Thyroid Nodule Surveillance in Transfusion-Dependent Thalassemia: A Comparative Ultrasonographic Study
Abstract
1. Introduction
2. Patients and Methods
3. Statistical Analysis
Sample Size
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grani, G.; Sponziello, M.; Filetti, S.; Durante, C. Thyroid nodules: Diagnosis and management. Nat. Rev. Endocrinol. 2024, 20, 715–728. [Google Scholar] [CrossRef]
- Alexander, E.K.; Cibas, E.S. Diagnosis of thyroid nodules. Lancet Diabetes Endocrinol. 2022, 10, 533–539. [Google Scholar] [CrossRef]
- Dobruch-Sobczak, K.; Adamczewski, Z.; Szczepanek-Parulska, E.; Migda, B.; Woliński, K.; Krauze, A.; Prostko, P.; Ruchała, M.; Lewiński, A.; Jakubowski, W.; et al. Histopathological Verification of the Diagnostic Performance of the EU-TIRADS Classification of Thyroid Nodules-Results of a Multicenter Study Performed in a Previously Iodine-Deficient Region. J. Clin. Med. 2019, 8, 1781. [Google Scholar] [CrossRef] [PubMed]
- Hekimsoy, İ.; Öztürk, E.; Ertan, Y.; Orman, M.N.; Kavukçu, G.; Özgen, A.G.; Özdemir, M.; Özbek, S.S. Diagnostic performance rates of the ACR-TIRADS and EU-TIRADS based on histopathological evidence. Diagn. Interv. Radiol. 2021, 27, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Magri, F.; Chytiris, S.; Croce, L.; Molteni, M.; Bendotti, G.; Gruosso, G.; Tata Ngnitejeu, S.; Agozzino, M.; Rotondi, M.; Chiovato, L. Performance of the ACR TI-RADS and EU TI-RADS scoring systems in the diagnostic work-up of thyroid nodules in a real-life series using histology as reference standard. Eur. J. Endocrinol. 2020, 183, 521–528. [Google Scholar]
- Russ, G.; Royer, B.; Bigorgne, C.; Rouxel, A.; Bienvenu-Perrard, M.; Leenhardt, L. Prospective evaluation of thyroid imaging reporting and data system on 4550 nodules with and without elastography. Eur. J. Endocrinol. 2013, 168, 649–655. [Google Scholar] [CrossRef]
- Castellana, M.; Castellana, C.; Treglia, G.; Giorgino, F.; Giovanella, L.; Russ, G.; Trimboli, P. Performance of Five Ultrasound Risk Stratification Systems in Selecting Thyroid Nodules for FNA. J. Clin. Endocrinol. Metab. 2020, 105, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Origa, R.; Issa, L. Beta Thalassemia in Children: Established Approaches, Old Issues, New Non-Curative Therapies, and Perspectives on Healing. J. Clin. Med. 2024, 13, 6966. [Google Scholar] [CrossRef]
- Casale, M.; Meloni, A.; Filosa, A.; Cuccia, L.; Caruso, V.; Palazzi, G.; Gamberini, M.R.; Pitrolo, L.; Putti, M.C.; D’Ascola, D.G.; et al. Multiparametric Cardiac Magnetic Resonance Survey in Children with Thalassemia Major: A Multicenter Study. Circ. Cardiovasc. Imaging 2015, 8, e003230. [Google Scholar] [CrossRef]
- He, L.N.; Chen, W.; Yang, Y.; Xie, Y.J.; Xiong, Z.Y.; Chen, D.Y.; Lu, D.; Liu, N.Q.; Yang, Y.H.; Sun, X.F. Elevated Prevalence of Abnormal Glucose Metabolism and Other Endocrine Disorders in Patients with β-Thalassemia Major: A Meta-Analysis. BioMed Res. Int. 2019, 2019, 6573497. [Google Scholar] [CrossRef]
- Pepe, A.; Meloni, A.; Filosa, A.; Pistoia, L.; Borsellino, Z.; D’Ascola, D.G.; Lisi, R.; Putti, M.C.; Allò, M.; Gamberini, M.R.; et al. Prospective CMR Survey in Children with Thalassemia Major: Insights From a National Network. JACC Cardiovasc. Imaging 2020, 13, 1284–1286. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razek, A.R.; Abdel-Salam, A.; El-Sonbaty, M.M.; Youness, E.R. Study of thyroid function in Egyptian children with β-thalassemia major and β-thalassemia intermedia. J. Egypt. Public Health Assoc. 2013, 88, 148–152. [Google Scholar] [PubMed]
- Carsote, M.; Vasiliu, C.; Trandafir, A.I.; Albu, S.E.; Dumitrascu, M.C.; Popa, A.; Mehedintu, C.; Petca, R.C.; Petca, A.; Sandru, F. New Entity-Thalassemic Endocrine Disease: Major Beta-Thalassemia and Endocrine Involvement. Diagnostics 2022, 12, 1921. [Google Scholar]
- Casale, M.; Citarella, S.; Filosa, A.; De Michele, E.; Palmieri, F.; Ragozzino, A.; Amendola, G.; Pugliese, U.; Tartaglione, I.; Della Rocca, F.; et al. Endocrine function and bone disease during long-term chelation therapy with deferasirox in patients with β-thalassemia major. Am. J. Hematol. 2014, 89, 1102–1106. [Google Scholar]
- Casale, M.; Forni, G.L.; Cassinerio, E.; Pasquali, D.; Origa, R.; Serra, M.; Campisi, S.; Peluso, A.; Renni, R.; Cattoni, A.; et al. Risk factors for endocrine complications in transfusion-dependent thalassemia patients on chelation therapy with deferasirox: A risk assessment study from a multi-center nation-wide cohort. Haematologica 2022, 107, 467–477. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Elsedfy, H.; Yaarubi, S.A.; Skordis, N.; Khater, D.; El Kholy, M.; Stoeva, I.; Fiscina, B.; Angastiniotis, M.; et al. The ICET-A Recommendations for the Diagnosis and Management of Disturbances of Glucose Homeostasis in Thalassemia Major Patients. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016058. [Google Scholar] [CrossRef]
- Gianesin, B.; Piel, F.B.; Musallam, K.M.; Barella, S.; Casale, M.; Cassinerio, E.; Di Maggio, R.; Gigante, A.; Gamberini, M.R.; Graziadei, G.; et al. Prevalence and mortality trends of hemoglobinopathies in Italy: A nationwide study. Haematologica 2025, 110, 1211–1216. [Google Scholar]
- Punzo, F.; Bertoli-Avella, A.M.; Scianguetta, S.; Della Ragione, F.; Casale, M.; Ronzoni, L.; Cappellini, M.D.; Forni, G.; Oostra, B.A.; Perrotta, S. Congenital dyserythropoietic anemia type II: Molecular analysis and expression of the SEC23B gene. Orphanet J. Rare Dis. 2011, 6, 89. [Google Scholar] [CrossRef]
- Taher, A.T.; Musallam, K.M.; Cappellini, M.D. Guidelines for the Management of Non-Transfusion-Dependent β-Thalassaemia; Thalassaemia International Federation: Nicosia, Cyprus, 2023. [Google Scholar]
- Baldini, M.; Serafino, S.; Zanaboni, L.; Cappellini, M.D. Thyroid cancer in β-thalassemia. Hemoglobin 2012, 36, 407–408. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Canatan, D.; Yassin, M.A.; Daar, S.; Elsedfy, H.; Di Maio, S.; Raiola, G.; Corrons, J.V.; Kattamis, C. Thyroid Disorders in Homozygous β-Thalassemia: Current Knowledge, Emerging Issues and Open Problems. Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019029. [Google Scholar] [CrossRef] [PubMed]
- Choleva, L.; Wilkes, M. Papillary Thyroid Carcinoma in a Pediatric Patient with β-Thalassemia. JCEM Case Rep. 2023, 1, luad131. [Google Scholar] [CrossRef]
- Morales, M.; Xue, X. Targeting iron metabolism in cancer therapy. Theranostics 2021, 11, 8412–8429. [Google Scholar] [CrossRef]
- Casale, M.; Baldini, M.I.; Del Monte, P.; Gigante, A.; Grandone, A.; Origa, R.; Poggi, M.; Gadda, F.; Lai, R.; Marchetti, M.; et al. Good Clinical Practice of the Italian Society of Thalassemia and Haemoglobinopathies (SITE) for the Management of Endocrine Complications in Patients with Haemoglobinopathies. J. Clin. Med. 2022, 11, 1826. [Google Scholar] [CrossRef]
- Casale, M.; Baldini, M.; Giusti, A.; Grandone, A.; Poggi, M. Growth Abnormalities, Endocrine, and Bone Disease. In Guidelines for the Management of Transfusion-Dependent β-Thalassaemia (TDT); Taher, A.T., Farmakis, D., Porter, J.B., Cappellini, M.D., Musallam, K.M., Eds.; Thalassaemia International Federation: Nicosia, Cyprus, 2025. [Google Scholar]
- Govoni, M.R.; Sprocati, M.; Fabbri, E.; Zanforlin, N.; De Sanctis, V. Papillary thyroid cancer in thalassaemia. Pediatr. Endocrinol. Rev. PER 2011, 8 (Suppl. 2), 314–321. [Google Scholar] [PubMed]
- Torregrossa, L.; Poma, A.M.; Macerola, E.; Rago, T.; Vignali, P.; Romani, R.; Proietti, A.; Di Stefano, I.; Scuotri, G.; Ugolini, C.; et al. The Italian Consensus for the Classification and Reporting of Thyroid Cytology: Cytohistologic and Molecular Correlations on 37,371 Nodules from a Single Institution. Cancer Cytopathol. 2022, 130, 899–912. [Google Scholar] [CrossRef]
- Haghpanah, S.; Pishdad, P.; Zarei, T.; Shahsavani, A.; Amirmoezi, F.; Ilkhanipoor, H.; Ilkhanipoor, H.; Safaei, S.; Setoodegan, F.; De Sanctis, V.; et al. Frequency of thyroid nodules in patients with β-thalassemias in Southern Iran. Acta Endocrinol. 2020, 16, 68–73. [Google Scholar]
- Albattah, A.; Imam, Y.; Saleh, A.O.; Ahmed, K.; Aboursheid, T.; Kohla, S.; Mohamed, S. Case Report: Papillary Thyroid Cancer in a Patient with Celiac Disease and Thalassemia Trait. Case Rep. Oncol. 2020, 13, 1364–1367. [Google Scholar] [CrossRef]
- Ahmadi, S.; Herbst, R.; Oyekunle, T.; Jiang, X.; Strickland, K.; Roman, S.; Sosa, J.A. Using the ATA and ACR TI-RADS Sonographic Classifications as Adjunctive Predictors of Malignancy for Indeterminate Thyroid Nodules. Endocr. Pract. 2019, 25, 908–917. [Google Scholar] [PubMed]
| N1 | N2 | Actual Power | Test Assumptions | |||||
|---|---|---|---|---|---|---|---|---|
| Power | Risk Difference | Risk Ratio | Odds Ratio | Sig. | ||||
| Test for Proportion Difference a | 34 | 34 | 0.811 | 0.8 | −0.260 | 0.161 | 0.117 | 0.05 |
| Characteristic | Group | Test | ||
|---|---|---|---|---|
| Healthy Controls | Patients with Thalassemia | Statistic | p-Value | |
| Thyroid ultrasound performed | 101 | 156 | ||
| Age 1 | 39.54 (16.17) | 44.60 (10.30) | 3283 | 0.006 |
| Prevalence of thyroid nodules 2 | 35/101 (34.65%) | 55 3/156 (35.26%) | 3.11 × 10−30 | >0.999 |
| Number of suspected nodules | 1.00 (0.00) | 1.31 (0.86) | ||
| Maximum diameter of the largest nodule (mm) 1 | 16.09 (10.77) | 9.16 (5.43) | 1094 | 0.003 |
| Moderate and highly suspicious nodule (TIRADS 4–TIRADS 5) diameter (mm) 1 | 19.29 (12.05) | 10.89 (4.52) | 190.5 | 0.038 |
| Age (if thyroid nodules) 1 | 47.48 (18.13) | 46.42 (10.45) | 630 | 0.545 |
| Characteristic | Group | Test | ||
|---|---|---|---|---|
| Healthy Controls | Patients with Thalassemia 3 | Statistic | p-Value | |
| TIRADS 1 | 980 | 0.649 | ||
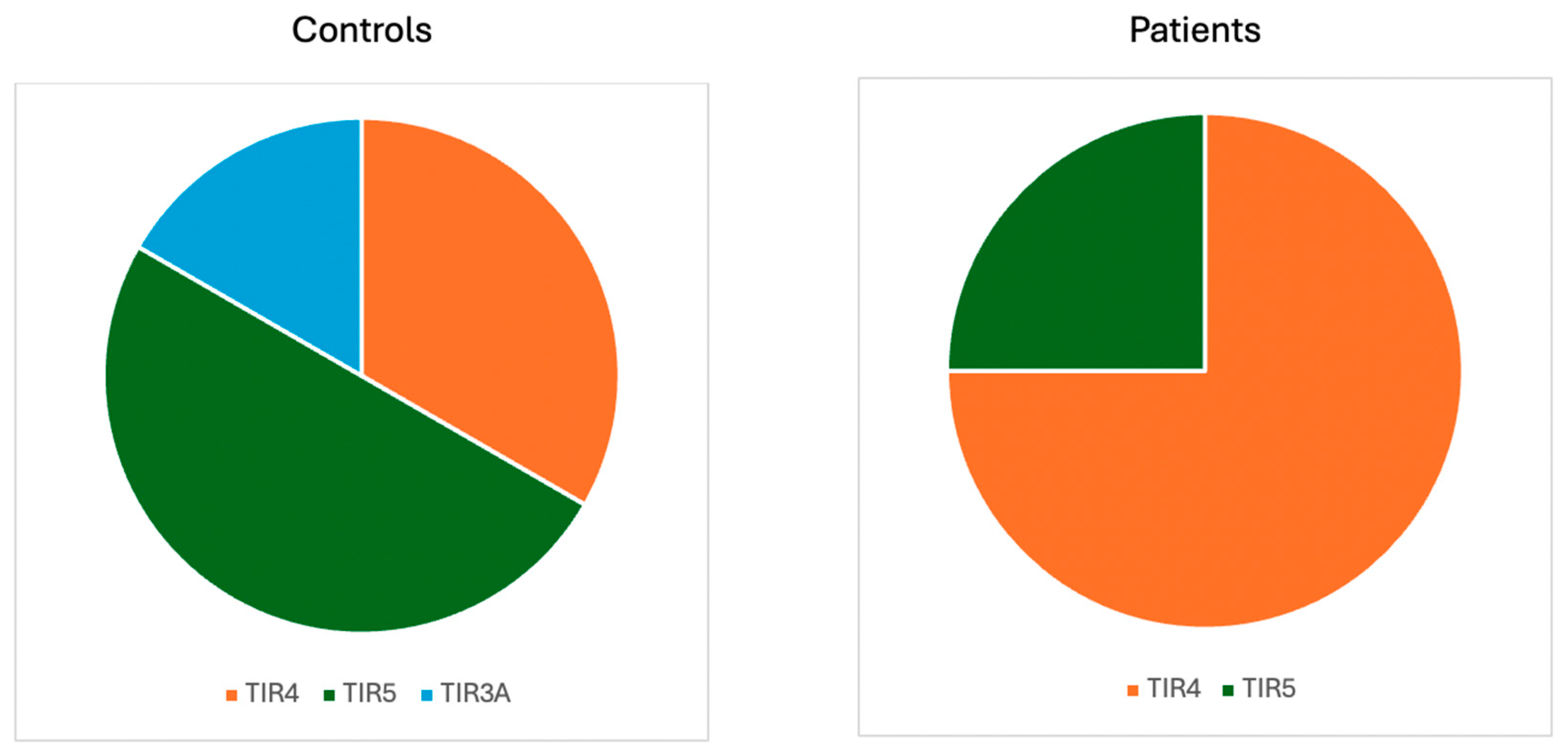
| TIRADS 1 | 3/35 (8.57%) | 10/53 (18.87%) | ||
| TIRADS 2 | 10/35 (28.57%) | 13/53 (24.53%) | ||
| TIRADS 3 | 8/35 (22.86%) | 10/53 (18.87%) | ||
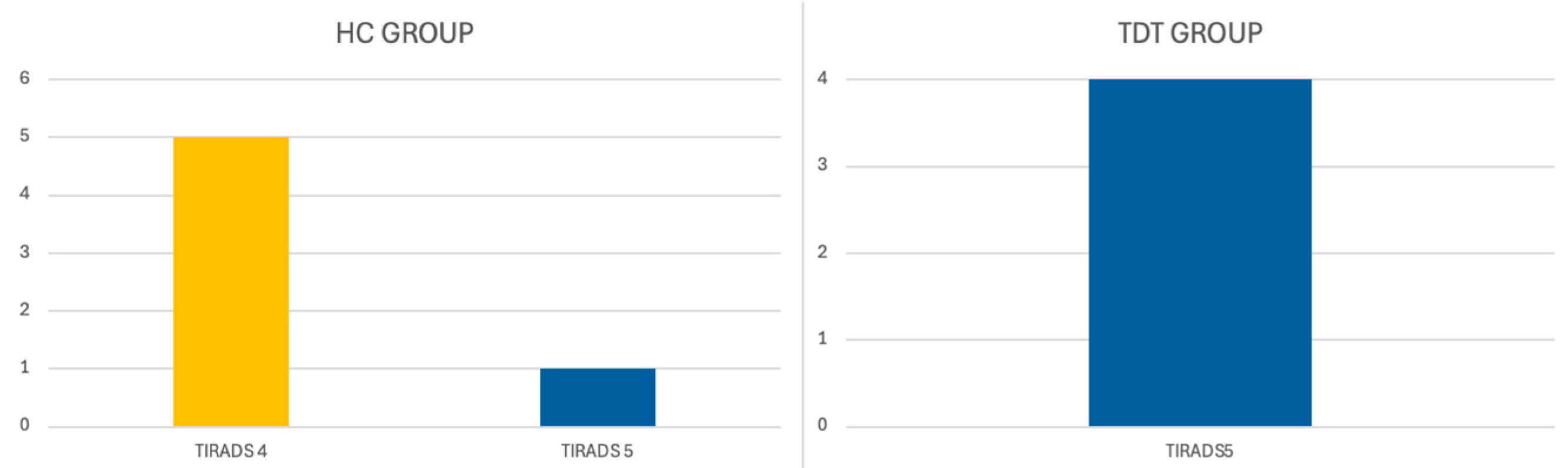
| TIRADS 4 | 11/35 (31.43%) | 12/53 (22.64%) | ||
| TIRADS 5 | 3/35 (8.57%) | 8/53 (15.09%) | ||
| TIR 1 | 90.5 | 0.796 | ||
| TIR1 | 0/16 (0.00%) | 0/12 (0.00%) | ||
| TIR2 | 10/16 (62.50%) | 6/12 (50.00%) | ||
| TIR3 | 1/16 (6.25%) | 2/12 (16.67%) | ||
| TIR4 | 2/16 (12.50%) | 3/12 (25.00%) | ||
| TIR5 | 3/16 (18.75%) | 1/12 (8.33%) | ||
| Fine-needle aspiration indicated but not performed | 1/22 (4.55%) | 7/21 (33.33%) | 0.021 | |
| Thyroid cancer 2 | 6/35 (17.14%) | 4/55 (7.27%) | 0.178 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casale, M.; Errico, M.; Origa, R.; Mureddu, P.; Allosso, F.; Digitale Selvaggio, L.; Grande, G.; Di Ludovico, C.; Navarra, R.; Roberti, D.; et al. Thyroid Nodule Surveillance in Transfusion-Dependent Thalassemia: A Comparative Ultrasonographic Study. J. Clin. Med. 2025, 14, 7265. https://doi.org/10.3390/jcm14207265
Casale M, Errico M, Origa R, Mureddu P, Allosso F, Digitale Selvaggio L, Grande G, Di Ludovico C, Navarra R, Roberti D, et al. Thyroid Nodule Surveillance in Transfusion-Dependent Thalassemia: A Comparative Ultrasonographic Study. Journal of Clinical Medicine. 2025; 14(20):7265. https://doi.org/10.3390/jcm14207265
Chicago/Turabian StyleCasale, Maddalena, Martina Errico, Raffaella Origa, Paolo Mureddu, Francesca Allosso, Lucia Digitale Selvaggio, Graziella Grande, Claudia Di Ludovico, Raffaele Navarra, Domenico Roberti, and et al. 2025. "Thyroid Nodule Surveillance in Transfusion-Dependent Thalassemia: A Comparative Ultrasonographic Study" Journal of Clinical Medicine 14, no. 20: 7265. https://doi.org/10.3390/jcm14207265
APA StyleCasale, M., Errico, M., Origa, R., Mureddu, P., Allosso, F., Digitale Selvaggio, L., Grande, G., Di Ludovico, C., Navarra, R., Roberti, D., Capellupo, M. C., Perrotta, S., & Pasquali, D. (2025). Thyroid Nodule Surveillance in Transfusion-Dependent Thalassemia: A Comparative Ultrasonographic Study. Journal of Clinical Medicine, 14(20), 7265. https://doi.org/10.3390/jcm14207265






