Preoxygenation in the ICU
Abstract
1. Introduction
2. Rationale for Preoxygenation
| Efficacy |
| Inspired oxygen concentration |
| Presence of leak |
| Patient-ventilation interface |
| Fresh gas flow |
| Tidal volume vs. deep breathing |
| Duration of preoxygenation |
| Alveolar ventilation/functional residual capacity ratio |
| Positive pressure (NIV or CPAP vs. Facemask or HFNC) |
| Efficiency |
| Oxygen volume in lungs |
| FRC |
| Alveolar oxygen tension |
| System oxygen supply vs. demand balance |
| Arterial oxygen content |
| Cardiac output |
| VO2 (whole body oxygen consumption) |
3. Strategies for Pre-Oxygenation
3.1. Conventional Preoxygenation
3.2. Preoxygenation with Non-Invasive Ventilation (NIV)
3.3. Preoxygenation with High-Flow Nasal Cannula (HFNC)
3.4. Combined Preoxygenation Strategies
3.5. Sedation-Assisted Preoxygenation
| Population | Strategy | Practical Settings | Key Evidence | Alternatives/Add-ons | Caveats |
|---|---|---|---|---|---|
| Severe hypoxemia (PaO2/FiO2 < 200) | NIV | PS ~5–10 cmH2O; PEEP ~5 cmH2O; FiO2 1.0; tight mask | [33,34,35,37] | NIV + HFNC [43] If contra indications to NIV: HFNC or HFNC + BVM [41] Gentle mask ventilation during apnea [44] Apneic oxygenation [43] | Interface intolerance |
| Moderate hypoxemia (PaO2/FiO2 200–300) | NIV or HFNC | HFNC 40–60 L·min−1, FiO2 1.0; or NIV as above | [33,39,45] | Gentle mask ventilation during apnea [44] Apneic oxygenation [43] | Interface intolerance |
| Mild or no hypoxemia | NIV or HFNC or BVM | Facemask, reservoir; or HFNC as above or NIV as above | ∅ | ∅ | Leakage reduces FiO2 |
| Obesity (BMI ≥ 30 kg·m−2) | NIV (Or HFNC) | PS 5–10 cmH2O + PEEP ~5 cmH2O; FiO2 1.0; ramped position HFNC as above | [30,46,47] Warning: OR data extrapolated | NIV + HFNC or BVM + HFNC [41] if severe hypoxemia | ∅ |
| High aspiration risk active vomiting | HFNC | HFNC as above | Pragmatic safety choice | Apneic oxygenation [43] | Rapid sequence essential Avoid aggressive BVM |
| Anticipated difficult laryngoscopy | NIV +/− HFNC | NIV as above; HFNC as above | [33,34,35,37] Pragmatic safety choice | Gentle mask ventilation during apnea [44] Apneic oxygenation [43] | Ensure rescue plan |
4. Peri-Intubation Oxygenation
4.1. Apneic Oxygenation
4.2. Mask Ventilation During the Apneic Period
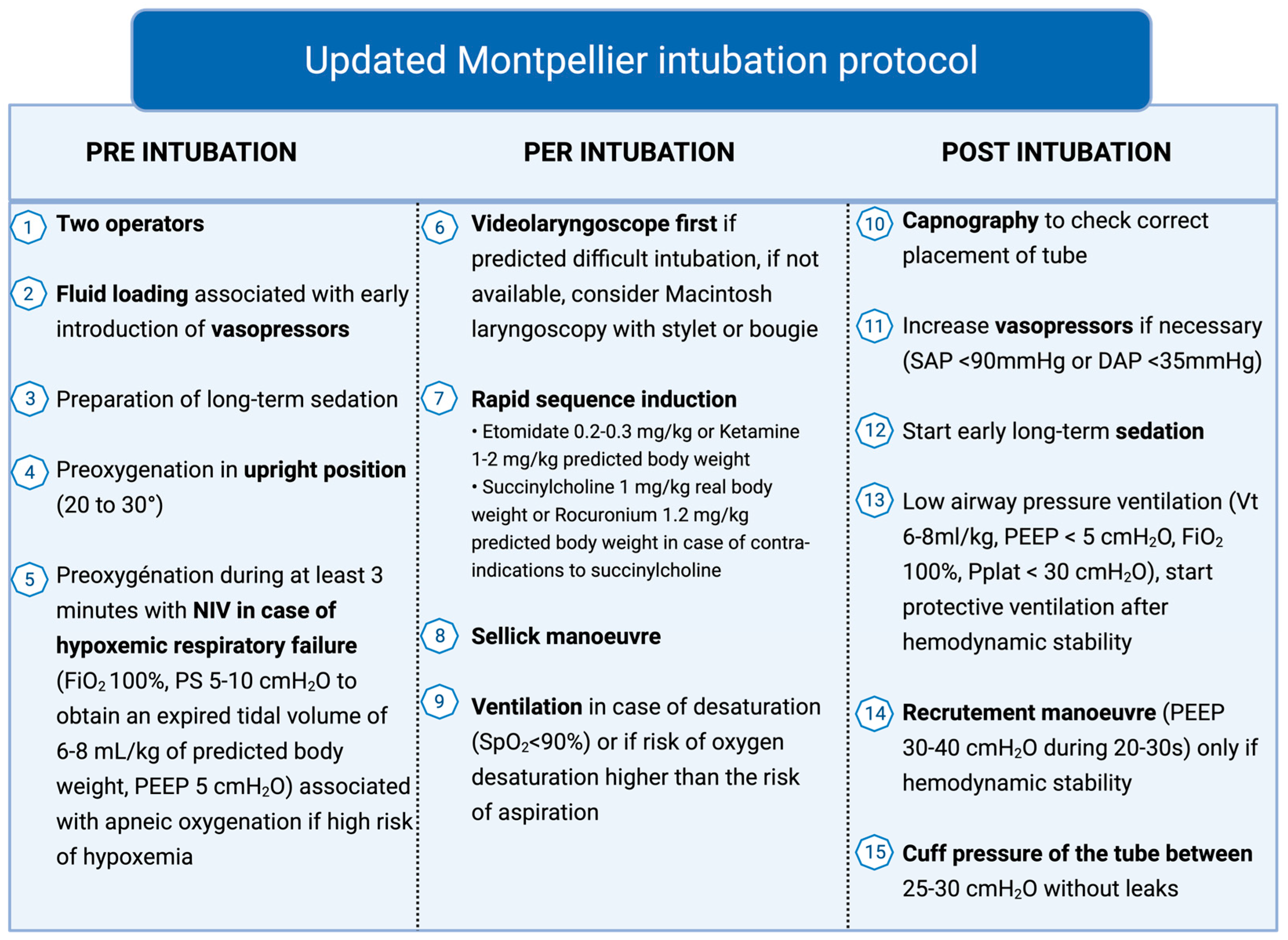
5. Intubation Protocol
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FRC | Functional Residual Capacity |
| FAO2 | Alveolar Fraction of Oxygen |
| HFNC | High-Flow Nasal Cannula |
| NIV | Non-Invasive Ventilation |
| PEEP | Positive End-Expiratory Pressure |
| FiO2 | Fraction of Inspired Oxygen |
| SpO2 | Peripheral Capillary Oxygen Saturation |
| PaO2 | Arterial Partial Pressure of Oxygen |
| BMV | Bag–Valve–Mask |
References
- Critical Care Statistics|SCCM. In Society of Critical Care Medicine. Available online: https://sccm.org/communications/critical-care-statistics?utm_source=chatgpt.com (accessed on 22 September 2025).
- De Jong, A.; Rolle, A.; Molinari, N.; Paugam-Burtz, C.; Constantin, J.-M.; Lefrant, J.-Y.; Asehnoune, K.; Jung, B.; Futier, E.; Chanques, G.; et al. Cardiac Arrest and Mortality Related to Intubation Procedure in Critically Ill Adult Patients: A Multicenter Cohort Study. Crit. Care Med. 2018, 46, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Russotto, V.; Myatra, S.N.; Laffey, J.G.; Tassistro, E.; Antolini, L.; Bauer, P.; Lascarrou, J.B.; Szuldrzynski, K.; Camporota, L.; Pelosi, P.; et al. Intubation Practices and Adverse Peri-intubation Events in Critically Ill Patients From 29 Countries. JAMA 2021, 325, 1164–1172. [Google Scholar] [CrossRef]
- Griesdale, D.E.; Bosma, T.L.; Kurth, T.; Isac, G.; Chittock, D.R. Complications of endotracheal intubation in the critically ill. Intensive Care Med. 2008, 34, 1835–1842. [Google Scholar] [CrossRef]
- Jaber, S.; Jung, B.; Corne, P.; Sebbane, M.; Muller, L.; Chanques, G.; Verzilli, D.; Jonquet, O.; Eledjam, J.-J.; Lefrant, J.-Y. An intervention to decrease complications related to endotracheal intubation in the intensive care unit: A prospective, multiple-center study. Intensive Care Med. 2010, 36, 248–255. [Google Scholar] [CrossRef]
- Russotto, V.; Tassistro, E.; Myatra, S.N.; Parotto, M.; Antolini, L.; Bauer, P.; Lascarrou, J.B.; Szułdrzyński, K.; Camporota, L.; Putensen, C.; et al. Peri-intubation Cardiovascular Collapse in Patients Who Are Critically Ill: Insights from the INTUBE Study. Am. J. Respir. Crit. Care Med. 2022, 206, 449–458. [Google Scholar] [CrossRef]
- Karamchandani, K.; Nasa, P.; Jarzebowski, M.; Brewster, D.J.; De Jong, A.; Bauer, P.R.; Berkow, L.; Brown, C.A.; Cabrini, L.; Casey, J.; et al. Tracheal intubation in critically ill adults with a physiologically difficult airway. An international Delphi study. Intensive Care Med. 2024, 50, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Taboada, M.; Doldan, P.; Calvo, A.; Almeida, X.; Ferreiroa, E.; Baluja, A.; Cariñena, A.; Otero, P.; Caruezo, V.; Naveira, A.; et al. Comparison of Tracheal Intubation Conditions in Operating Room and Intensive Care Unit: A Prospective, Observational Study. Anesthesiology 2018, 129, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.M.; Woodall, N.; Harper, J.; Benger, J.; Fourth National Audit Project. Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: Intensive care and emergency departments. Br. J. Anaesth. 2011, 106, 632–642. [Google Scholar] [CrossRef]
- Cook, T.M.; Woodall, N.; Frerk, C.; Fourth National Audit Project. Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: Anaesthesia. Br. J. Anaesth. 2011, 106, 617–631. [Google Scholar] [CrossRef]
- Pluijms, W.A.; van Mook, W.N.; Wittekamp, B.H.; Bergmans, D.C. Postextubation laryngeal edema and stridor resulting in respiratory failure in critically ill adult patients: Updated review. Crit. Care 2015, 19, 295. [Google Scholar] [CrossRef]
- Engidaw, M.S.; Tekeba, B.; Amare, H.T.; Belay, K.A.; Ayana, Y.; Liyew, B. Incidence and predictors of extubation failure among adult intensive care unit patients in Northwest Amhara comprehensive specialized hospitals. Sci. Rep. 2025, 15, 20957. [Google Scholar] [CrossRef]
- Ho, U.-C.; Hsieh, C.-J.; Lu, H.-Y.; Huang, A.P.-H.; Kuo, L.-T. Predictors of extubation failure and prolonged mechanical ventilation among patients with intracerebral hemorrhage after surgery. Respir. Res. 2024, 25, 19. [Google Scholar] [CrossRef]
- Walsh, A.; Peesay, T.; Newark, A.; Shearer, S.; Parsa, K.; Pierce, M.; Gao, W.Z. Association of Severe Tongue Edema With Prone Positioning in Patients Intubated for COVID-19. Laryngoscope 2022, 132, 287–289. [Google Scholar] [CrossRef]
- Edgcombe, H.; Carter, K.; Yarrow, S. Anaesthesia in the prone position. Br. J. Anaesth. 2008, 100, 165–183. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Gordillo-Escobar, E.; Trenado, J.; Gordo, F.; Fisac, L.; García-Prieto, E.; López-Martin, C.; Abella, A.; Jiménez, J.R.; García-Garmendia, J.L.; et al. A Nationwide, Prospective Study of Tracheal Intubation in Critically Ill Adults in Spain: Management, Associated Complications, and Outcomes. Crit. Care Med. 2024, 52, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Kelly, F.E. Airway challenges in critical care. Anaesthesia 2011, 66, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, M.; Becker, T.K.; Gries, A.; Knapp, J.; Wenzel, V. The First Shot Is Often the Best Shot: First-Pass Intubation Success in Emergency Airway Management. Anesth. Analg. 2015, 121, 1389. [Google Scholar] [CrossRef]
- De Jong, A.; Molinari, N.; Terzi, N.; Mongardon, N.; Arnal, J.-M.; Guitton, C.; Allaouchiche, B.; Paugam-Burtz, C.; Constantin, J.-M.; Lefrant, J.-Y.; et al. Early identification of patients at risk for difficult intubation in the intensive care unit: Development and validation of the MACOCHA score in a multicenter cohort study. Am. J. Respir. Crit. Care Med. 2013, 187, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Apfelbaum, J.L.; Hagberg, C.A.; Connis, R.T.; Abdelmalak, B.B.; Agarkar, M.; Dutton, R.P.; Fiadjoe, J.E.; Greif, R.; Klock, P.A.; Mercier, D.; et al. 2022 American Society of Anesthesiologists Practice Guidelines for Management of the Difficult Airway. Anesthesiology 2022, 136, 31–81. [Google Scholar] [CrossRef]
- De Jong, A.; Jaber, S. Do Not Throw the Intubation Checklist out with the Bath Water! Chest 2018, 153, 771–773. [Google Scholar] [CrossRef]
- Hansel, J.; Rogers, A.M.; Lewis, S.R.; Cook, T.M.; Smith, A.F. Videolaryngoscopy versus direct laryngoscopy for adults undergoing tracheal intubation: A Cochrane systematic review and meta-analysis update. Br. J. Anaesth. 2022, 129, 612–623. [Google Scholar] [CrossRef]
- Acquisto, N.M.; Mosier, J.M.; Bittner, E.A.; Patanwala, A.E.; Hirsch, K.G.; Hargwood, P.; Oropello, J.M.; Bodkin, R.P.; Groth, C.M.; Kaucher, K.A. Society of Critical Care Medicine Clinical Practice Guidelines for Rapid Sequence Intubation in the Critically Ill Adult Patient. Crit. Care Med. 2023, 51, 1411–1430. [Google Scholar] [CrossRef] [PubMed]
- De Jong, A.; Myatra, S.N.; Roca, O.; Jaber, S. How to improve intubation in the intensive care unit. Update on knowledge and devices. Intensive Care Med. 2022, 48, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Janz, D.R.; Semler, M.W.; Joffe, A.M.; Casey, J.D.; Lentz, R.J.; de Boisblanc, B.P.; Khan, Y.A.; Santanilla, J.I.; Bentov, I.; Rice, T.W.; et al. A Multicenter Randomized Trial of a Checklist for Endotracheal Intubation of Critically Ill Adults. Chest 2018, 153, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Azam Danish, M. Preoxygenation and Anesthesia: A Detailed Review. Cureus 2021, 13, e13240. [Google Scholar] [CrossRef]
- Nimmagadda, U.; Salem, M.R.; Crystal, G.J. Preoxygenation: Physiologic Basis, Benefits, and Potential Risks. Anesth. Analg. 2017, 124, 507–517. [Google Scholar] [CrossRef]
- Mosier, J.M.; Hypes, C.D.; Sakles, J.C. Understanding preoxygenation and apneic oxygenation during intubation in the critically ill. Intensive Care Med. 2017, 43, 226–228. [Google Scholar] [CrossRef]
- Puig, J.; Charco, P.; Reviriego, L.; Belda, F.J. Is preoxygenation still important? New concepts. Trends Anaesth. Crit. Care 2017, 16, 46–53. [Google Scholar] [CrossRef]
- Delay, J.M.; Sebbane, M.; Jung, B.; Nocca, D.; Verzilli, D.; Pouzeratte, Y.; El Kamel, M.; Fabre, J.M.; Eledjam, J.J.; Jaber, S. The effectiveness of noninvasive positive pressure ventilation to enhance preoxygenation in morbidly obese patients: A randomized controlled study. Anesth. Analg. 2008, 107, 1707–1713. [Google Scholar] [CrossRef]
- Hagberg, C.A. (Ed.) Preoxygenation. In Benumof and Hagberg’s Airway Management (Third Edition); W.B. Saunders: Philadelphia, PA, USA, 2013; pp. i–iii. [Google Scholar] [CrossRef]
- Charles, A.; Jaffre, S.; Lakhal, K.; Cinotti, R.; Lejus-Bourdeau, C. Evaluation of preoxygenation devices using a lung simulator mimicking normal adult spontaneous breathing. Anaesth. Crit. Care Pain Med. 2024, 43, 101378. [Google Scholar] [CrossRef]
- Pitre, T.; Liu, W.; Zeraatkar, D.; Dionne, J.C.; Gibbs, K.W.; Ginde, A.A.; Needham-Nethercott, N.; Rice, T.W.; Semler, M.W.; Rochwerg, B. Preoxygenation strategies for intubation of patients who are critically ill: A systematic review and network meta-analysis of randomised trials. Lancet Respir. Med. 2025, 13, 585–596. [Google Scholar] [CrossRef]
- Baillard, C.; Fosse, J.-P.; Sebbane, M.; Chanques, G.; Vincent, F.; Courouble, P.; Cohen, Y.; Eledjam, J.-J.; Adnet, F.; Jaber, S. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am. J. Respir. Crit. Care Med. 2006, 174, 171–177. [Google Scholar] [CrossRef]
- Gibbs, K.W.; Semler, M.W.; Driver, B.E.; Seitz, K.P.; Stempek, S.B.; Taylor, C.; Resnick-Ault, D.; White, H.D.; Gandotra, S.; Doerschug, K.C.; et al. Noninvasive Ventilation for Preoxygenation during Emergency Intubation. N. Engl. J. Med. 2024, 390, 2165–2177. [Google Scholar] [CrossRef]
- Frat, J.-P.; Grieco, D.L.; De Jong, A.; Gibbs, K.; Carteaux, G.; Roca, O.; Lemiale, V.; Piquilloud, L.; Rittayamai, N.; Pisani, L.; et al. Noninvasive respiratory supports in ICU. Intensive Care Med. 2025, 51, 1476–1489. [Google Scholar] [CrossRef]
- Frat, J.-P.; Ricard, J.-D.; Quenot, J.-P.; Pichon, N.; Demoule, A.; Forel, J.-M.; Mira, J.-P.; Coudroy, R.; Berquier, G.; Voisin, B.; et al. Non-invasive ventilation versus high-flow nasal cannula oxygen therapy with apnoeic oxygenation for preoxygenation before intubation of patients with acute hypoxaemic respiratory failure: A randomised, multicentre, open-label trial. Lancet Respir. Med. 2019, 7, 303–312. [Google Scholar] [CrossRef]
- Pensier, J.; Guerrero, M.A.; Berger-Estilita, J.; Borgstedt, L.; Zaher, A.M.S.; Jaber, S.; De Jong, A. Perioperative ventilation support, what clinicians and searchers must know. Anaesth. Crit. Care Pain. Med. 2025, 44, 101554. [Google Scholar] [CrossRef] [PubMed]
- Guitton, C.; Ehrmann, S.; Volteau, C.; Colin, G.; Maamar, A.; Jean-Michel, V.; Mahe, P.-J.; Landais, M.; Brule, N.; Bretonnière, C.; et al. Nasal high-flow preoxygenation for endotracheal intubation in the critically ill patient: A randomized clinical trial. Intensive Care Med. 2019, 45, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Jaber, S.; Monnin, M.; Girard, M.; Conseil, M.; Cisse, M.; Carr, J.; Mahul, M.; Delay, J.M.; Belafia, F.; Chanques, G.; et al. Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: The single-centre, blinded, randomised controlled OPTINIV trial. Intensive Care Med. 2016, 42, 1877–1887. [Google Scholar] [CrossRef]
- Jaber, S.; De Jong, A.; Schaefer, M.S.; Zhang, J.; Ma, X.; Hao, X.; Zhou, S.; Lv, S.; Banner-Goodspeed, V.; Niu, X.; et al. Preoxygenation with standard facemask combining apnoeic oxygenation using high flow nasal cannula versuss standard facemask alone in patients with and without obesity: The OPTIMASK international study. Ann. Intensive Care 2023, 13, 26. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Kumar, P.; Jafra, A.; Thakur, H.; Yaddanapudi, L.N.; Jain, K. Peri-Intubation Hypoxia Af-ter Delayed Versus Rapid Sequence Intubation in Critically Injured Patients on Arrival to Trauma Triage: A Randomized Controlled Trial. Anesth. Analg. 2023, 136, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Semler, M.W.; Janz, D.R.; Lentz, R.J.; Matthews, D.T.; Norman, B.C.; Assad, T.R.; Keriwala, R.D.; Ferrell, B.A.; Noto, M.J.; McKown, A.C.; et al. Randomized Trial of Apneic Oxygenation during Endotracheal Intubation of the Critically Ill. Am. J. Respir. Crit. Care Med. 2016, 193, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.D.; Janz, D.R.; Russell, D.W.; Vonderhaar, D.J.; Joffe, A.M.; Dischert, K.M.; Brown, R.M.; Zouk, A.N.; Gulati, S.; Heideman, B.E.; et al. Bag-Mask Ventilation during Tracheal Intubation of Critically Ill Adults. N. Engl. J. Med. 2019, 380, 811–821. [Google Scholar] [CrossRef]
- Vourc’h, M.; Asfar, P.; Volteau, C.; Bachoumas, K.; Clavieras, N.; Egreteau, P.-Y.; Asehnoune, K.; Mercat, A.; Reignier, J.; Jaber, S.; et al. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: A randomized controlled clinical trial. Intensive Care Med. 2015, 41, 1538–1548. [Google Scholar] [CrossRef]
- Vourc’h, M.; Baud, G.; Feuillet, F.; Blanchard, C.; Mirallie, E.; Guitton, C.; Jaber, S.; Asehnoune, K. High-flow Nasal Cannulae Versus Non-invasive Ventilation for Preoxygenation of Obese Patients: The PREOPTIPOP Randomized Trial. eClinicalMedicine 2019, 13, 112–119. [Google Scholar] [CrossRef]
- Rodriguez, M.; Ragot, S.; Coudroy, R.; Quenot, J.-P.; Vignon, P.; Forel, J.-M.; Demoule, A.; Mira, J.-P.; Ricard, J.-D.; Nseir, S.; et al. Noninvasive ventilation vs. high-flow nasal cannula oxygen for preoxygenation before intubation in patients with obesity: A post hoc analysis of a randomized controlled trial. Ann. Intensive Care 2021, 11, 114. [Google Scholar] [CrossRef]
- Fayed, M.; Maroun, W.; Patel, N.; Galusca, D. Apneic Oxygenation: A Summarized Review and Stepwise Approach. Cureus 2023, 15, e50916. [Google Scholar] [CrossRef] [PubMed]
- Lyons, C.; Callaghan, M. Uses and mechanisms of apnoeic oxygenation: A narrative review. Anaesthesia 2019, 74, 497–507. [Google Scholar] [CrossRef]
- Corl, K.A.; Dado, C.; Agarwal, A.; Azab, N.; Amass, T.; Marks, S.J.; Levy, M.M.; Merchant, R.C.; Aliotta, J. A modified Montpellier protocol for intubating intensive care unit patients is associated with an increase in first-pass intubation success and fewer complications. J. Crit. Care 2018, 44, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.S.; Bucca, A.W.; Propst, S.L.; Ellender, T.J.; Sarmiento, E.J.; Menard, L.M.; Hunter, B.R. Association of Checklist Use in Endotracheal Intubation With Clinically Important Outcomes: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e209278. [Google Scholar] [CrossRef]
- Monet, C.; Capdevila, M.; Lakbar, I.; Cuny, A.; Raimbert, C.; Briane, C.; Jaber, S.; De Jong, A. Intubation difficile en réanimation. Méd Intensive Réa 2025, 34. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monet, C.; Capdevila, M.; Lakbar, I.; Aarab, Y.; Pensier, J.; De Jong, A.; Jaber, S. Preoxygenation in the ICU. J. Clin. Med. 2025, 14, 7305. https://doi.org/10.3390/jcm14207305
Monet C, Capdevila M, Lakbar I, Aarab Y, Pensier J, De Jong A, Jaber S. Preoxygenation in the ICU. Journal of Clinical Medicine. 2025; 14(20):7305. https://doi.org/10.3390/jcm14207305
Chicago/Turabian StyleMonet, Clément, Mathieu Capdevila, Inès Lakbar, Yassir Aarab, Joris Pensier, Audrey De Jong, and Samir Jaber. 2025. "Preoxygenation in the ICU" Journal of Clinical Medicine 14, no. 20: 7305. https://doi.org/10.3390/jcm14207305
APA StyleMonet, C., Capdevila, M., Lakbar, I., Aarab, Y., Pensier, J., De Jong, A., & Jaber, S. (2025). Preoxygenation in the ICU. Journal of Clinical Medicine, 14(20), 7305. https://doi.org/10.3390/jcm14207305