One Shot, One Rhythm: Termination of Refractory Persistent Atrial Fibrillation in a Young Patient via Single Pulmonary Vein Application: A Case Report
Abstract
1. Introduction
2. Materials and Methods
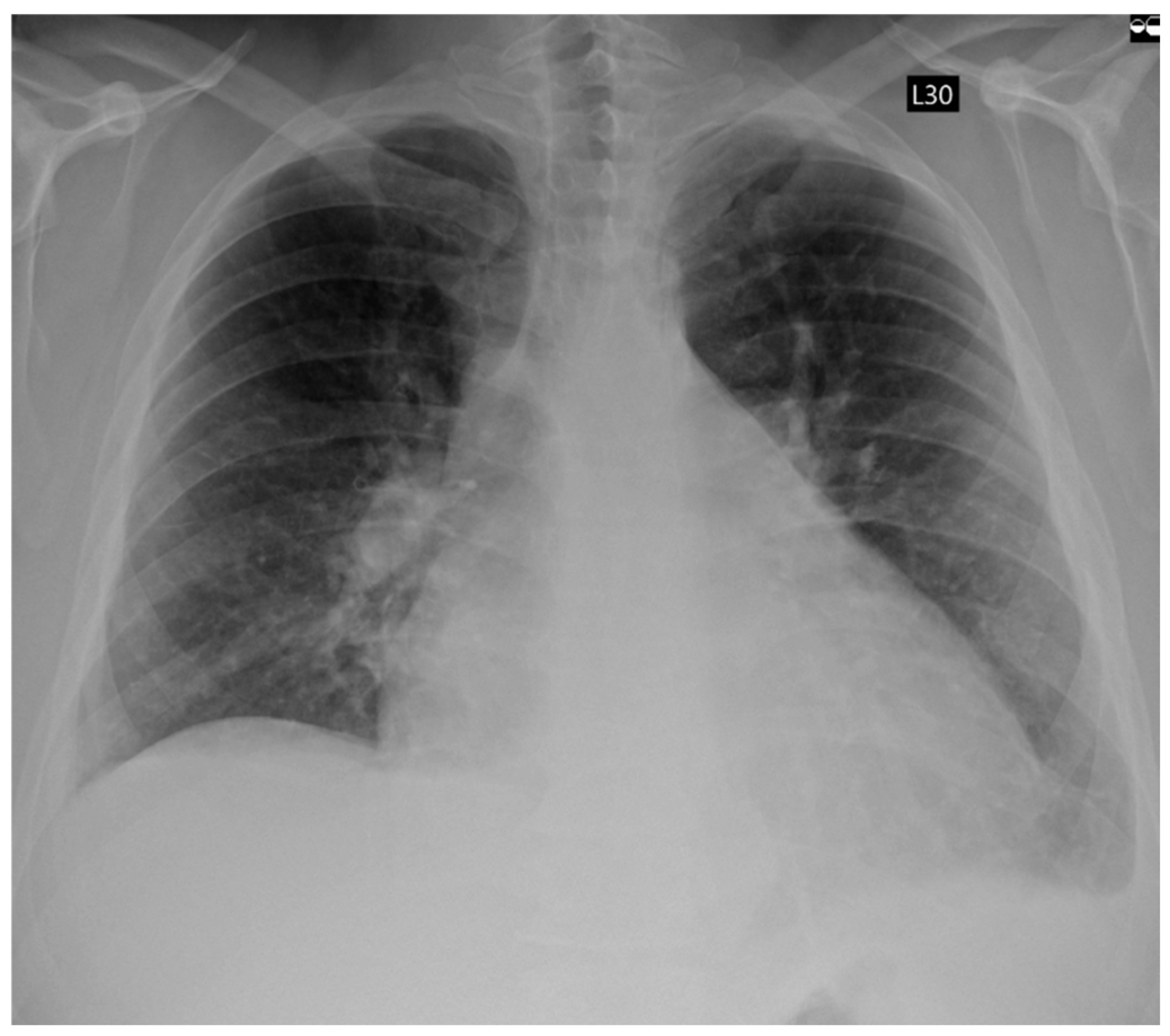
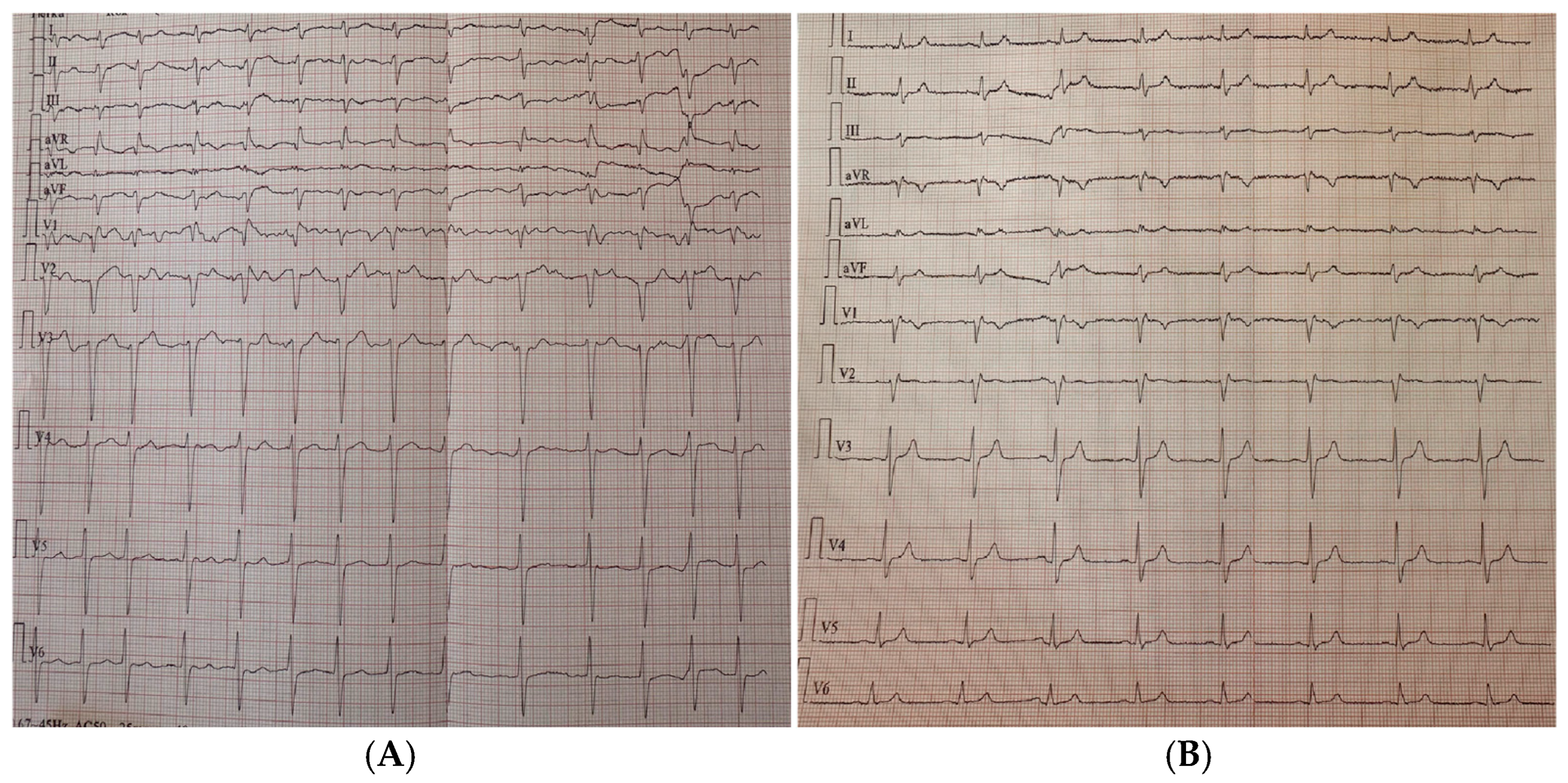
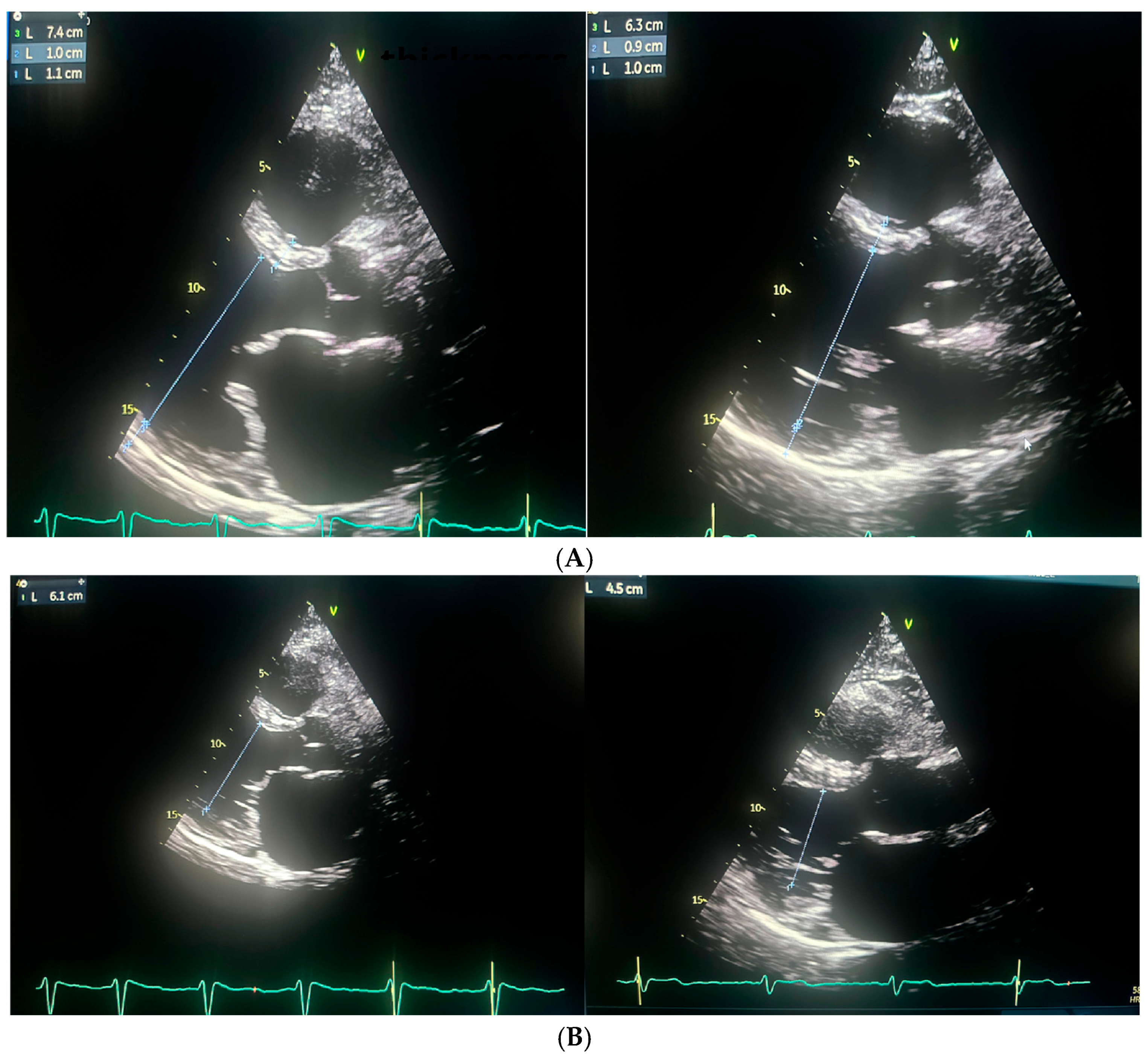
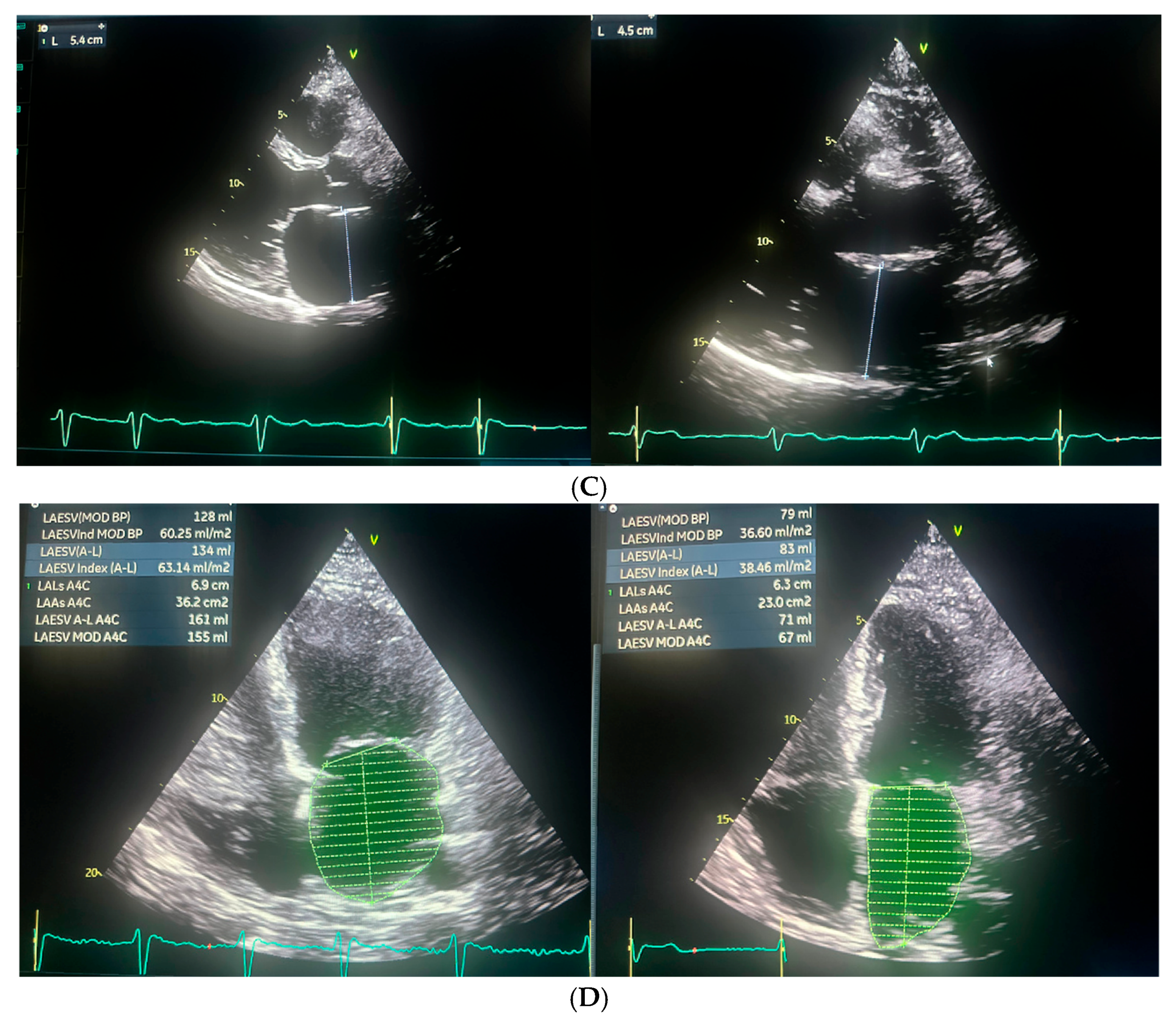
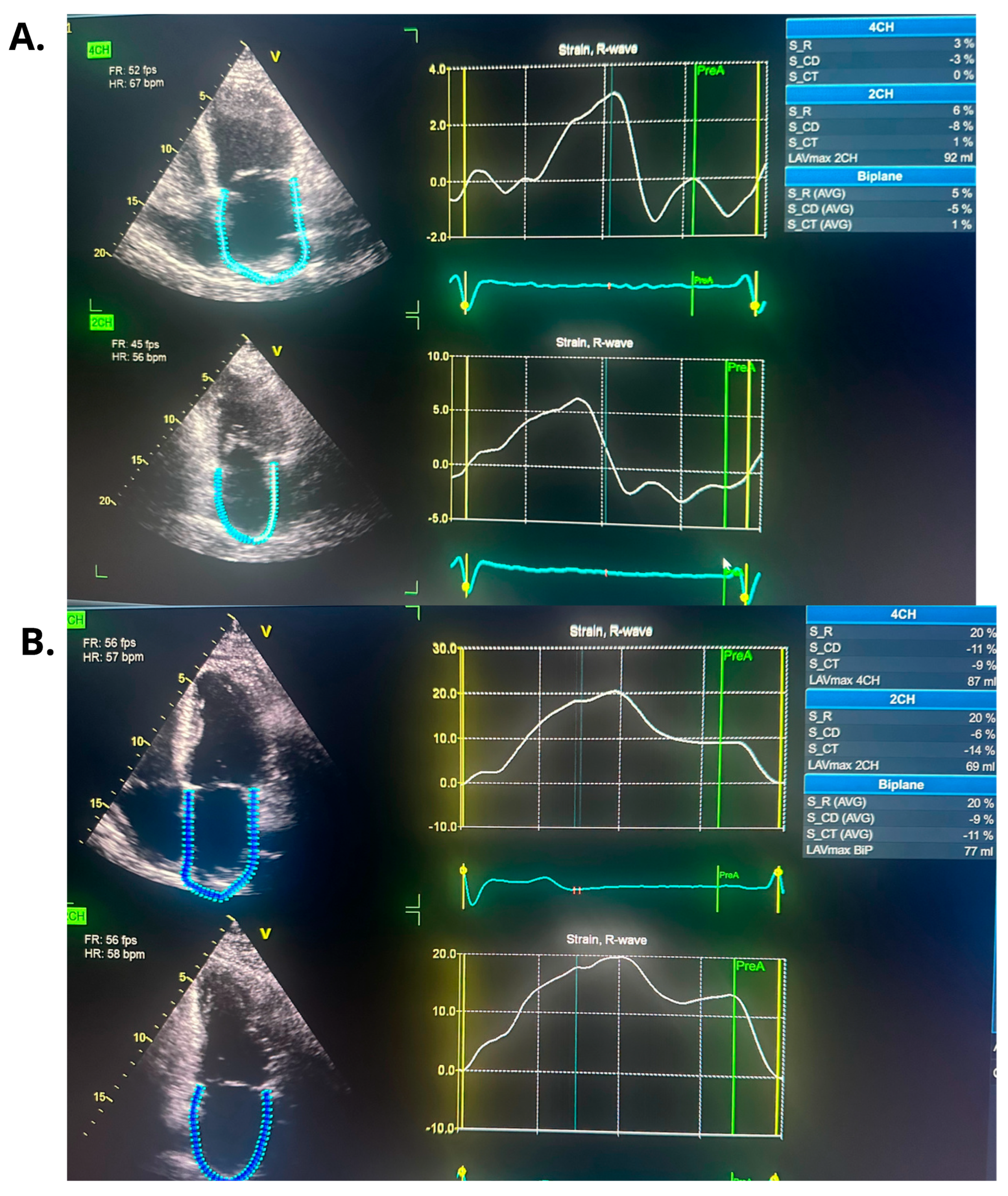
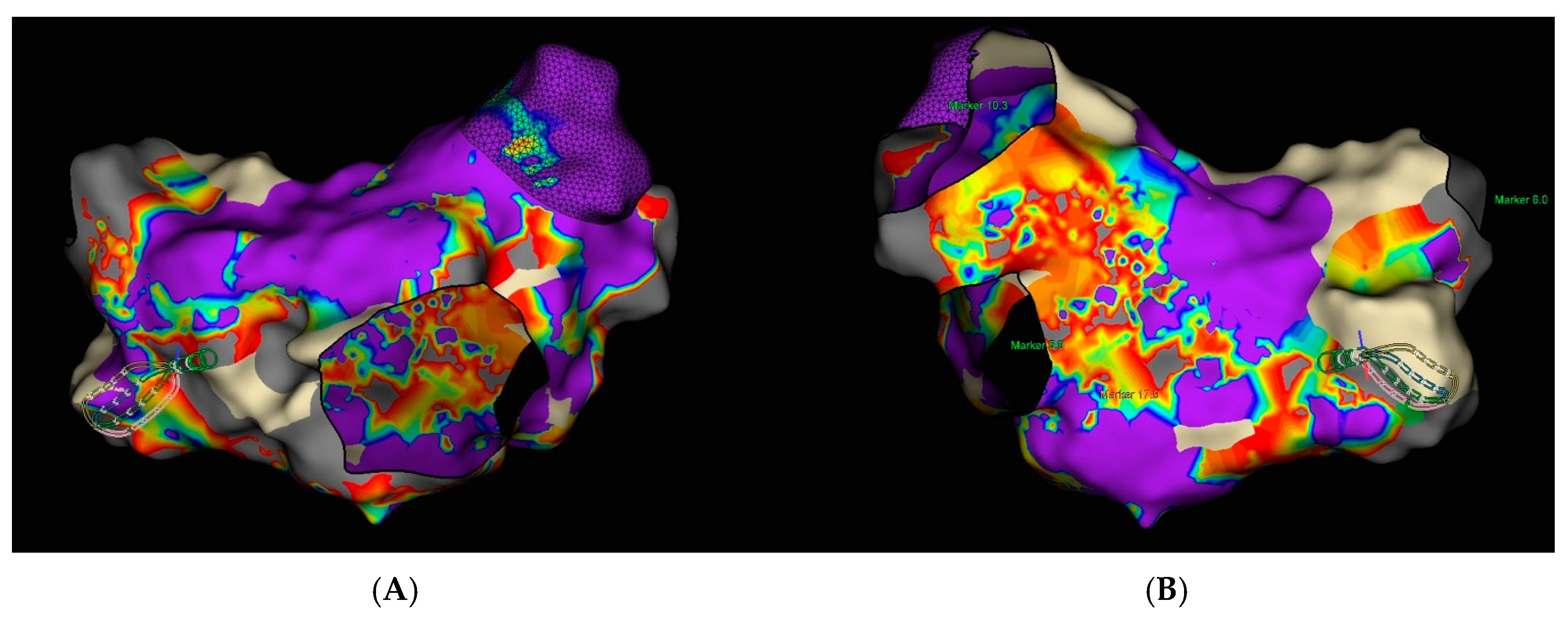
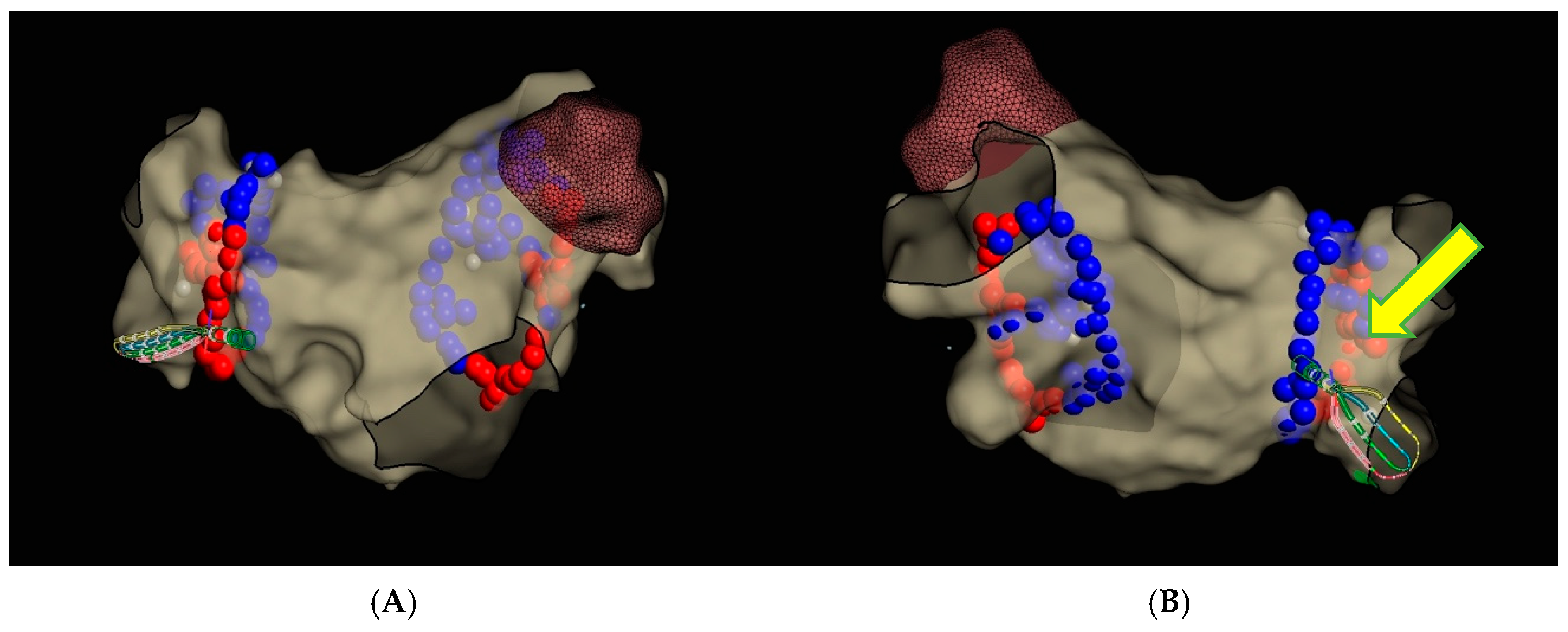
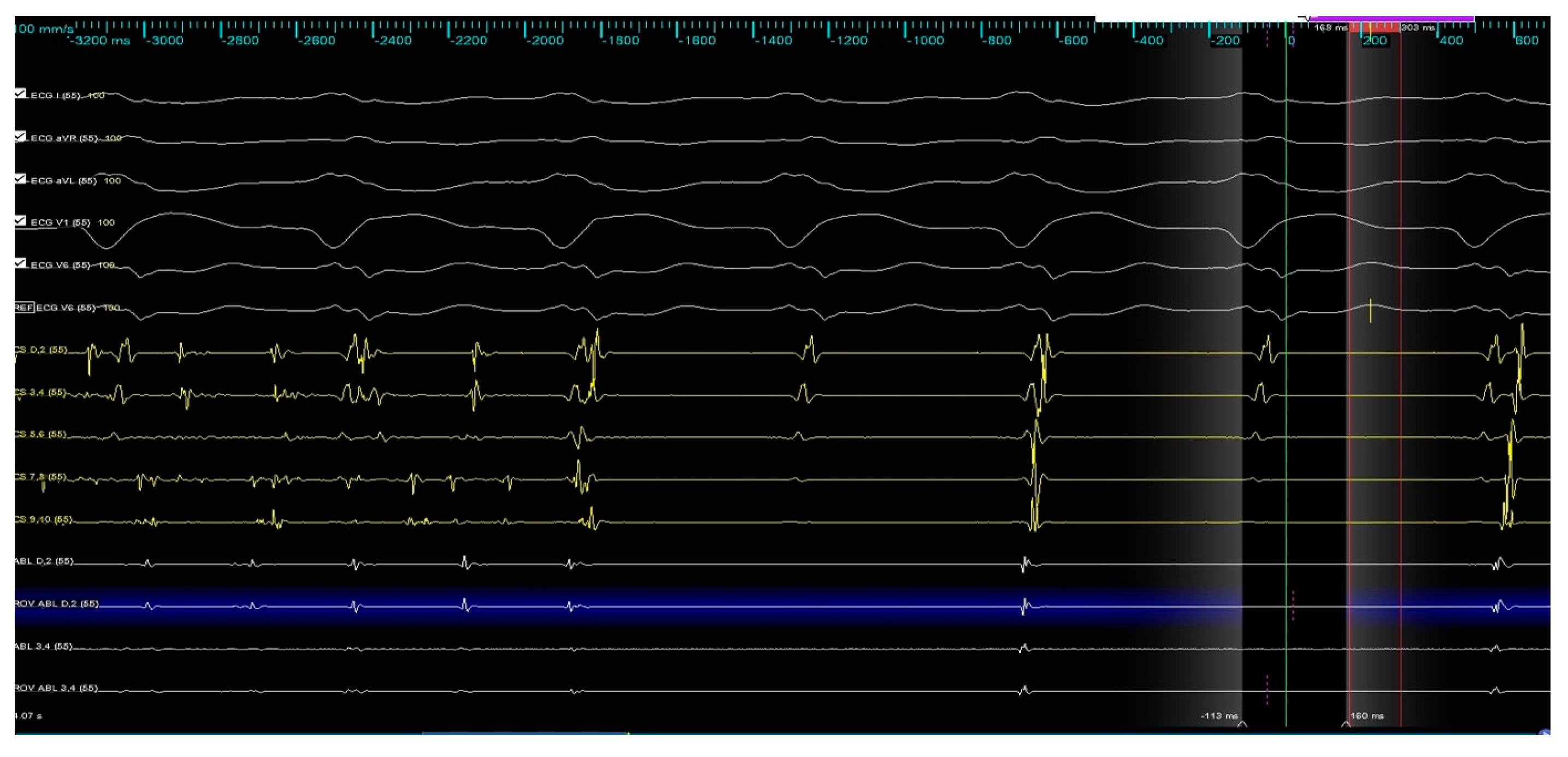
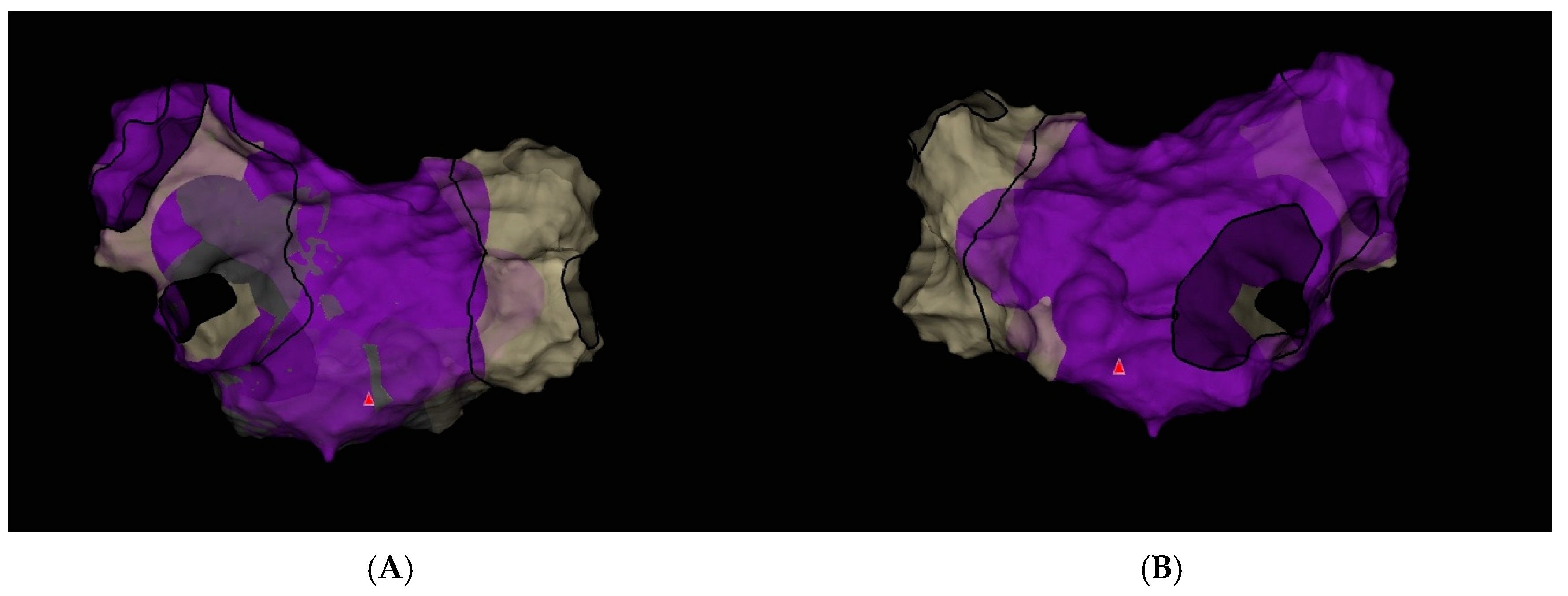
3. Case Report
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, H.; Zhang, Q.; Much, A.A.; Maor, E.; Segev, A.; Beinart, R.; Adawi, S.; Lu, Y.; Bragazzi, N.L.; Wu, J. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: Results from the Global Burden of Disease Study 2017. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 574–582. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tzeis, S.; Gerstenfeld, E.P.; Kalman, J.; Saad, E.B.; Shamloo, A.S.; Andrade, J.G.; Barbhaiya, C.R.; Baykaner, T.; Boveda, S.; Calkins, H.; et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm 2024, 21, e31–e149. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Jiang, C.Y.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Johner, N.; Namdar, M.; Shah, D.C. Individualised approaches for catheter ablation of AF: Patient selection and procedural endpoints. Arrhythmia Electrophysiol. Rev. 2019, 8, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Rolf, S.; Kircher, S.; Arya, A.; Eitel, C.; Sommer, P.; Richter, S.; Gaspar, T.; Bollmann, A.; Altmann, D.; Piedra, C.; et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ. Arrhythmia Electrophysiol. 2014, 7, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Gaspar, T.; Schönbauer, R.; Wójcik, M.; Fiedler, L.; Roithinger, F.X.; Martinek, M.; Pürerfellner, H.; Kirstein, B.; Richter, U.; et al. Low-Voltage Myocardium-Guided Ablation Trial of Persistent Atrial Fibrillation. NEJM Evid. 2022, 1, EVIDoa2200141. [Google Scholar] [CrossRef] [PubMed]
- Rappel, W.J.; Zaman, J.A.; Narayan, S.M. Mechanisms for the termination of atrial fibrillation by localized ablation: Computational and clinical studies. Circ. Arrhythmia Electrophysiol. 2015, 8, 1325–1333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elayi, C.S.; Di Biase, L.; Barrett, C.; Ching, C.K.; al Aly, M.; Lucciola, M.; Bai, R.; Horton, R.; Fahmy, T.S.; Verma, A.; et al. Atrial fibrillation termination as a procedural endpoint during ablation in long-standing persistent atrial fibrillation. Heart Rhythm 2010, 7, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Liu, X.; Zhang, Y.; Jiang, W.F.; Zhou, L.; Qin, M.; Zhang, D.; Zhang, X.; Wu, S.; Xu, K. Optimal endpoint for catheter ablation of longstanding persistent atrial fibrillation: A randomized clinical trial. Pacing Clin. Electrophysiol. 2018, 41, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017, 14, e275–e444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wyler von Ballmoos, M.C.; Hui, D.S.; Mehaffey, J.H.; Malaisrie, S.C.; Vardas, P.N.; Gillinov, A.M.; Sundt, T.M.; Badhwar, V. The Society of Thoracic Surgeons 2023 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann. Thorac. Surg. 2024, 118, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Herweg, B.; Kowalski, M.; Steinberg, J.S. Termination of persistent atrial fibrillation resistant to cardioversion by a single radiofrequency application. Pacing Clin. Electrophysiol. 2003, 26, 1420–1423. [Google Scholar] [CrossRef] [PubMed]
- Dasí, A.; Nagel, C.; Pope, M.T.B.; Wijesurendra, R.S.; Betts, T.R.; Sachetto, R.; Loewe, A.; Bueno-Orovio, A.; Rodriguez, B. In Silico TRials Guide Optimal Stratification of ATrIal FIbrillation Patients to Catheter Ablation and Pharmacological medicaTION: The i-STRATIFICATION Study. Europace 2024, 26, euad150. [Google Scholar] [CrossRef] [PubMed]
- Deisenhofer, I.; Albenque, J.-P.; Busch, S.; Gitenay, E.; Mountantonakis, S.E.; Roux, A.; Horvilleur, J.; Bakouboula, B.; Oza, S.; Abbey, S.; et al. Artificial Intelligence for Individualized Treatment of Persistent Atrial Fibrillation: A Randomized Controlled Trial. Nat. Med. 2025, 31, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Sotomi, Y.; Hikoso, S.; Nakatani, D.; Mizuno, H.; Okada, K.; Dohi, T.; Kitamura, T.; Sunaga, A.; Kida, H.; et al. DR-FLASH Score Is Useful for Identifying Patients With Persistent Atrial Fibrillation Who Require Extensive Catheter Ablation Procedures. J. Am. Heart Assoc. 2022, 11, e024916. [Google Scholar] [CrossRef] [PubMed]
- Providência, R.; Faustino, A.; Ferreira, M.J.; Gonçalves, L.; Trigo, J.; Botelho, A.; Barra, S.; Boveda, S. Evaluation of left atrial deformation to predict left atrial stasis in patients with non-valvular atrial fibrillation—A pilot-study. Cardiovasc. Ultrasound 2013, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Negishi, K.; Kurosawa, K.; Tateno, R.; Tange, S.; Arai, M.; Amano, M.; Kurabayashi, M. Left atrial strain provides incremental value for embolism risk stratification over CHA2DS2-VASc score and indicates prognostic impact in patients with atrial fibrillation. J. Am. Soc. Echocardiogr. 2014, 27, 709–716.e4. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, A.; Genovesi, S.; Sonaglioni, A.; Binda, G.; Rigamonti, E.; Lombardo, M.; Anzà, C. Mechanical atrial recovery after cardioversion in persistent atrial fibrillation evaluated by bidimensional speckle tracking echocardiography. J. Cardiovasc. Med. 2019, 20, 745–751. [Google Scholar] [CrossRef] [PubMed]
| Ablation Parameters | Value |
|---|---|
| Skin to skin time (s) | 168 |
| Left atrium dwell time (s) | 141 |
| Ablation time (s) | 83 |
| Mean ACT time (s) | 310 |
| Fluoroscopy time (s) | 682 |
| Dose area product (mG) | 215 |
| General Parameters | First Admission | Ablation | Follow-Up |
|---|---|---|---|
| Day of analysis | 0 | 60 | 529 |
| Weight (kg) | 123 | 98 | 100 |
| BMI (kg/m2) | 40.16 | 32 | 32.65 |
| NYHA class | IV | III | I |
| EHRA | IV | III | I |
| NTproBNP (pg/pL) | 3948 | 4164 | 213 |
| Hemoglobin (g/dL) | 14.6 | 17.3 | 14.8 |
| Creatinine (mg/dL) | 1.06 | 1.2 | 0.99 |
| Potassium (mmol/L) | 4.5 | 4.2 | 4.4 |
| CRP (mg/L) | 12.2 | 4.9 | 0.8 |
| Echocardiographic Parameters | First Admission | Ablation | Follow-Up |
|---|---|---|---|
| LVEF (%) | 25 | 27 | 54 |
| LVEDD (mm) | 61 | 74 | 63 |
| LVESD (mm) | 55 | 61 | 45 |
| LA (mm) | 48 | 54 | 45 |
| LAA (cm2) | - | 36.2 | 23 |
| LAVI (ml/m2) | - | 60.25 | 36.6 |
| RAA (cm2) | - | 22.8 | 21.5 |
| TAPSE (mm) | - | 18 | 23 |
| PALS (%) | - | 5 | 20 |
| RVSP (mmHg) | 37 | 40 | - |
| Component | Points |
|---|---|
| Diabetes mellitus | 1 |
| Renal dysfunction | 1 |
| Persistent atrial fibrillation | 1 |
| Left atrial diameter > 45 mm | 1 |
| Age > 65 years | 1 |
| Female sex | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozielski, J.; Dąbrowska-Kugacka, A.; Daniłowicz-Szymanowicz, L.; Szołkiewicz, M. One Shot, One Rhythm: Termination of Refractory Persistent Atrial Fibrillation in a Young Patient via Single Pulmonary Vein Application: A Case Report. J. Clin. Med. 2025, 14, 7297. https://doi.org/10.3390/jcm14207297
Kozielski J, Dąbrowska-Kugacka A, Daniłowicz-Szymanowicz L, Szołkiewicz M. One Shot, One Rhythm: Termination of Refractory Persistent Atrial Fibrillation in a Young Patient via Single Pulmonary Vein Application: A Case Report. Journal of Clinical Medicine. 2025; 14(20):7297. https://doi.org/10.3390/jcm14207297
Chicago/Turabian StyleKozielski, Jonasz, Alicja Dąbrowska-Kugacka, Ludmiła Daniłowicz-Szymanowicz, and Marek Szołkiewicz. 2025. "One Shot, One Rhythm: Termination of Refractory Persistent Atrial Fibrillation in a Young Patient via Single Pulmonary Vein Application: A Case Report" Journal of Clinical Medicine 14, no. 20: 7297. https://doi.org/10.3390/jcm14207297
APA StyleKozielski, J., Dąbrowska-Kugacka, A., Daniłowicz-Szymanowicz, L., & Szołkiewicz, M. (2025). One Shot, One Rhythm: Termination of Refractory Persistent Atrial Fibrillation in a Young Patient via Single Pulmonary Vein Application: A Case Report. Journal of Clinical Medicine, 14(20), 7297. https://doi.org/10.3390/jcm14207297