, P.; Ding, L. Cardiovascular Magnetic Resonance Imaging Analysis Using Neural Networks. J. Radiat. Res. Appl. Sci. 2025, 18, 101874. [Google Scholar] [CrossRef]

| Indication for CMR | Class | LoE | Society |
|---|---|---|---|
| Acute coronary syndrome 1 | IIa | A | ESC |
| Chronic coronary syndrome (moderate-high probability of obstructive CAD) | I | B | ESC |
| Chronic coronary syndrome (inconclusive echocardiographic examination) | IIb | C | ESC |
| MINOCA | I | B | ACC/AHA, ESC |
| Myocarditis (general, cancer therapy-related) | I | B | ACC/AHA, ESC |
| Myocarditis (follow up, ICI-related) | I | C | ESC |
| Pericarditis (undiagnosed with clinical criteria) | I | B | ESC |
| Cardiomyopathies 2 (initial assessment) | I | B | AHA/ACC, ESC |
| Cardiomyopathies 2 (follow-up) | IIa | C | ESC |
| Aortic regurgitation 3 | I | ACC/AHA | B |
| Mitral regurgitation (primary) 3 | I | B | ACC/AHA |
| Mitral regurgitation (secondary) 3 | I | C | ACC/AHA |
| Adult congenital heart disease (shunt lesions 4, ToF, coarctation of aorta) | I | B | ACC/AHA |
| Adult congenital heart disease (dTGA, single ventricle and Fontan circulation) | I | C | ACC/AHA |
| Pulmonary hypertension (symptomatic systemic sclerosis patients) | IIb | C | ESC |
| Right ventricular morpho-functional evaluation | I | B | ACC/AHA, EACVI/ESC |
| Cancer therapy-related LV dysfunction follow-up | IIa | C | ESC |
| Indication for CMR | Class |
|---|---|
| Coronary artery disease | I |
| MINOCA | I |
| Myocarditis | I |
| Pericardial inflammation and constriction | I |
| Cardiomyopathies 1 | I |
| Aortic, mitral and tricuspid regurgitation 2 | II |
| Pulmonary regurgitation and stenosis 2 | I |
| Aortic stenosis (general) 2 | II |
| Aortic stenosis (sub- and supravalvular stenosis) 2 | I |
| Mitral and tricuspid stenosis 2 | III |
| Adult congenital heart disease 3 | I |
| Right ventricular morpho-functional evaluation | I |
| Cardiac mass assessment | I |
| Clinical Task | Echocardiography | CMR | CT/Nuclear Imaging |
|---|---|---|---|
| Morphology, function and valvular evaluation | First line for morphology and LV/RV function; may underestimate volumes and regurgitant severity | Reference standard for ventricular volumes, mass, and flow quantification | CT offers excellent valvular anatomy, but limited hemodynamics |
| Tissue characterization and myocardial scarring | Indirect signs only (wall thickening, strain changes) | LGE and quantitative mapping detect scar, edema, and infiltration | Nuclear scans show perfusion and metabolism |
| Vascular anatomy | Limited use | MR angiography (selected cases) | CTA is the reference for noninvasive coronary imaging |
| Ischemia detection | Stress echo provides functional ischemia testing | Stress perfusion CMR is highly accurate and cost-effective | SPECT is widely available, but less accurate |
| Radiation/contrast | No radiation and widely available, but operator-dependent | No radiation, but requires gadolinium use and longer exam times | Involves ionizing radiation and uses iodinated contrast (CT) or tracers (SPECT) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tudela Martínez, J.I.; Alcaraz Pérez, P.; Martínez Encarnación, L.; González-Carrillo, J.; Rodríguez Sánchez, D.; Sarabia Tirado, F.; Jiménez Sánchez, A.F.; Guzmán-Aroca, F.; Berna Mestre, J.d.D. Cardiac Magnetic Resonance in Adults: An Updated Review of the Diagnostic Approach to Major Heart Diseases. J. Clin. Med. 2025, 14, 7323. https://doi.org/10.3390/jcm14207323
Tudela Martínez JI, Alcaraz Pérez P, Martínez Encarnación L, González-Carrillo J, Rodríguez Sánchez D, Sarabia Tirado F, Jiménez Sánchez AF, Guzmán-Aroca F, Berna Mestre JdD. Cardiac Magnetic Resonance in Adults: An Updated Review of the Diagnostic Approach to Major Heart Diseases. Journal of Clinical Medicine. 2025; 14(20):7323. https://doi.org/10.3390/jcm14207323
Chicago/Turabian StyleTudela Martínez, José Ignacio, Pablo Alcaraz Pérez, Lourdes Martínez Encarnación, Josefa González-Carrillo, Daniel Rodríguez Sánchez, Francisco Sarabia Tirado, Andrés Francisco Jiménez Sánchez, Florentina Guzmán-Aroca, and Juan de Dios Berna Mestre. 2025. "Cardiac Magnetic Resonance in Adults: An Updated Review of the Diagnostic Approach to Major Heart Diseases" Journal of Clinical Medicine 14, no. 20: 7323. https://doi.org/10.3390/jcm14207323
APA StyleTudela Martínez, J. I., Alcaraz Pérez, P., Martínez Encarnación, L., González-Carrillo, J., Rodríguez Sánchez, D., Sarabia Tirado, F., Jiménez Sánchez, A. F., Guzmán-Aroca, F., & Berna Mestre, J. d. D. (2025). Cardiac Magnetic Resonance in Adults: An Updated Review of the Diagnostic Approach to Major Heart Diseases. Journal of Clinical Medicine, 14(20), 7323. https://doi.org/10.3390/jcm14207323






