1. Introduction
Tooth wear (TW) refers to the loss of superficial dental tissue due to chemical (erosion) and/or mechanical processes (attrition and abrasion) that are not related to dental caries, trauma, or developmental disorders [
1]. The prevalence of TW ranges from 30% to 50% in primary dentition and between 20% and 45% in permanent dentition [
2].
Chemical wear may be caused by stomach acids and by exogenous acids from acidic foods and beverages [
3,
4]. Populations affected by gastroesophageal reflux disease (GERD) and individuals with eating disorders (EDs), particularly those who engage in self-induced vomiting, exhibit not only a higher prevalence of chemical TW, but also more severe TW [
5,
6,
7,
8,
9]. Intrinsic mechanical wear results from grinding between upper and lower teeth, as observed in sleep bruxism (SB) or awake bruxism (AB) [
10], whereas extrinsic mechanical wear is mainly associated with aggressive tooth brushing [
11]. Notably, the most recent meta-analysis reports a global bruxism (sleep and awake combined) prevalence of 22.22%, with sleep bruxism at 21% and awake bruxism at 23%, underscoring the magnitude of the problem [
12]. Therefore, several risk factors for TW have been identified, including gastroesophageal reflux disease, EDs, specific diets, acidic beverages, alcohol consumption, other legal and illegal substances, medications particularly, oral dryness-inducing agents and inhaled corticosteroids for asthma management, as well as occupational and sports-related factors [
13,
14]. Individuals affected by gambling disorder (GD) and other addictive behaviors may experience poorer sleep quality, higher stress levels, and increased anxiety [
15]. Since these psychological states are strongly associated with AB and, to a lesser extent, SB, persons with GD, especially those experiencing higher addiction-related distress, may be more prone to jaw clenching or teeth grinding during wakefulness. However, whether individuals with GD represent risk group of TW is not well established, since no study has assessed the severity of mechanical or chemical TW of this population [
13].
In the general population, several risk factors have been associated with TW, and those assessed by questionnaires or interview are categorized into several domains, including sociodemographic/socioeconomic factors, medical history, drinking habits, eating habits, oral hygiene habits, dental factors, bruxism and temporomandibular disorder (TMD), behavioral or leisure-related factors, and stress [
16,
17]. Age, male gender, regurgitation, and dietary factors seem to be the key factors for chemical TW, whereas oral hygiene habits and bruxism are the main factors for mechanical TW [
16,
17,
18,
19]. The reason why men have more TW are not yet understood, but it has been hypothesized that men consume more acidic drinks resulting in more chemical TW [
20,
21]. In addition, men have higher bite force, leading to more occlusal contact area and mechanical TW [
20,
22,
23]. On the other hand, saliva is recognized as one of the most important protective factors against dental erosion due to its buffering capacity, remineralization potential, and role in the formation of the acquired enamel pellicle [
24,
25]. In conditions with frequent intrinsic acid exposure, altered salivary parameters have been described with heterogenous results. One recent systematic review and meta-analysis found that patients with gastroesophageal reflux disease exhibit significantly lower salivary pH, reduced stimulated flow rates, and diminished buffering capacity compared with healthy controls [
26]. Similarly, another systematic review stated that individuals with eating disorders—especially those with bulimia—often experience decreased unstimulated salivary flow, lower pH, impaired buffering ability, and frequent xerostomia, with severity worsening alongside longer illness duration and concurrent medication use [
8]. It remains unclear how stimulated and unstimulated salivary flow, pH, and buffering capacity contribute to protection against TW in individuals exposed to acid due to GERD or self-induced vomiting, as well as in individuals with either SB or AB [
21,
25,
27].
The relationship between different salivary characteristics and the presence of TW has been studied recently in different populations, from a general population of young adults [
28,
29] to patients with moderate or severe TW [
18,
21]. However, it is still unknown what the role of salivary characteristics is in the severity of mechanical or chemical TW in individuals of different at-risk populations. Since several risk factors have correlated each other, several statistical approaches have been recommended to provide a stronger basis for causal inference, such as multivariable regression models, quantitative assessment of systematic bias, and conditional process analysis [
30,
31,
32].
This study aimed to investigate the relationship between salivary characteristics and the severity of chemical and mechanical TW across different at-risk groups, including GERD, SB and mental disorders such as EDs and GD. Additionally, it also aimed to identify the predictors more strongly associated with the severity of chemical and mechanical TW. The null hypothesis was that there is no relationship between any salivary characteristic and the degree of TW.
4. Discussion
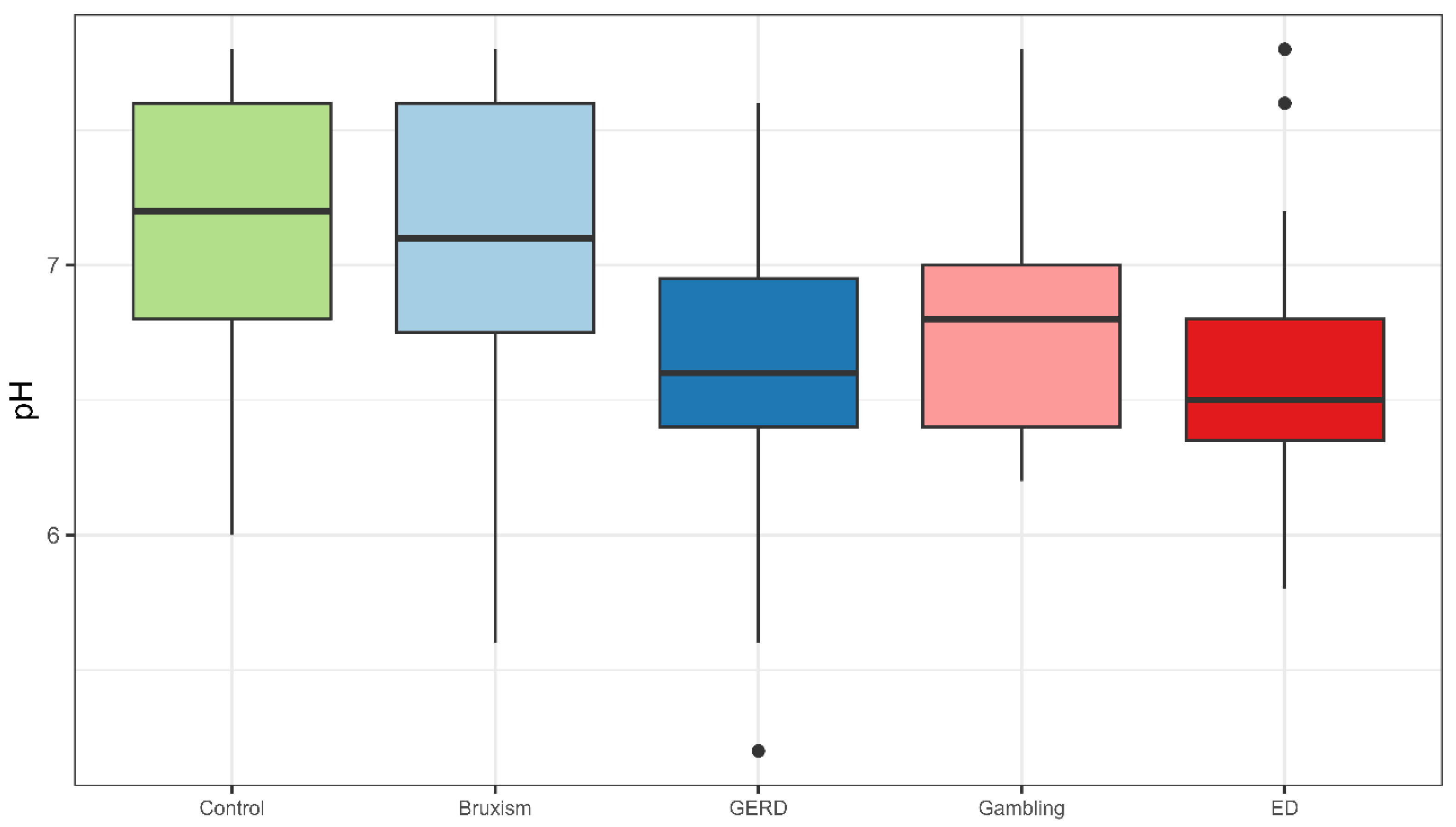
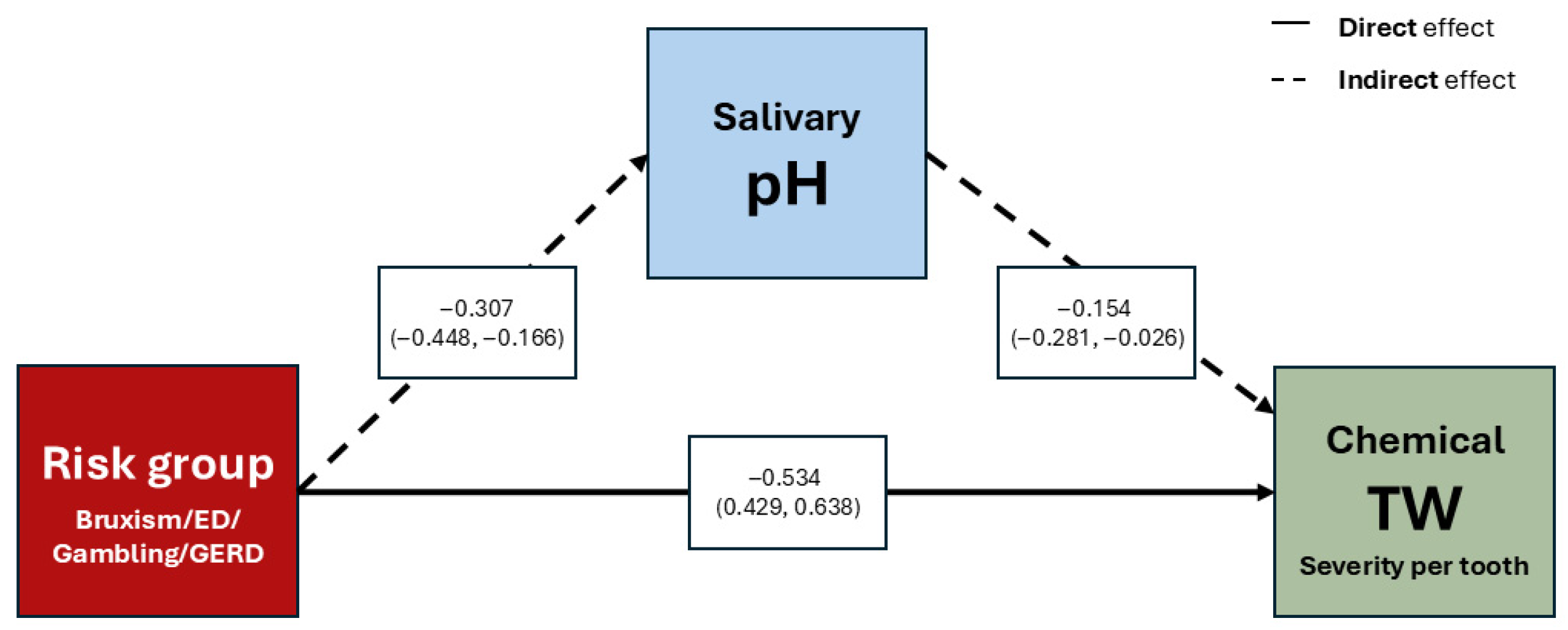
This study shows that belonging to an at-risk group and exhibiting lower salivary pH are the variables most strongly associated with chemical TW. Accordingly, the null hypothesis of no relationship between salivary characteristics and TW is partially rejected, as salivary pH showed a significant association with chemical TW, whereas salivary flow rate and buffer capacity did not.
Both GERD and ED risk groups exhibited lower salivary pH, and GERD patients also showed the lowest stimulated salivary flow rate, while buffering capacity did not differ significantly among groups. These findings align with previous research linking intrinsic acid exposure to altered salivary parameters, although results remain inconsistent due to methodological limitations [
26,
27,
49]. The qualitative assessment of the systematic review by Madariaga et al. [
25] reported an inverse correlation between salivary pH and flow and TW, but found no clear association with buffering capacity, likely due to limitations of colorimetric strip methods. Similarly, a meta-analysis restricted to patients with GERD concluded that salivary pH, resting flow, and buffering capacity may be reduced, although evidence remains uncertain due to variability in GERD diagnosis and salivary testing protocols [
26].
GERD is a well-known risk factor for TW, reflecting alterations in salivary protective function. Moazzez et al. [
50] reported reduced buffering capacity and lower stimulated salivary flow in patients with hoarseness. Similarly, Sujatha et al. [
51] and Bechir et al. [
52] found decreased salivary pH, flow, and buffering capacity associated with TW. Proton pump inhibitor use, and chronic acid exposure may further impair salivary gland function [
26].
Similarly, patients with EDs and self-induced vomiting experience recurrent acid exposure and salivary dysfunction [
6,
53]. Evidence on salivary alterations in EDs shows heterogeneous results. While some studies reported lower oral pH [
54] or reduced unstimulated salivary flow associated with greater erosion severity [
55], others identified decreased pH, bicarbonate, and phosphate levels leading to reduced buffering capacity [
56]. A systematic review confirmed that, despite methodological differences, reduced salivary pH and flow are frequent findings and represent key risk factors for TW in these patients [
53]. In the present study, however, no significant differences in salivary flow or buffering capacity were observed in this group, possibly due to the lower chronicity of ED and the small sample size [
25].
Regarding the SB group, salivary parameters were within normal ranges, aligning with previous findings [
18,
29,
57]. These results might suggest that saliva plays a limited role in mechanical wear under normal conditions but may become relevant in patients with comorbidities or reduced salivary flow, which could contribute to TW by decreasing oral lubrication [
10,
58].
Interestingly, the group with GD also exhibited significantly lower salivary pH despite normal flow and buffering capacity. This is the first report on salivary parameters and TW in GD, a population characterized by stress dysregulation, psychiatric comorbidities, all known to affect salivary composition [
59,
60,
61].
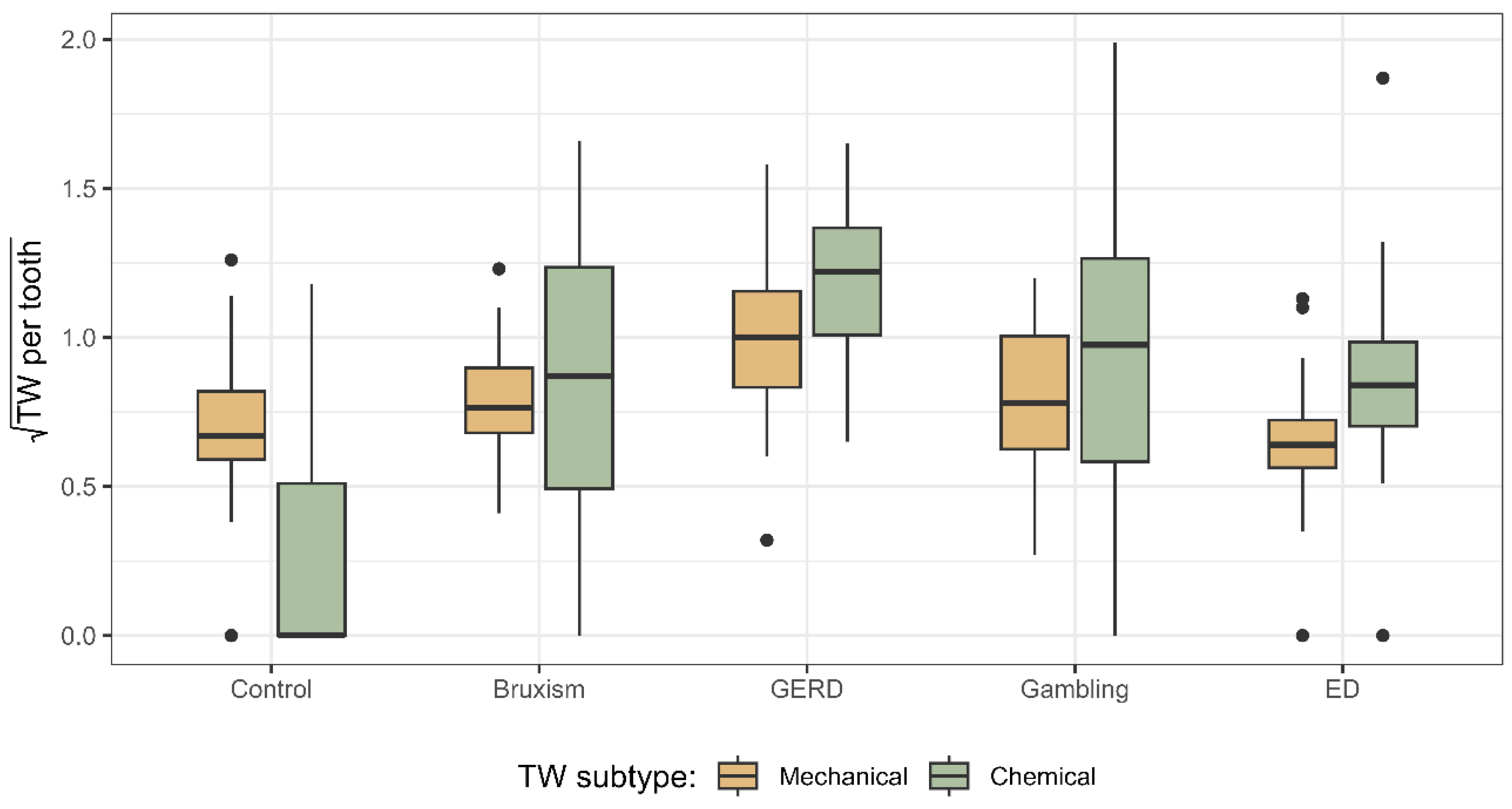
In terms of TW severity, notable differences were observed depending on the underlying aetiology. Patients with GERD presented the greatest severity of both chemical and mechanical wear, suggesting a synergistic effect between acid erosion and attrition, as acid-mediated demineralization increases susceptibility of dental surfaces to forces from mastication, bruxism, and parafunctional habits [
10,
62,
63]. GERD is frequently associated with SB, conferring a 4.7-fold increased risk of severe TW [
63]. Additionally, GERD-associated salivary dysfunction and older age may contribute to greater loss [
64].
By contrast, ED, Gambling, and Sleep Bruxism groups showed mechanical TW severity comparable to controls. Although these populations are associated with stress, anxiety, and bruxism, the bruxism itself tends to be intermittent and when it occurs predominantly in a centric pattern, the limited duration of tooth contact may reduce clinically detectable mechanical wear [
10,
65,
66]. The predominance of chemical wear in the sleep bruxism group supports earlier findings that erosion may amplify occlusal TW [
67]. Differentiating mechanical and chemical components remains challenging given the lack of standardized diagnostic criteria and limited clinical validation of wear patterns [
43].
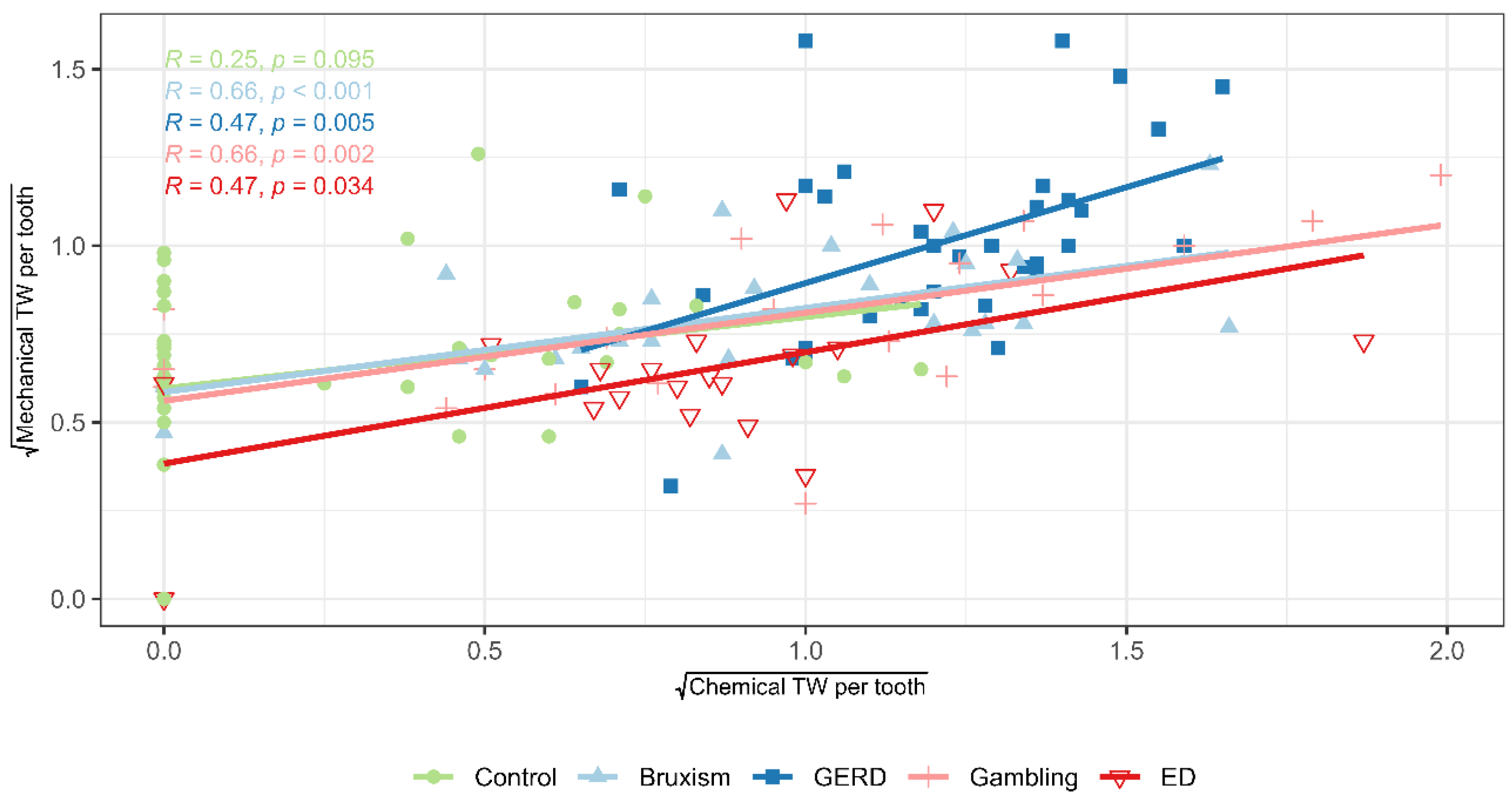
The results showed a strong association between the severity of chemical and mechanical TW per tooth, especially in high-risk groups, consistent with previous studies. Rius-Bonet et al. [
29] found erosion increased attrition risk 6.3-fold in healthy young adults, highlighting the importance of preventive strategies targeting both processes. Chemical and mechanical factors act synergistically, as acid-induced softening enhances susceptibility to mechanical forces [
27,
67]. Clinically, this underscores the need to minimize acid exposure (e.g., diet, reflux, ED, salivary dysfunction) while controlling mechanical risks (e.g., bruxism, parafunctions, brushing technique).
Analysis of the salivary parameters revealed physiologically expected interrelationships. Physiologically, salivary flow rate and pH are inversely related to viscosity, as increased flow enhances bicarbonate secretion, elevates pH, and reduces mucin concentration, thereby decreasing viscosity. In contrast, buffering capacity primarily depends on the proportion of bicarbonates, phosphates, and specific proteins, and does not vary in direct proportion to flow rate or viscosity [
27,
68].
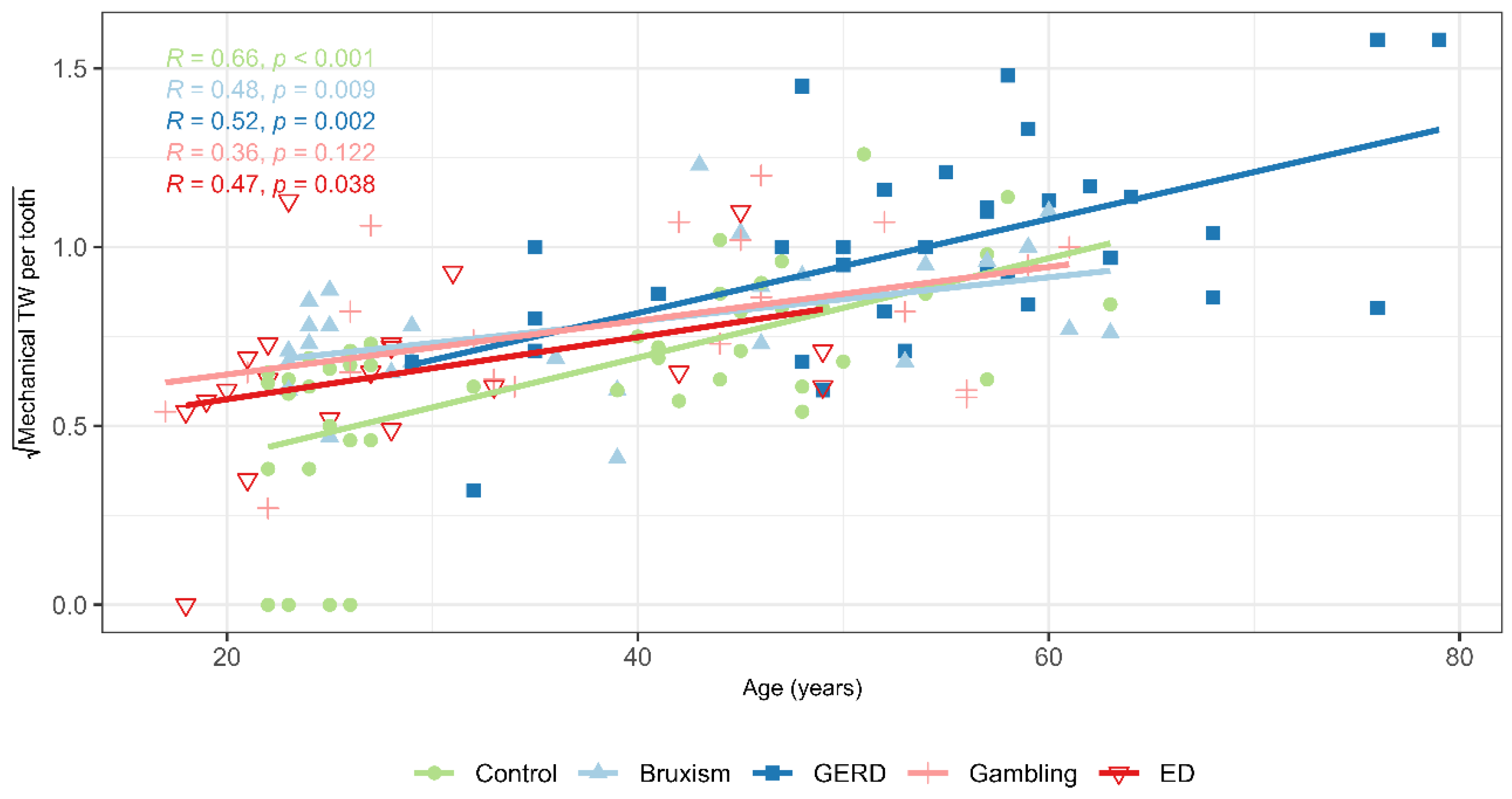
Chemical TW severity increased with age and BMI and decreased with the number of remaining teeth. Age-related cumulative acid exposure and reduced salivary protection, along with concentrated occlusal loads from tooth loss, increase susceptibility [
10,
27,
69]. Higher BMI, often associated with GERD and acidic dietary patterns, also contributes to wear [
17]. However, no association with acidic diet was observed, likely due to underreporting, cross-sectional design, or low exposure levels [
14,
70].
The severity of mechanical TW was positively associated with age, male sex, brushing force, and bruxism, and negatively with the number of teeth and frequency of toothbrushing. Aging increases cumulative exposure to masticatory forces and oral habits, while tooth loss concentrates functional load on fewer occlusal surfaces, amplifying attrition [
10,
71]. Literature has shown that inadequate brushing technique and excessive brushing force are associated with cervical abrasion [
67,
72]. However, there is no evidence that lower brushing frequency contributes to greater TW through improper technique or excessive force. Finally, bruxism, particularly involving eccentric tooth contact, has been linked to increased mechanical wear severity due to repetitive occlusal loading [
10,
73]. However, most evidence is cross-sectional, underscoring the need for longitudinal studies using standardized criteria for bruxism and TW assessment [
19].
Most studies on TW do not differentiate chemical from mechanical processes. In contrast, our study separated these components and found no sex-related differences in chemical wear, but significantly greater mechanical wear in men, associated with higher maximum bite force and BMI. These results support previous evidence linking greater occlusal load from higher bite force in men to increased mechanical TW [
23,
74,
75].
In the present study, age was positively associated with BMI, acidic dietary habits, SB, salivary dehydration, and increased viscosity, and while it was negatively associated with the number of teeth, perceived stress, salivary pH, and flow rate. Trends in overweight status, behavioural factors, and age-related physiological changes in salivary function may contribute to an elevated risk of TW in older adults [
10,
21,
76]. Younger adults, despite higher stress, generally have more efficient salivary function [
29,
77].
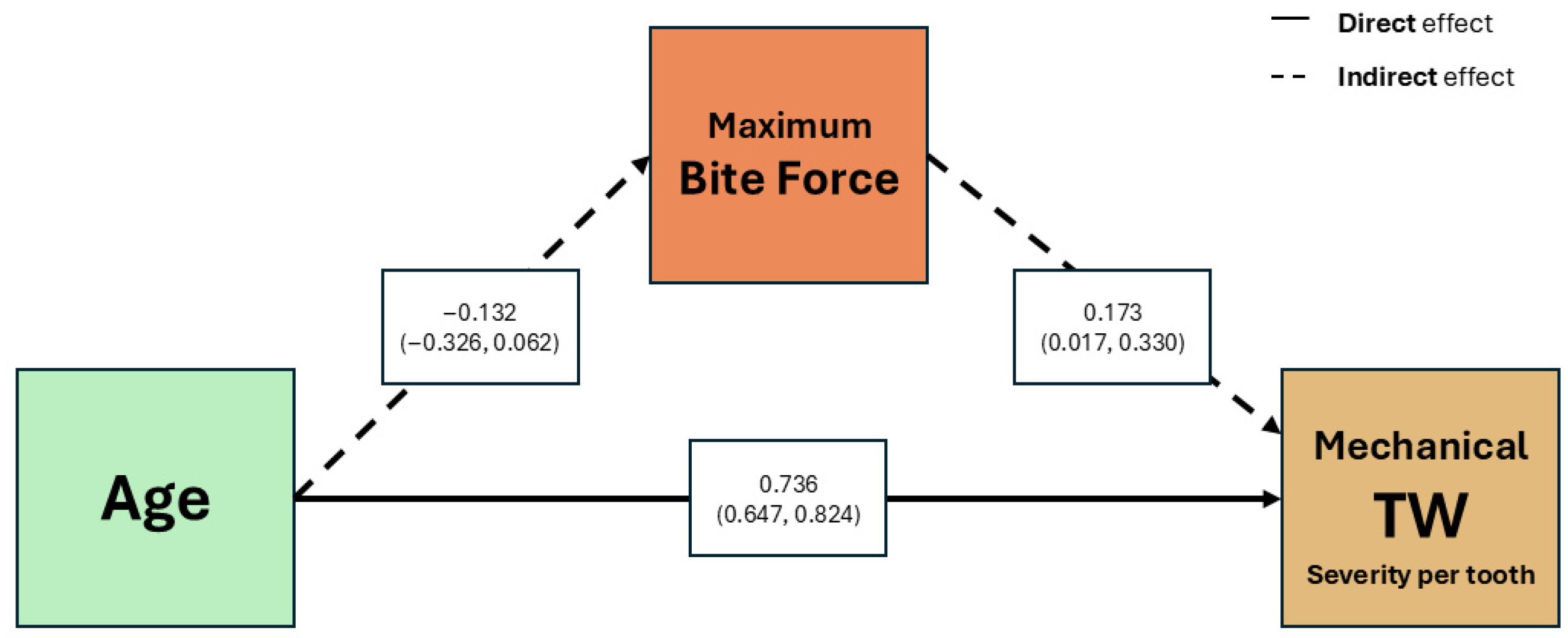
Multivariate analysis adjusted for age and sex identified at-risk group membership as directly associated with chemical TW, with salivary pH mediating this relationship. Studies incorporating salivary parameters confirm that not all components have equal weight as risk factors. Madariaga et al. [
21] reported that stimulated salivary pH had the strongest negative association with wear severity after adjusting for age, sex, and risk group, consistent with Nobre et al. [
28], who identified reduced pH as a key predictor of dental erosion. Likewise, Mulic et al. [
78] found risk group membership, such as GERD, to be a significant determinant of dental erosion. Collectively, these findings suggest that salivary pH may be a potential indicator of TW risk. The mediation model assessing maximum bite force as a link between age and mechanical TW showed both age and bite force positively associated with wear per tooth, even after adjusting for sex. However, no significant indirect effect of age through bite force was found. These findings are consistent with previous evidence identifying age as a marker of cumulative exposure and bite force as an indicator of occlusal load intensity, both exerting separate effects on structural dental loss [
69,
75]. It should be emphasized that the absence of bite force data for some participants at random could influence the interpretation of the mediation model; however, this study is designed to explore variable associations rather than to draw causal inferences.
Certain limitations in this study must be acknowledged. Technical and logistical challenges—such as strict eligibility criteria, limited participant availability, and extended recruitment timelines—prevented attainment of the planned sample size, especially for the TCA and ED groups. Consequently, statistical power may be reduced, and findings in these groups should be viewed as preliminary. The cross-sectional design precludes temporal analysis and limits causal inference. High variability among available TW assessment tools and methodological inconsistencies in salivary testing pose challenges for comparisons across studies and interpretation of findings. Although chair-side saliva test kits are convenient and user-friendly, their reliance on colorimetric pH and buffering evaluation provides only semi-quantitative results. Readings may fluctuate based on operator interpretation, which could affect precision. Additionally, reliance on self-reported data, potential residual confounding beyond age and sex, and recruitment from a tertiary care setting may affect external validity. Nevertheless, key strengths include rigorous group classification with standardized criteria, inclusion of a healthy control group, and novel data on GD as possible emerging at-risk profile. Exploratory modelling, along with efforts to separately assess chemical and mechanical wear, contributed to a better distinction of possible etiological factors. Understanding the predominant type of wear in each risk group supports the development of more targeted preventive and therapeutic strategies.