Dysregulation of Treg/Th17 Balance and Intracellular Expression of IL-21 and IL-22 in the Pathogenesis of Gestational Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Group Characteristics
- Chronic hypertension,
- Kidney and liver disease,
- Autoimmune diseases,
- Active or previous cancer,
- Multiple pregnancies,
- Preterm births,
- Evidence of systemic infection at the time of blood sampling.
2.2. Biological Sample Collection
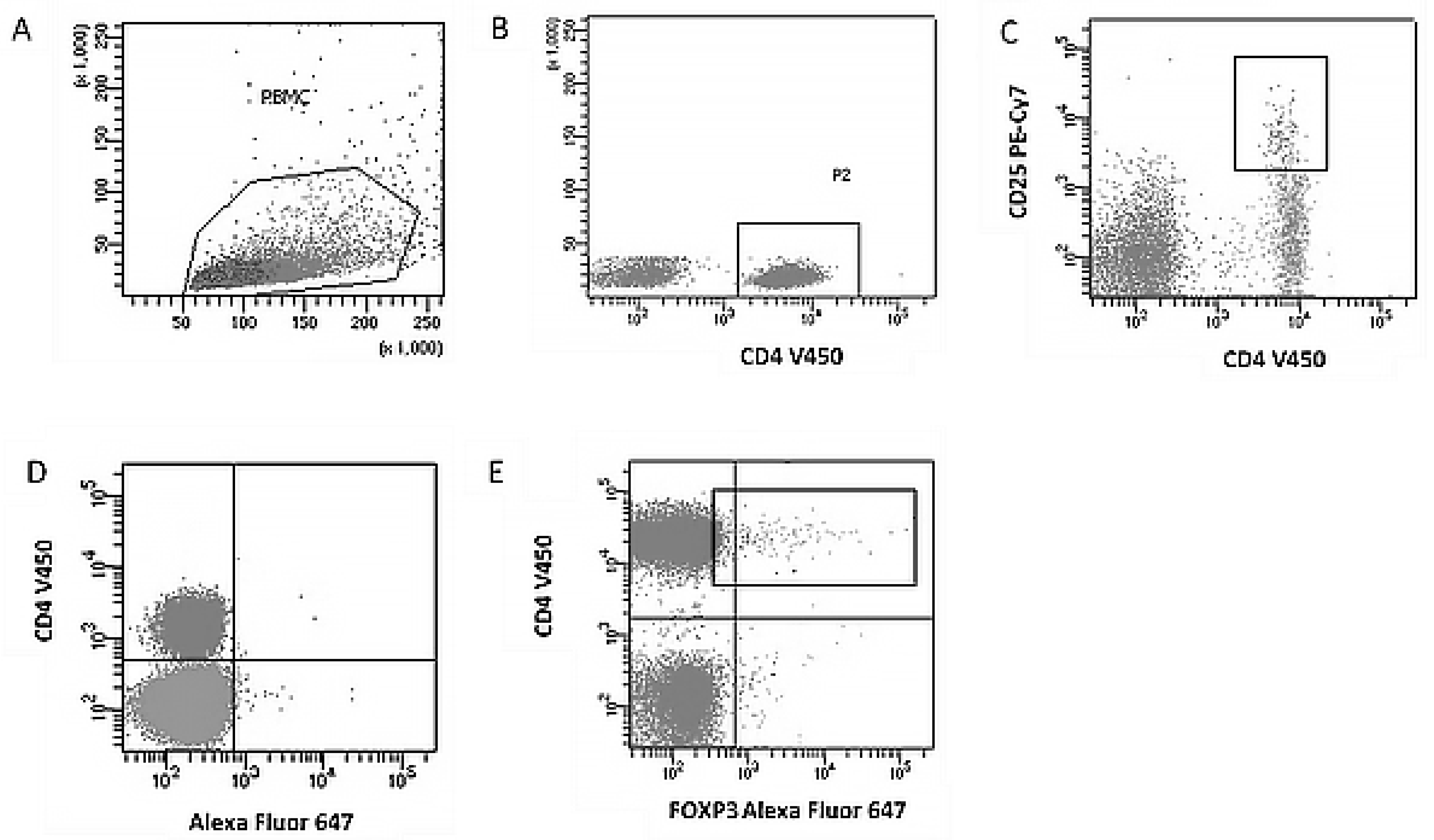
2.3. Immunophenotyping of Regulatory T Cells (Treg)
- Alexa Fluor® 647 Mouse anti-Human FoxP3 (BD Biosciences, San Diego, CA, USA),
- V450 Mouse anti-Human CD4 (BD Biosciences, San Diego, CA, USA),
- PE-Cy™7 Mouse anti-Human CD25 (BD Biosciences, San Diego, CA, USA).
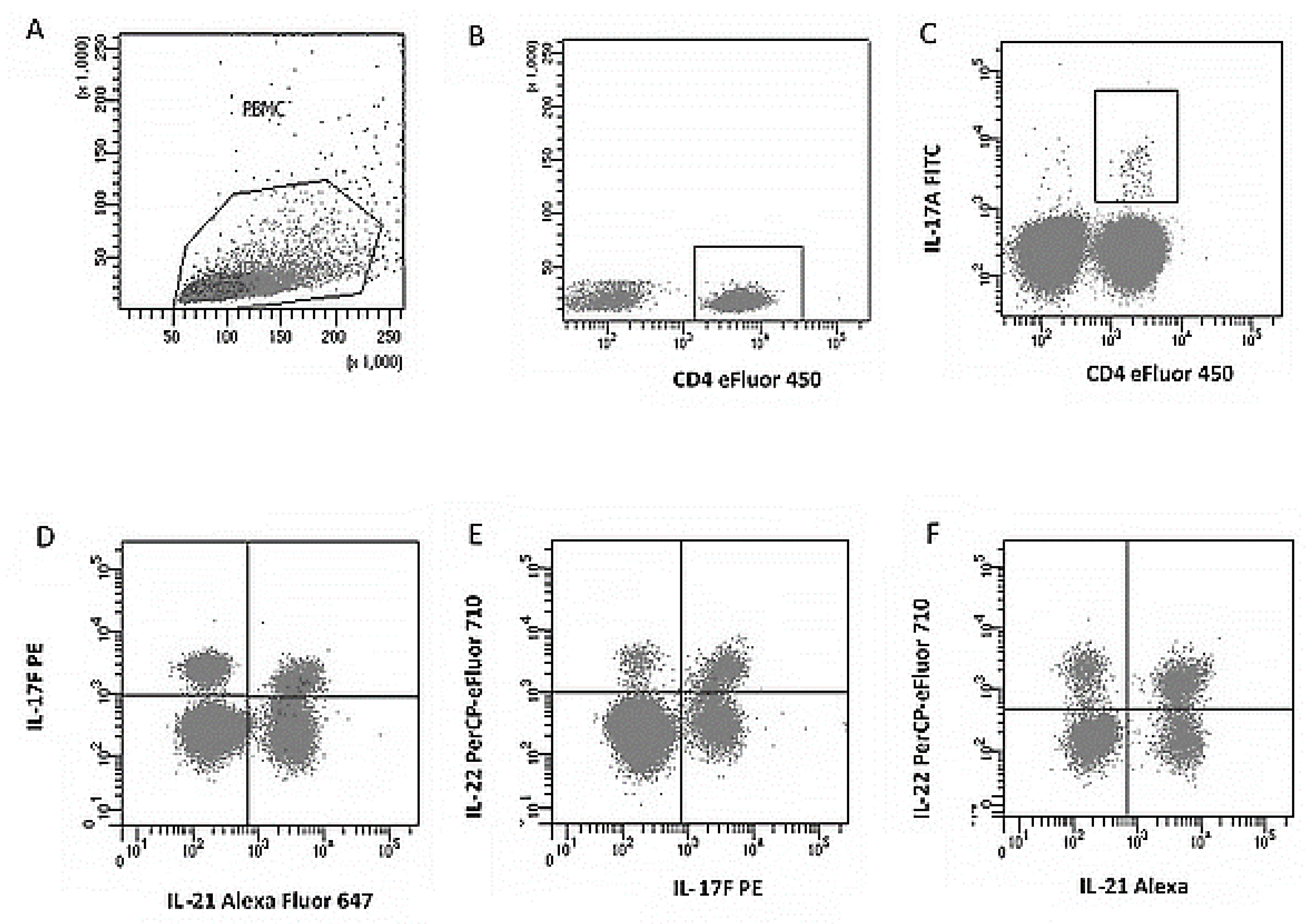
2.4. Analysis of Th17 Subpopulations and Cytokine Expression
- FITC anti-human IL-17A (eBio64Dec17),
- PE anti-human IL-17F (SHLR17),
- PerCP-eFluor® 710 anti-human IL-22 (22URTI),
- Alexa Fluor® 647 anti-human IL-21 (FFA21),
- eFluor® 450 anti-human CD4 (RPA-T4).
2.5. Statistical Analysis
- Mann–Whitney U test—for comparisons between groups.
- Spearman’s rank correlation test—for the analysis of relationships between continuous variables. The strength of the correlation between two variables in a given test was described based on the correlation coefficient (rho). Rho values between 0.1 and 0.3 are considered weak. Values between 0.3 and 0.5 are considered moderate, while coefficients above 0.5 suggest a strong correlation.
- Categorical data were presented as absolute numbers and percentages (%).
3. Results
3.1. Clinical and Demographic Characteristics of the Patients, Family History, and Blood Pressure Levels
3.2. Immune Profile—Th17 and Treg Lymphocyte Subpopulations
3.3. Correlation Analysis Between Immunological Parameters and Demographic and Clinical Variables
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| L-17A | Interleukin-17A |
| IL-17F | Interleukin-17F |
| IL-21 | Interleukin-21 |
| IL-22 | Interleukin-22 |
| PBMC | Peripheral Blood Mononuclear Cells |
| PE | Preeclampsia |
| PIH | Pregnancy-Induced Hypertension |
| Th17 | T helper 17 cells |
| Treg | Regulatory T cells |
References
- Krop, J.; Heidt, S.; Claas, F.H.J.; Eikmans, M. Regulatory T Cells in Pregnancy: It Is Not All About FoxP3. Front. Immunol. 2020, 11, 1182. [Google Scholar] [CrossRef]
- Liu, W.; Almo, S.C.; Zang, X. Co-Stimulate or Co-Inhibit Regulatory T Cells, Which Side to Go? Immunol. Investig. 2016, 45, 813–831. [Google Scholar] [CrossRef]
- Saito, S.; Nakashima, A.; Shima, T.; Ito, M. Th1/Th2/Th17 and Regulatory T-Cell Paradigm in Pregnancy. Am. J. Reprod. Immunol. 2010, 63, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Ma, X. (Ed.) Regulation of Cytokine Gene Expression in Immunity and Diseases. In Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2016; ISBN 978-94-024-0919-2. [Google Scholar]
- Figueiredo, A.S.; Schumacher, A. The T Helper Type 17/Regulatory T Cell Paradigm in Pregnancy. Immunology 2016, 148, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. The Role of Transforming Growth Factor β in T Helper 17 Differentiation. Immunology 2018, 155, 24–35. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Wing, K.; Miyara, M. Regulatory T Cells—A Brief History and Perspective. Eur. J. Immunol. 2007, 37 (Suppl. S1), S116–123. [Google Scholar] [CrossRef]
- Hosseini, A.; Dolati, S.; Hashemi, V.; Abdollahpour-Alitappeh, M.; Yousefi, M. Regulatory T and T Helper 17 Cells: Their Roles in Preeclampsia. J. Cell Physiol. 2018, 233, 6561–6573. [Google Scholar] [CrossRef]
- Xu, L.; Kitani, A.; Fuss, I.; Strober, W. Cutting Edge: Regulatory T Cells Induce CD4+CD25-Foxp3- T Cells or Are Self-Induced to Become Th17 Cells in the Absence of Exogenous TGF-Beta. J. Immunol. 2007, 178, 6725–6729. [Google Scholar] [CrossRef]
- Ziegler, S.F.; Buckner, J.H. FOXP3 and the Regulation of Treg/Th17 Differentiation. Microbes Infect. 2009, 11, 594–598. [Google Scholar] [CrossRef]
- Korn, T.; Oukka, M.; Kuchroo, V.; Bettelli, E. Th17 Cells: Effector T Cells with Inflammatory Properties. Semin. Immunol. 2007, 19, 362–371. [Google Scholar] [CrossRef]
- Tiemessen, M.M.; Jagger, A.L.; Evans, H.G.; van Herwijnen, M.J.C.; John, S.; Taams, L.S. CD4+CD25+Foxp3+ Regulatory T Cells Induce Alternative Activation of Human Monocytes/Macrophages. Proc. Natl. Acad. Sci. USA 2007, 104, 19446–19451. [Google Scholar] [CrossRef]
- Zhou, L.; Lopes, J.E.; Chong, M.M.W.; Ivanov, I.I.; Min, R.; Victora, G.D.; Shen, Y.; Du, J.; Rubtsov, Y.P.; Rudensky, A.Y.; et al. TGF-Beta-Induced Foxp3 Inhibits T(H)17 Cell Differentiation by Antagonizing RORgammat Function. Nature 2008, 453, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Borsellino, G.; Kleinewietfeld, M.; Di Mitri, D.; Sternjak, A.; Diamantini, A.; Giometto, R.; Höpner, S.; Centonze, D.; Bernardi, G.; Dell’Acqua, M.L.; et al. Expression of Ectonucleotidase CD39 by Foxp3+ Treg Cells: Hydrolysis of Extracellular ATP and Immune Suppression. Blood 2007, 110, 1225–1232. [Google Scholar] [CrossRef]
- Szulc-Dąbrowska, L.; Gieryńska, M.; Depczyńska, D.; Schollenberger, A.; Toka, F.N. Th17 lymphocytes in bacterial infections. Postep. Hig. Med. Dosw. (Online) 2015, 69, 398–417. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.J.; Nurieva, R.I.; Yang, X.O.; Dong, C. Regulation and Function of Proinflammatory TH17 Cells. Ann. N. Y. Acad. Sci. 2008, 1143, 188–211. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.; Lee, J.; Hillsamer, P.; Kim, C.H. Human Th17 Cells Share Major Trafficking Receptors with Both Polarized Effector T Cells and FOXP3+ Regulatory T Cells. J. Immunol. 2008, 180, 122–129. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Zhou, L.; Littman, D.R. Transcriptional Regulation of Th17 Cell Differentiation. Semin. Immunol. 2007, 19, 409–417. [Google Scholar] [CrossRef]
- Hirota, K.; Martin, B.; Veldhoen, M. Development, Regulation and Functional Capacities of Th17 Cells. Semin. Immunopathol. 2010, 32, 3–16. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Wang, X.-Y.; Lu, K.-M.; Yu, C.-H.; Su, M.-T.; Kang, L.; Hsu, K.-F.; Chen, P.-F.; Lin, S.-H. Maternal Th17/Treg Cytokines and Small Extracellular Vesicles in Plasma as Potential Biomarkers for Preeclampsia. Int. J. Med. Sci. 2022, 19, 1672–1679. [Google Scholar] [CrossRef]
- Harmon, A.C.; Cornelius, D.C.; Amaral, L.M.; Faulkner, J.L.; Cunningham, M.W.; Wallace, K.; LaMarca, B. The Role of Inflammation in the Pathology of Preeclampsia. Clin. Sci. 2016, 130, 409–419. [Google Scholar] [CrossRef]
- Peluso, I.; Fantini, M.C.; Fina, D.; Caruso, R.; Boirivant, M.; MacDonald, T.T.; Pallone, F.; Monteleone, G. IL-21 Counteracts the Regulatory T Cell-Mediated Suppression of Human CD4+ T Lymphocytes. J. Immunol. 2007, 178, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.R.; Zhu, Z.; Hansen, D.M.; Bai, Q.; Fang, Y. The Role of IL-21 in Immunity and Cancer. Cancer Lett. 2015, 358, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.Y.; Abdalkareem, E.A.; Khoo, B.Y. Functional Roles of Cytokines in Infectious Disease Associated Colorectal Carcinogenesis. Mol. Biol. Rep. 2022, 49, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Zenewicz, L.A.; Flavell, R.A. IL-22 and Inflammation: Leukin’ through a Glass Onion. Eur. J. Immunol. 2008, 38, 3265–3268. [Google Scholar] [CrossRef]
- Dudakov, J.A.; Hanash, A.M.; van den Brink, M.R.M. Interleukin-22: Immunobiology and Pathology. Annu. Rev. Immunol. 2015, 33, 747–785. [Google Scholar] [CrossRef]
- Zhao, K.; Zhao, D.; Huang, D.; Yin, L.; Chen, C.; Pan, B.; Wu, Q.; Li, Z.; Yao, Y.; Shen, E.; et al. Interleukin-22 Aggravates Murine Acute Graft-versus-Host Disease by Expanding Effector T Cell and Reducing Regulatory T Cell. J. Interferon Cytokine Res. 2014, 34, 707–715. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Shi, Y.; Xu, N.; Wang, Y.; Li, A.; Song, W. Increased Circulating Th22 Cells Correlated with Th17 Cells in Patients with Severe Preeclampsia. Hypertens Pregnancy 2017, 36, 100–107. [Google Scholar] [CrossRef]
- Prejbisz, A.; Dobrowolski, P.; Kosiński, P.; Bomba-Opoń, D.; Adamczak, M.; Bekiesińska-Figatowska, M.; Kądziela, J.; Konopka, A.; Kostka-Jeziorny, K.; Kurnatowska, I.; et al. Management of Hypertension in Pregnancy—Prevention, Diagnosis, Treatment and Long-Term Prognosis. A Position Statement of the Polish Society of Hypertension, Polish Cardiac Society and Polish Society of Gynaecologists and Obstetricians. Arter. Hypertens. 2019, 23, 117–182. [Google Scholar] [CrossRef]
- Malinowski, A.; Wilczyński, J.R. Immunological mechanisms of maintaining pregnancy. Ginekol. Prakt. 2003, 11, 47–56. [Google Scholar]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and Pregnancy: The Role of the Immune System at the Implantation Site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef]
- Mor, G.; Aldo, P.; Alvero, A.B. The Unique Immunological and Microbial Aspects of Pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-X.; Jin, L.-P.; Xu, B.; Liang, S.-S.; Li, D.-J. Decidual Stromal Cells Recruit Th17 Cells into Decidua to Promote Proliferation and Invasion of Human Trophoblast Cells by Secreting IL-17. Cell Mol. Immunol. 2014, 11, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Somerset, D.A.; Zheng, Y.; Kilby, M.D.; Sansom, D.M.; Drayson, M.T. Normal Human Pregnancy Is Associated with an Elevation in the Immune Suppressive CD25+ CD4+ Regulatory T-Cell Subset. Immunology 2004, 112, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Darmochwal-Kolarz, D.; Kludka-Sternik, M.; Tabarkiewicz, J.; Kolarz, B.; Rolinski, J.; Leszczynska-Gorzelak, B.; Oleszczuk, J. The Predominance of Th17 Lymphocytes and Decreased Number and Function of Treg Cells in Preeclampsia. J. Reprod. Immunol. 2012, 93, 75–81. [Google Scholar] [CrossRef]
- Taylor, E.B.; Sasser, J.M. Natural Killer Cells and T Lymphocytes in Pregnancy and Pre-Eclampsia. Clin. Sci. 2017, 131, 2911–2917. [Google Scholar] [CrossRef]
- Cao, C.; Ma, T.; Chai, Y.-F.; Shou, S.-T. The Role of Regulatory T Cells in Immune Dysfunction during Sepsis. World J. Emerg. Med. 2015, 6, 5–9. [Google Scholar] [CrossRef]
- Ribeiro, V.R.; Romao-Veiga, M.; Nunes, P.R.; de Oliveira, L.R.C.; Romagnoli, G.G.; Peracoli, J.C.; Peracoli, M.T.S. Silibinin Downregulates the Expression of the Th1 and Th17 Profiles by Modulation of STATs and Transcription Factors in Pregnant Women with Preeclampsia. Int. Immunopharmacol. 2022, 109, 108807. [Google Scholar] [CrossRef]
- Miller, D.; Motomura, K.; Galaz, J.; Gershater, M.; Lee, E.D.; Romero, R.; Gomez-Lopez, N. Cellular Immune Responses in the Pathophysiology of Preeclampsia. J. Leukoc. Biol. 2022, 111, 237–260. [Google Scholar] [CrossRef]
- Toldi, G.; Saito, S.; Shima, T.; Halmos, A.; Veresh, Z.; Vásárhelyi, B.; Rigó Jr, J.; Molvarec, A. The Frequency of Peripheral Blood CD4+ CD25high FoxP3+ and CD4+ CD25− FoxP3+ Regulatory T Cells in Normal Pregnancy and Pre-Eclampsia. Am. J. Reprod. Immunol. 2012, 68, 175–180. [Google Scholar] [CrossRef]
- Zenclussen, A.C.; Gerlof, K.; Zenclussen, M.L.; Sollwedel, A.; Bertoja, A.Z.; Ritter, T.; Kotsch, K.; Leber, J.; Volk, H.-D. Abnormal T-Cell Reactivity against Paternal Antigens in Spontaneous Abortion. Am. J. Pathol. 2005, 166, 811–822. [Google Scholar] [CrossRef]
- Mottet, C.; Golshayan, D. CD4+CD25+Foxp3+ Regulatory T Cells: From Basic Research to Potential Therapeutic Use. Swiss Med. Wkly. 2007, 137, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Eghbal-Fard, S.; Yousefi, M.; Heydarlou, H.; Ahmadi, M.; Taghavi, S.; Movasaghpour, A.; Jadidi-Niaragh, F.; Yousefi, B.; Dolati, S.; Hojjat-Farsangi, M.; et al. The Imbalance of Th17/Treg Axis Involved in the Pathogenesis of Preeclampsia. J. Cell Physiol. 2019, 234, 5106–5116. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhang, X.; Liu, C.; Lyu, J.; Liu, X.; Zhong, S.; Liang, Y.; Liu, P.; Huang, L.; Xiao, Z.; et al. Phenotypic and Functional Alteration of CD45+ Immune Cells in the Decidua of Preeclampsia Patients Analyzed by Mass Cytometry (CyTOF). Front. Immunol. 2022, 13, 1047986. [Google Scholar] [CrossRef] [PubMed]
- Mora-Palazuelos, C.; Bermúdez, M.; Aguilar-Medina, M.; Ramos-Payan, R.; Ayala-Ham, A.; Romero-Quintana, J.G. Cytokine-Polymorphisms Associated with Preeclampsia: A Review. Medicine 2022, 101, e30870. [Google Scholar] [CrossRef]
- Santner-Nanan, B.; Peek, M.J.; Khanam, R.; Richarts, L.; Zhu, E.; Fazekas de St Groth, B.; Nanan, R. Systemic Increase in the Ratio between Foxp3+ and IL-17-Producing CD4+ T Cells in Healthy Pregnancy but Not in Preeclampsia. J. Immunol. 2009, 183, 7023–7030. [Google Scholar] [CrossRef]
- Jonsson, Y.; Rubèr, M.; Matthiesen, L.; Berg, G.; Nieminen, K.; Sharma, S.; Ernerudh, J.; Ekerfelt, C. Cytokine Mapping of Sera from Women with Preeclampsia and Normal Pregnancies. J. Reprod. Immunol. 2006, 70, 83–91. [Google Scholar] [CrossRef]
- Li, H.; Yu, L.; Ding, Y.; Nie, Y.; Yang, M. Yin Yang 1 Impacts upon Preeclampsia by Regulating Treg/TH17 Cells and PI3K/AKT Pathway. J. Immunotoxicol. 2023, 20, 2228420. [Google Scholar] [CrossRef]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal Developmental Pathways for the Generation of Pathogenic Effector TH17 and Regulatory T Cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef]
- Luppi, P.; DeLoia, J.A. Monocytes of Preeclamptic Women Spontaneously Synthesize Pro-Inflammatory Cytokines. Clin. Immunol. 2006, 118, 268–275. [Google Scholar] [CrossRef]
- Pennington, K.A.; Schlitt, J.M.; Jackson, D.L.; Schulz, L.C.; Schust, D.J. Preeclampsia: Multiple Approaches for a Multifactorial Disease. Dis. Model. Mech. 2012, 5, 9–18. [Google Scholar] [CrossRef]
- Kim, D.-H.; Shin, S.H.; Kim, E.-K.; Kim, H.-S. Association of Increased Cord Blood Soluble Endoglin with the Development of Bronchopulmonary Dysplasia in Preterm Infants with Maternal Preeclampsia. Pregnancy Hypertens 2018, 13, 148–153. [Google Scholar] [CrossRef] [PubMed]
- English, F.A.; Kenny, L.C.; McCarthy, F.P. Risk Factors and Effective Management of Preeclampsia. Integr. Blood Press Control. 2015, 8, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Schoonderwoerd, M.J.A.; Goumans, M.-J.T.H.; Hawinkels, L.J.A.C. Endoglin: Beyond the Endothelium. Biomolecules 2020, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- George, E.M.; Granger, J.P. Endothelin: Key Mediator of Hypertension in Preeclampsia. Am. J. Hypertens. 2011, 24, 964–969. [Google Scholar] [CrossRef]
- Boulanger, H.; Bounan, S.; Mahdhi, A.; Drouin, D.; Ahriz-Saksi, S.; Guimiot, F.; Rouas-Freiss, N. Immunologic Aspects of Preeclampsia. AJOG Glob. Rep. 2024, 4, 100321. [Google Scholar] [CrossRef]
- Redman, C.W.G.; Sargent, I.L. Circulating Microparticles in Normal Pregnancy and Pre-Eclampsia. Placenta 2008, 29, 73–77. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, L.; Tang, Q.; Xu, Y.; Liu, S.; Li, H. Circulating Levels of IFN-γ, IL-1, IL-17 and IL-22 in Pre-Eclampsia: A Systematic Review and Meta-Analysis. Eur. J. Obstet Gynecol. Reprod. Biol. 2020, 248, 211–221. [Google Scholar] [CrossRef]
- Care, A.S.; Bourque, S.L.; Morton, J.S.; Hjartarson, E.P.; Robertson, S.A.; Davidge, S.T. Reduction in Regulatory T Cells in Early Pregnancy Causes Uterine Artery Dysfunction in Mice. Hypertension 2018, 72, 177–187. [Google Scholar] [CrossRef]
- Pasiński, J.; Świerczewski, A.; Estemberg, D.; Kowalska-Koprek, U.; Karowicz-Bilińska, A. The Influence of Vitamin C and E Use on Concentration of Endothelin-1 and Lipid Peroxides in the Serum of Pregnant Women with Arterial Hypertension. Ginekol. Pol. 2013, 84, 32–37. [Google Scholar] [CrossRef]
- Heimrath, J.; Czekański, A.; Krawczenko, A.; Duś, D. The role of endothelium in the pathogenesis of pregnancy-induced hypertension. Postep. Hig. Med. Dosw. (Online) 2007, 61, 48–57. [Google Scholar]
- Logiodice, F.; Lombardelli, L.; Kullolli, O.; Haller, H.; Maggi, E.; Rukavina, D.; Piccinni, M.-P. Decidual Interleukin-22-Producing CD4+ T Cells (Th17/Th0/IL-22+ and Th17/Th2/IL-22+, Th2/IL-22+, Th0/IL-22+), Which Also Produce IL-4, Are Involved in the Success of Pregnancy. Int. J. Mol. Sci. 2019, 20, 428. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, B.; Li, M.-Q.; Li, D.-J.; Jin, L.-P. IL-22 Secreted by Decidual Stromal Cells and NK Cells Promotes the Survival of Human Trophoblasts. Int. J. Clin. Exp. Pathol. 2013, 6, 1781–1790. [Google Scholar]
- Stefańska, K.; Zieliński, M.; Jankowiak, M.; Zamkowska, D.; Sakowska, J.; Adamski, P.; Jassem-Bobowicz, J.; Piekarska, K.; Leszczyńska, K.; Świątkowska-Stodulska, R.; et al. Cytokine Imprint in Preeclampsia. Front. Immunol. 2021, 12, 667841. [Google Scholar] [CrossRef]
- Wei, L.; Laurence, A.; Elias, K.M.; O’Shea, J.J. IL-21 Is Produced by Th17 Cells and Drives IL-17 Production in a STAT3-Dependent Manner. J. Biol. Chem. 2007, 282, 34605–34610. [Google Scholar] [CrossRef] [PubMed]
- Onoda, T.; Rahman, M.; Nara, H.; Araki, A.; Makabe, K.; Tsumoto, K.; Kumagai, I.; Kudo, T.; Ishii, N.; Tanaka, N.; et al. Human CD4+ Central and Effector Memory T Cells Produce IL-21: Effect on Cytokine-Driven Proliferation of CD4+ T Cell Subsets. Int. Immunol. 2007, 19, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Poordast, T.; Najib, F.S.; Baharlou, R.; Bijani, A.; Alamdarloo, S.M.; Poordast, A. Assessment of T Helper 17-Associated Cytokines in Third Trimester of Pregnancy. Iran. J. Immunol. 2017, 14, 172–179. [Google Scholar] [PubMed]
- Saifi, B.; Rezaee, S.A.; Tajik, N.; Ahmadpour, M.E.; Ashrafi, M.; Vakili, R.; SoleimaniAsl, S.; Aflatoonian, R.; Mehdizadeh, M. Th17 Cells and Related Cytokines in Unexplained Recurrent Spontaneous Miscarriage at the Implantation Window. Reprod. Biomed. Online 2014, 29, 481–489. [Google Scholar] [CrossRef]
- Gershater, M.; Romero, R.; Arenas-Hernandez, M.; Galaz, J.; Motomura, K.; Tao, L.; Xu, Y.; Miller, D.; Pique-Regi, R.; Martinez, G.; et al. Interleukin-22 Plays a Dual Role in the Amniotic Cavity: Tissue Injury and Host Defense against Microbes in Preterm Labor. J. Immunol. 2022, 208, 1595–1615. [Google Scholar] [CrossRef]
- Abrahams, V.M.; Kim, Y.M.; Straszewski, S.L.; Romero, R.; Mor, G. Macrophages and Apoptotic Cell Clearance during Pregnancy. Am. J. Reprod. Immunol. 2004, 51, 275–282. [Google Scholar] [CrossRef]
- Bersani, I.; De Carolis, M.P.; Foell, D.; Weinhage, T.; Rossi, E.D.; De Carolis, S.; Rubortone, S.A.; Romagnoli, C.; Speer, C.P. Interleukin-22: Biomarker of Maternal and Fetal Inflammation? Immunol. Res. 2015, 61, 4–10. [Google Scholar] [CrossRef]
- Mihu, D.; Razvan, C.; Malutan, A.; Mihaela, C. Evaluation of Maternal Systemic Inflammatory Response in Preeclampsia. Taiwan J. Obstet. Gynecol. 2015, 54, 160–166. [Google Scholar] [CrossRef]
| Variable | Control Group (n = 48) | Study Group (n = 60) | p-Value |
|---|---|---|---|
| Family history | |||
| 41 (85.4%) | 41 (85.4%) | 41 (68.3%) |
| 7 (14.6%) | 19 (31.7%) | 0.0662 |
| Pre-pregnancy systolic blood pressure (SBP) [mmHg] | Median: 120 IQR: [110–120] Range: 90–140 | Median: 120 IQR: [120–130] Range: 102–145 | 0.0014 * |
| Diastolic blood pressure before pregnancy (DBP) [mmHg] | Median: 71.5 IQR: [68–80] Range: 50–90 | Median: 80 IQR: [80–85] Range: 60–115 | <0.0001 * |
| Systolic blood pressure in pregnancy (SBP) [mmHg] | Median: 120 IQR: [113.5–122.5] Range: 90–140 | Median: 155 IQR: [145–165] Range: 130–210 | <0.0001 * |
| Diastolic blood pressure in pregnancy (DBP) [mmHg] | Median: 80 IQR: [70–80] Range: 60–90 | Median: 95.5 IQR: [90–105] Range: 85–180 | <0.0001 * |
| Parameter | Control Group (n = 48) | Study Group (n = 60) | p |
|---|---|---|---|
| Percentage of peripheral blood lymphocytes [%] | Median: 45.3 IQR: [37.0–60.9] Range: (14.6–72.9) | Median: 47.5 IQR: [39.4–54.7] Range: (16.6–79.1) | 0.9501 |
| Treg (CD4+CD25+FoxP3+) [%] | Median: 2.4 IQR: [0.9–3.9] Range: (0.2–19.0) | Median: 1.9 IQR: [0.8–3.4] Range: (0.2–8.1) | 0.1677 |
| Th17 (CD4+IL-17A+) [%] | Median: 1.8 IQR: [1.2–2.8] Range: (0.6–9.4) | Median: 6.8 IQR: [3.8–9.8] Range: (0.5–22.6) | <0.0001 * |
| Th17 (IL-17F+IL-21+) [%] | Median: 1.2 IQR: [0.7–2.1] Range: (0.1–8.1) | Median: 1.2 IQR: [0.6–3.1] Range: (0.3–7.5) | 0.8368 |
| Th17 (IL-17F+IL-22+) [%] | Median: 0.7 IQR: [0.3–2.1] Range: (0–8.2) | Median: 2.7 IQR: [1.6–5.7] Range: (0.1–43.1) | <0.0001 * |
| Th17 (IL-21+IL-22+) [%] | Median: 0.8 IQR: [0.2–1.5] Range: (0–10.3) | Median: 1.7 IQR: [0.8–5.8] Range: (0.1–28.8) | 0.0001 * |
| Treg/Th17 ratio | Median: 19.3 IQR: [13.0–32.2] Range: (3.2–66.8) | Median: 5.5 IQR: [3.7–8.5] Range: (1.2–87.8) | <0.0001 * |
| Variable | Percentage of Peripheral Blood Lymphocytes | Percentage of Activated Th17 Lymphocytes (CD4+IL-17A+) | Percentage of Th17 Lymphocytes with Intracellular Expression of IL17F and IL21 | Percentage of Th17 Lymphocytes with Intracellular Expression of IL17F and IL22 | Percentage of Th17 Lymphocytes with Intracellular Expression of IL21 and IL22 | Treg/Th17 Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rho | p | Rho | p | Rho | p | Rho | p | Rho | p | Rho | p | |
| Mother’s age [years] | 0.043 | 0.7485 | −0.116 | 0.3922 | −0.157 | 0.2434 | 0.193 | 0.1512 | 0.335 | 0.0109 * | 0.108 | 0.4246 |
| Number of pregnancies | −0.077 | 0.5617 | −0.025 | 0.8560 | −0.139 | 0.3016 | 0.077 | 0.5669 | 0.236 | 0.0774 | −0.003 | 0.9846 |
| Number of births | −0.057 | 0.6654 | 0.013 | 0.9220 | −0.153 | 0.2551 | 0.020 | 0.8831 | 0.178 | 0.1858 | −0.019 | 0.8910 |
| Number of miscarriages in the history | −0.102 | 0.4458 | −0.082 | 0.5457 | −0.030 | 0.8256 | 0.136 | 0.3161 | 0.199 | 0.1414 | 0.055 | 0.6874 |
| Week of pregnancy (hbd) on the day of sample collection | 0.149 | 0.2591 | −0.003 | 0.9823 | 0.263 | 0.0484 * | 0.325 | 0.0136 * | 0.124 | 0.3592 | 0.037 | 0.7840 |
| Week of pregnancy (hbd) on the day of delivery | 0.185 | 0.1612 | −0.011 | 0.9331 | 0.234 | 0.0803 | 0.333 | 0.0114 * | 0.133 | 0.3236 | 0.041 | 0.7627 |
| Newborn body weight [g] | 0.221 | 0.0926 | −0.072 | 0.5962 | 0.082 | 0.5436 | 0.328 | 0.0128 * | 0.157 | 0.2448 | 0.096 | 0.4791 |
| Newborn’s body length [cm] | 0.153 | 0.2514 | 0.022 | 0.8737 | 0.175 | 0.1969 | 0.404 | 0.0020 * | 0.177 | 0.1931 | −0.006 | 0.9671 |
| APGAR in 1 min | 0.310 | 0.0213 * | 0.123 | 0.3807 | −0.176 | 0.2062 | 0.226 | 0.1029 | 0.065 | 0.6441 | −0.067 | 0.6358 |
| APGAR in 3 min | 0.331 | 0.0177 * | 0.054 | 0.7105 | −0.013 | 0.9283 | 0.265 | 0.0627 | 0.049 | 0.7376 | −0.002 | 0.9894 |
| APGAR in 5 min | 0.410 | 0.0019 * | 0.136 | 0.3333 | 0.047 | 0.7393 | 0.182 | 0.1932 | 0.022 | 0.8747 | −0.037 | 0.7941 |
| Mother’s weight before pregnancy [kg] | 0.248 | 0.0588 | −0.076 | 0.5723 | −0.321 | 0.0150 * | −0.057 | 0.6722 | −0.057 | 0.6756 | 0.164 | 0.2221 |
| Current mother weight (at hbd of material collection) [kg] | 0.175 | 0.1848 | 0.003 | 0.9802 | −0.132 | 0.3272 | −0.140 | 0.2979 | −0.082 | 0.5459 | 0.065 | 0.6333 |
| BMI before pregnancy [kg/m2] | 0.207 | 0.1161 | −0.077 | 0.5673 | −0.174 | 0.1953 | −0.092 | 0.4981 | −0.139 | 0.3015 | 0.166 | 0.2167 |
| BMI during pregnancy (at the time of material collection) [kg/m2] | 0.140 | 0.2896 | −0.027 | 0.8443 | −0.062 | 0.6459 | −0.165 | 0.2208 | −0.146 | 0.2787 | 0.069 | 0.6103 |
| SBP before pregnancy [mmHg] | −0.231 | 0.0809 | 0.134 | 0.3230 | 0.092 | 0.4989 | 0.235 | 0.0811 | 0.014 | 0.9167 | −0.210 | 0.1206 |
| DBP before pregnancy [mmHg] | −0.154 | 0.2488 | 0.100 | 0.4632 | −0.021 | 0.8799 | 0.103 | 0.4498 | −0.055 | 0.6897 | −0.103 | 0.4481 |
| SBP in pregnancy [mmHg] | −0.016 | 0.9056 | −0.011 | 0.9328 | −0.145 | 0.2818 | −0.057 | 0.6750 | 0.015 | 0.9123 | −0.131 | 0.3325 |
| DBP in pregnancy [mmHg] | −0.175 | 0.1850 | 0.133 | 0.3256 | −0.250 | 0.0604 | 0.025 | 0.8510 | 0.072 | 0.5962 | −0.135 | 0.3163 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatek, M.; Kwaśniewski, W.; Gęca, T.; Grywalska, E.; Rahnama-Hezavah, M.; Mertowski, S.; Urbanowicz, T.; Kowalkowska, M.E.; Krasiński, M.; Kwaśniewska, A.; et al. Dysregulation of Treg/Th17 Balance and Intracellular Expression of IL-21 and IL-22 in the Pathogenesis of Gestational Hypertension. J. Clin. Med. 2025, 14, 7288. https://doi.org/10.3390/jcm14207288
Kwiatek M, Kwaśniewski W, Gęca T, Grywalska E, Rahnama-Hezavah M, Mertowski S, Urbanowicz T, Kowalkowska ME, Krasiński M, Kwaśniewska A, et al. Dysregulation of Treg/Th17 Balance and Intracellular Expression of IL-21 and IL-22 in the Pathogenesis of Gestational Hypertension. Journal of Clinical Medicine. 2025; 14(20):7288. https://doi.org/10.3390/jcm14207288
Chicago/Turabian StyleKwiatek, Maciej, Wojciech Kwaśniewski, Tomasz Gęca, Ewelina Grywalska, Mansur Rahnama-Hezavah, Sebastian Mertowski, Tomasz Urbanowicz, Magdalena Ewa Kowalkowska, Maciej Krasiński, Anna Kwaśniewska, and et al. 2025. "Dysregulation of Treg/Th17 Balance and Intracellular Expression of IL-21 and IL-22 in the Pathogenesis of Gestational Hypertension" Journal of Clinical Medicine 14, no. 20: 7288. https://doi.org/10.3390/jcm14207288
APA StyleKwiatek, M., Kwaśniewski, W., Gęca, T., Grywalska, E., Rahnama-Hezavah, M., Mertowski, S., Urbanowicz, T., Kowalkowska, M. E., Krasiński, M., Kwaśniewska, A., & Brązert, M. (2025). Dysregulation of Treg/Th17 Balance and Intracellular Expression of IL-21 and IL-22 in the Pathogenesis of Gestational Hypertension. Journal of Clinical Medicine, 14(20), 7288. https://doi.org/10.3390/jcm14207288






