Different Duration of Prone Positioning Treatment for Patients with Acute Respiratory Distress Syndrome in Intensive Care Unit Patients: A Prospective Randomized Clinical Study
Abstract
1. Introduction
2. Materials and Methods
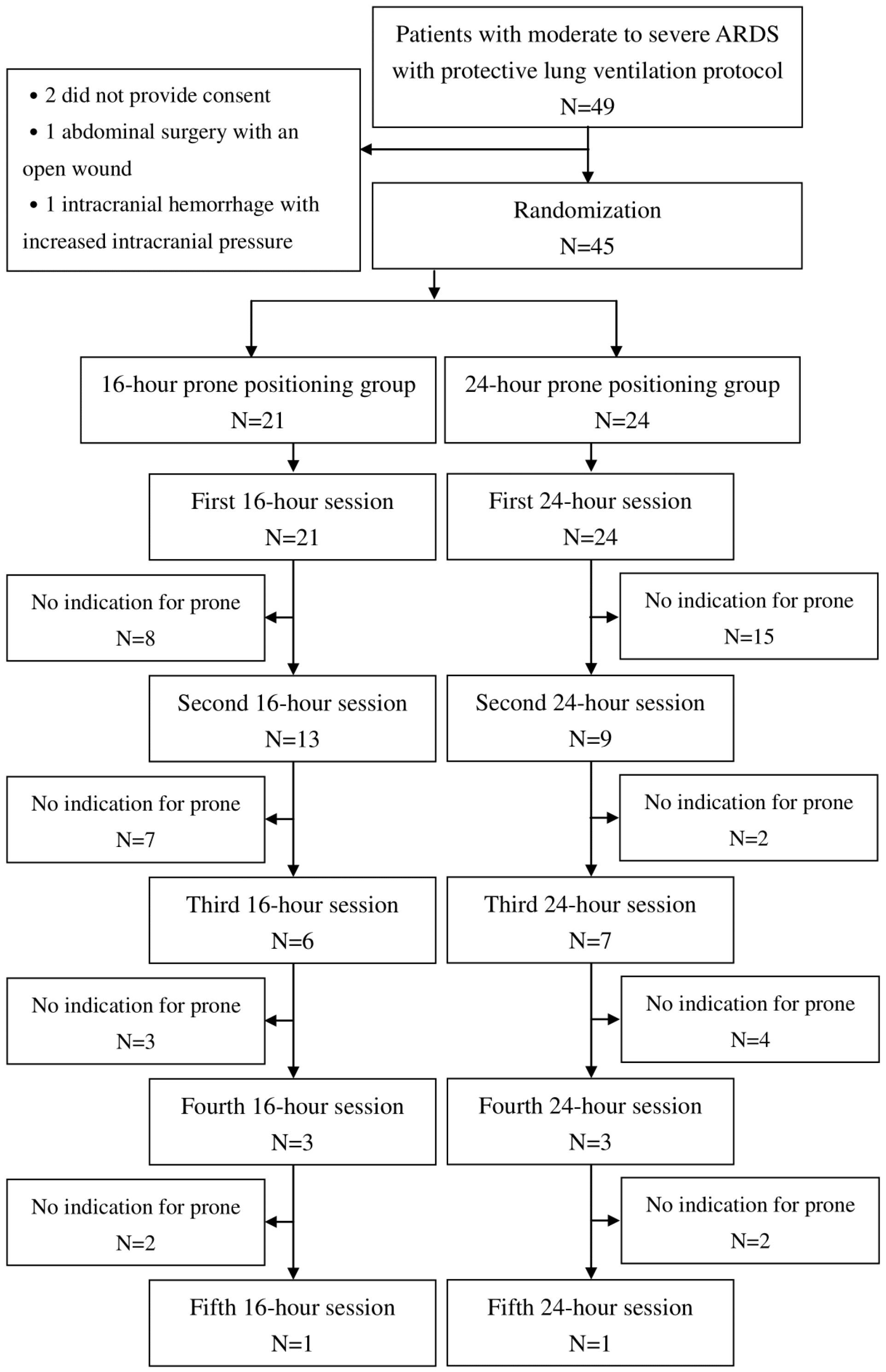
2.1. Enrollment
2.2. Randomization
2.3. Observations
2.4. Definitions
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Demographics
3.2. Primary Endpoints
3.3. Secondary Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.J.K.; Amato, M.B.P.; Branson, R.; Brower, R.G.; et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef]
- Bryan, A.C. Conference on the scientific basis of respiratory therapy. Pulmonary physiotherapy in the pediatric age group. Comments of a devil’s advocate. Am. Rev. Respir. Dis. 1974, 110, 143–144. [Google Scholar]
- Papazian, L.; Aubron, C.; Brochard, L.; Chiche, J.D.; Combes, A.; Dreyfuss, D.; Guérin, C.; Jaber, S.; Mekontso-Dessap, A.; Mercat, A.; et al. Formal guideline: Management of acute respiratory distress syndrome. Ann. Intensive Care 2019, 9, 69. [Google Scholar] [CrossRef]
- Yan, Y.; Boa, J.; Cai, S.; Zhoung, X.; Geng, P.; Liang, J.; Deng, J.; Chen, Z.; Qin, Z.; Hu, H.; et al. The effects of prolonged prone positioning on response and prognosis in patients with acute respiratory distress syndrome: A retrospective cohor study. J. Intensive Care 2025, 13, 24. [Google Scholar] [CrossRef]
- Abroug, F.; Ouanes-Besbes, L.; Dachraoui, F.; Ouanes, I.; Brochard, L. An updated study-level meta-analysis of randomised controlled trials on proning in ARDS and acute lung injury. Crit. Care 2011, 15, R6. [Google Scholar] [CrossRef] [PubMed]
- McAuley, D.F.; Giles, S.; Fichter, H.; Perkins, G.D.; Gao, F. What is the optimal duration of ventilation in the prone position in acute lung injury and acute respiratory distress syndrome? Intensive Care Med. 2002, 28, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, C.H.; Colantuoni, E.; Sahetya, S.K.; Eakin, M.N.; Fan, E.; Psoter, K.J.; Iwashyna, T.J.; Needham, D.M.; Hager, D.N. Extended versus standard proning duration for COVID-19-associated acute respiratory distress syndrome: A target trial emulation study. Ann. Am. Thorac. Soc. 2024, 21, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Guerin, C.; Reignier, J.; Richard, J.C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef]
- Amato, M.B.; Barbas, C.S.; Medeiros, D.M.; Magaldi, R.B.; Schettino, G.P.; Lorenzi-Filho, G.; Kairalla, R.A.; Deheinzelin, D.; Munoz, C.; Oliveira, R.; et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N. Engl. J. Med. 1998, 338, 347–354. [Google Scholar] [CrossRef]
- Ferguson, N.D.; Fan, E.; Camporota, L.; Antonelli, M.; Anzueto, A.; Beale, R.; Brochard, L.; Brower, R.; Esteban, A.; Gattinoni, L.; et al. The Berlin definition of ARDS: An expanded rational, justification, and supplementary material. Intensive Care Med. 2012, 38, 1573–1582. [Google Scholar] [CrossRef]
- Miller, P.R.; Johnson, J.C., 3rd; Karchmer, T.; Hoth, J.J.; Meredith, J.W.; Chang, M.C. National Nosocomial Infection Surveillance System: From benchmark to bedside in trauma patients. J. Trauma 2006, 60, 98–103. [Google Scholar] [CrossRef]
- Kallet, R.H. A Comprehensive Review of Prone Position in ARDS. Respir. Care 2015, 60, 1660–1687. [Google Scholar] [CrossRef] [PubMed]
- Albert, R.K.; Leasa, D.; Sanderson, M.; Robertson, H.T.; Hlastala, M.P. The prone position improves arterial oxygenation and reduces shunt in oleic-acid-induced acute lung injury. Am. Rev. Respir. Dis. 1987, 135, 628–633. [Google Scholar] [PubMed]
- Malbouisson, L.M.; Busch, C.J.; Puybasset, L.; Lu, Q.; Cluzel, P.; Rouby, J.J. Role of the heart in the loss of aeration characterizing lower lobes in acute respiratory distress syndrome. CT Scan ARDS Study Group. Am. J. Respir. Crit. Care Med. 2000, 161, 2005–2012. [Google Scholar] [CrossRef]
- Amato, M.B.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.; Mercat, A.; et al. Driving pressure and survival in the acute respiratory distress syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Ladha, K.; Vidal Melo, M.F.; McLean, D.J.; Wanderer, J.P.; Grabitz, S.D.; Kurth, T.; Eikermann, M. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: Hospital based registry study. BMJ 2015, 351, h3646. [Google Scholar] [CrossRef]
- Roca, O.; Peñuelas, O.; Muriel, A.; García-de-Acilu, M.; Laborda, C.; Sacanell, J.; Riera, J.; Raymondos, K.; Du, B.; Thille, A.W.; et al. Driving pressure is a risk factor for ARDS in mechanically ventilated subjects without ARDS. Respir. Care 2021, 66, 1505–1513. [Google Scholar] [CrossRef]
- Riad, Z.; Mezidi, M.; Subtil, F.; Louis, B.; Guérin, C. Short-term effects of the prone positioning manoeuver on lung and chest wall mechanics in ARDS Patients. Am. J. Respir. Crit. Care Med. 2018, 197, 1355–1358. [Google Scholar] [CrossRef]
- Munshi, L.; Del Sorbo, L.; Adhikari, N.K.J.; Hodgson, C.L.; Wunsch, H.; Meade, M.O.; Uleryk, E.; Mancebo, J.; Pesenti, A.; Ranier, V.M.; et al. Prone Position for Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2017, 14, S280–S288. [Google Scholar] [CrossRef]
- Kraut, J.A.; Madias, N.E. Lactic acidosis. N. Engl. J. Med. 2014, 371, 2309–2319. [Google Scholar] [CrossRef]
- Yoshida, T.; Tanaka, A.; Roldan, R.; Quispe, R.; Taenaka, H.; Uchiyama, A.; Fujino, Y. Prone position reduces spontaneous inspiratory effort in patients with acute respiratory distress syndrome: A bicenter study. Am. J. Respir. Crit. Care Med. 2021, 203, 1437–1440. [Google Scholar] [CrossRef]
- Johannigman, J.A.; Davis, K., Jr.; Miller, S.L.; Campbell, R.S.; Luchette, F.A.; Frame, S.B.; Branson, R.D. Prone positioning for acute respiratory distress syndrome in the surgical intensive care unit: Who when and how long? Surgery 2000, 128, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Vagginelli, F.; Carlesso, E.; Taccone, P.; Conte, V.; Chiumello, D.; Valenza, F.; Caironi, P.; Pesenti, A. Decrease in PaCO2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit. Care Med. 2003, 31, 2727–2733. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.L.; Chiang, H.T.; Lin, S.L.; Ger, L.P.; Kun, M.H.; Huang, Y.C. Prone position ventilation induces sustained improvement in oxygenation in patients with acute respiratory distress syndrome who have a large shunt. Crit. Care Med. 2002, 30, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- L’Her, E.; Renault, A.; Oger, E.; Robaux, M.A.; Boles, J.M. A prospective survey of early 12-h prone positioning effects in patients with acute respiratory distress syndrome. Intensive Care Med. 2002, 28, 570–575. [Google Scholar] [CrossRef]
- Girard, R.; Baboi, L.; Ayzac, L.; Richard, J.-C.; Guérin, C.; Group, P.T. The impact of patient positioning on pressure ulcers in patients with severe ARDS: Results from a multicentre randomised controlled trial on prone positioning. Intensive Care Med. 2014, 40, 397–403. [Google Scholar] [CrossRef]
- Lee, J.M.; Bae, W.; Lee, Y.J.; Cho, Y.-J. The efficacy and safety of prone positional ventilation in acute respiratory distress syndrome: Updated study-level meta-analysis of 11 randomized controlled trials. Crit. Care Med. 2014, 42, 1252–1262. [Google Scholar] [CrossRef]
- Page, D.B.; Russell, D.W.; Gandotra, S.; Dransfield, M.T. Prolonged prone position for COVID-19-induced acute respiratory distress syndrome: A randomized pilot clinical trial. Ann. Am. Thorac. Soc. 2022, 19, 685–687. [Google Scholar] [CrossRef]



| Timing | Within I week of a known clinical insult or new/worsening respiratory symptoms | ||
| Chest imaging (Chest X-ray or CT scan) | Bilateral opacities—not fully explained by effusions, lobar/lung collapse, or nodules | ||
| Origin of Edema | Respiratory failure not fully explained by cardiac failure or fluid overload; Need objective assessment (e.g., echocardiography)to exclude hydrostatic edema if no risk factor present | ||
| Mild | Moderate | Severe | |
| Oxygenation | 200 < PaO2/FiO2 ≤ 300 with PEEP or CPAP ≥ 5 cmH2O | 100 < PaO2/FiO2 ≤ 200 with PEEP ≥ 5 cmH2O | PaO2/FiO2 ≤ 100 with PEEP ≥ 5 cmH2O |
| Characteristic | 16 h (N = 21) | 24 h (N = 24) | p-Value |
|---|---|---|---|
| Gender (M/F) | 11/10 | 10/14 | 0.47 |
| Age, year | 71.1± 13.6 | 69.0 ± 11.2 | 0.59 |
| BMI (kg/m2) | 23.2 ± 3.3 | 24.9 ± 6.3 | 0.27 |
| Organ failure number | 2.3 ± 1.2 | 2.3 ± 1.1 | 1.00 |
| APACHE II score Invasive mechanical ventilation (%) | 26.6 ± 7.3 21 (100) | 27.4 ± 7.7 24 (100%) | 0.72 0.99 |
| Sedation (%) | 21 (100) | 24 (100) | 0.99 |
| Muscle relaxant (%) | 18 (85.7) | 24 (100) | 0.06 |
| Vasopressor (%) | 18 (85.7) | 17 (70.8) | 0.22 |
| Steroid (%) Intubation to prone day (median, IQR) | 17 (81.0) 2 (1–3) | 20 (83.3) 2 (1–3) | 0.81 0.36 |
| ARDS to prone day | 1.1 ± 1.9 | 1.0 ± 1.3 | 0.91 |
| Pulmonary ARDS (%) | 16 (76.2) | 20 (83.3) | 0.55 |
| Extrapulmonary ARDS (%) | 5 (23.8) | 4 (16.7) | 0.55 |
| Serum lactate (mmole/L) | 3.6 ± 3.5 | 2.1 ± 1.1 | 0.08 |
| Characteristic | 16 h (N = 21) | 24 h (N = 24) | p-Value |
|---|---|---|---|
| PaO2/FiO2 (mmHg) | 79.3 ± 31.9 | 89.7 ± 30.5 | 0.27 |
| PaO2 (mmHg) | 75.4 ± 28.9 | 78.9 ± 23.4 | 0.67 |
| PaCO2 (mmHg) | 46.3 ± 28.9 | 53.3 ± 18.5 | 0.12 |
| pH | 7.4 ± 0.1 | 7.3 ± 0.1 | 0.13 |
| Respiratory rate (breath/minute) | 23.1 ± 4.9 | 22.1 ± 5.8 | 0.55 |
| Tidal volume (mL) | 456.4 ± 120.4 | 462.8 ± 117.2 | 0.86 |
| PEEP (cmH2O) | 12.0 ± 3.1 | 11.5 ± 2.6 | 0.53 |
| Compliance (mL/cmH2O) | 23.8 ± 8.9 | 23.2 ± 8.8 | 0.82 |
| Driving pressure (cmH2O) | 19.5 ± 4.5 | 21.0 ± 4.2 | 0.25 |
| Mean airway pressure (mmHg) | 19.2 ± 3.1 | 19.3 ± 3.6 | 0.92 |
| Characteristic | 16 h (N = 21) | 24 h (N = 24) | p-Value |
|---|---|---|---|
| Session > 1 of prone positioning (%) Total prone positioning session per patient (median, IQR) | 13 (61.9) 2 (1–3) | 9 (37.5) 1 (1–3) | 0.06 0.77 |
| Change of P/F from prone to supine position (mmHg) | −111.4 ± 134.7 | −74.8 ± 71.9 | 0.28 |
| Tube dislodgement (%) | 1 (4.8) | 0 (0) | 0.28 |
| Endotracheal tube obstruction (%) | 1 (4.8) | 2 (8.3) | 0.63 |
| Pressure sore (%) | 1 (4.8) | 4 (16.7) | 0.13 |
| Ventilator-associated pneumonia (%) | 1 (4.8) | 3 (12.5) | 0.35 |
| Weaning ventilator (%) | 9 (42.9) | 9 (37.5) | 0.71 |
| Mortality at hospital discharge (%) | 12 (57.1) | 13 (54.2) | 0.86 |
| Change in PaCO2 in the first session (mmHg) | 3.2 ± 10.9 | 9.6 ± 15.3 | 0.12 |
| Change in P/F after prone positioning in the first session (mmHg) | 104.4 ± 84.9 | 123.1 ± 105.2 | 0.52 |
| PaO2/FiO2 responder (%) | 20 (95.2) | 23 (95.8) | 0.88 |
| Rescue ECMO (%) | 0 (0) | 0 (0) | 0.99 |
| 30-day outcomes | |||
| ICU-free days | 18.2 ± 7.7 | 14.4 ± 7.2 | 0.28 |
| Ventilator-free days | 16.1 ± 9.1 | 9.7 ± 7.3 | 0.16 |
| Alive and liberated from ventilator (%) | 8 (38.1) | 7 (29.2) | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-W.; Liu, S.-M.; Yang, C.-Y.; Sun, S.-F.; Kuo, S.-H.; Chu, K.-A. Different Duration of Prone Positioning Treatment for Patients with Acute Respiratory Distress Syndrome in Intensive Care Unit Patients: A Prospective Randomized Clinical Study. J. Clin. Med. 2025, 14, 7261. https://doi.org/10.3390/jcm14207261
Hsu C-W, Liu S-M, Yang C-Y, Sun S-F, Kuo S-H, Chu K-A. Different Duration of Prone Positioning Treatment for Patients with Acute Respiratory Distress Syndrome in Intensive Care Unit Patients: A Prospective Randomized Clinical Study. Journal of Clinical Medicine. 2025; 14(20):7261. https://doi.org/10.3390/jcm14207261
Chicago/Turabian StyleHsu, Chien-Wei, Shan-Mei Liu, Chin-Yao Yang, Shu-Fen Sun, Shu-Hung Kuo, and Kao-An Chu. 2025. "Different Duration of Prone Positioning Treatment for Patients with Acute Respiratory Distress Syndrome in Intensive Care Unit Patients: A Prospective Randomized Clinical Study" Journal of Clinical Medicine 14, no. 20: 7261. https://doi.org/10.3390/jcm14207261
APA StyleHsu, C.-W., Liu, S.-M., Yang, C.-Y., Sun, S.-F., Kuo, S.-H., & Chu, K.-A. (2025). Different Duration of Prone Positioning Treatment for Patients with Acute Respiratory Distress Syndrome in Intensive Care Unit Patients: A Prospective Randomized Clinical Study. Journal of Clinical Medicine, 14(20), 7261. https://doi.org/10.3390/jcm14207261






