Assessment of the Effectiveness of a Visual Coaching Device Combined with Auditory Instructions on Reproducibility and Stability in Deep Inspiration Breath-Hold Radiotherapy
Abstract
1. Introduction
2. Materials and Methods
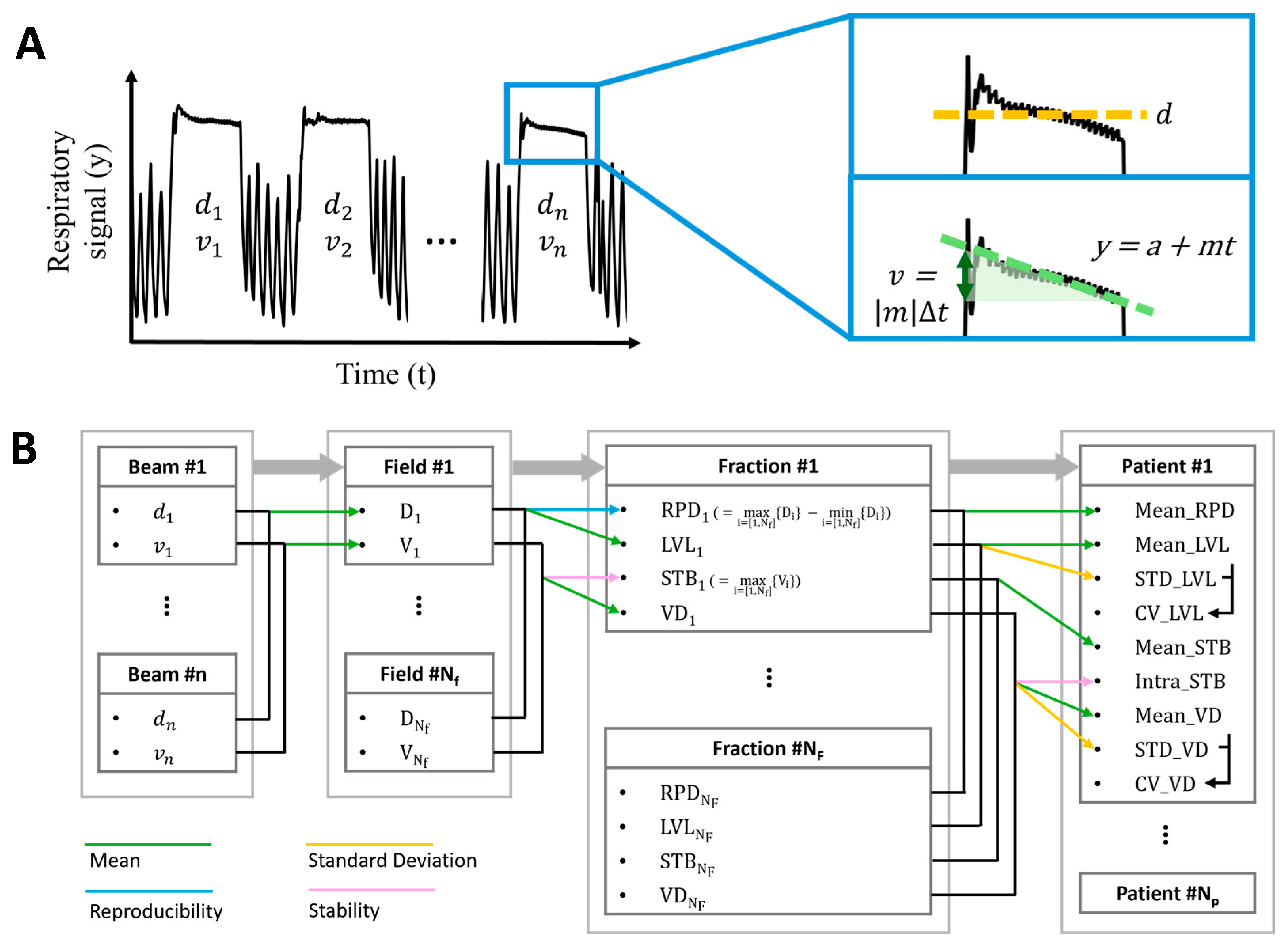
2.1. Data Acquisition and Processing
2.2. Evaluation Metrics
2.3. Statistical Analysis
3. Results
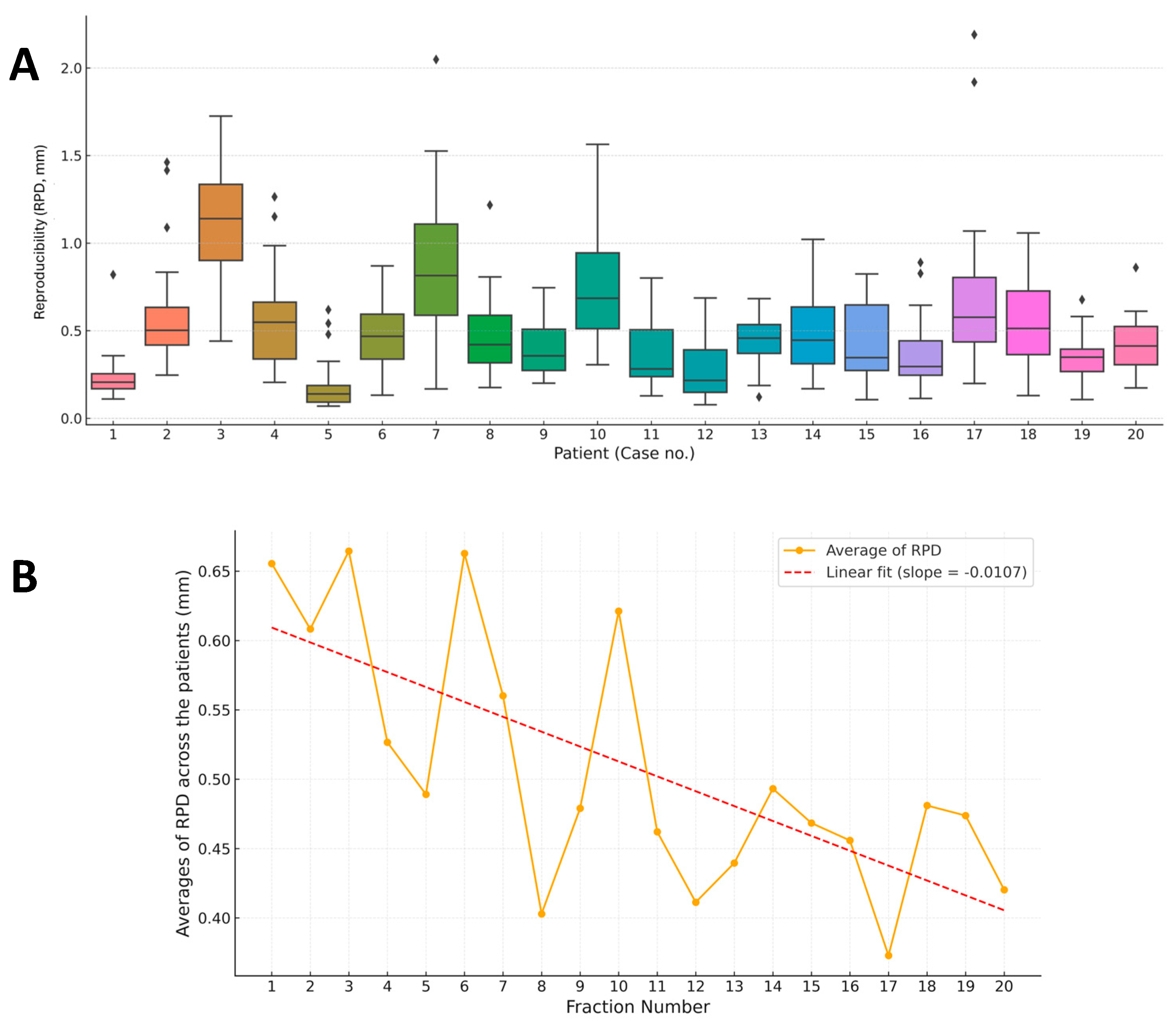
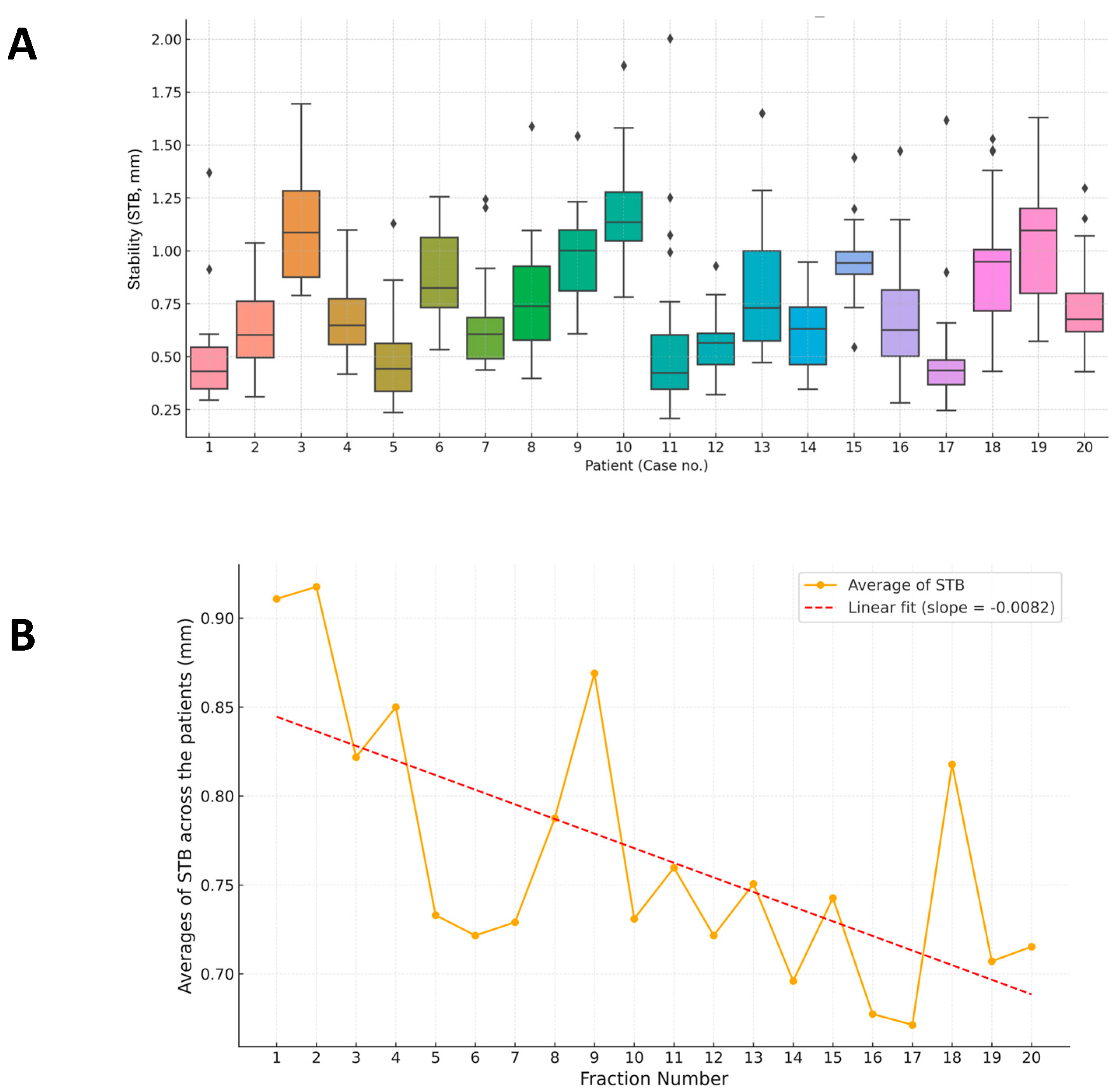
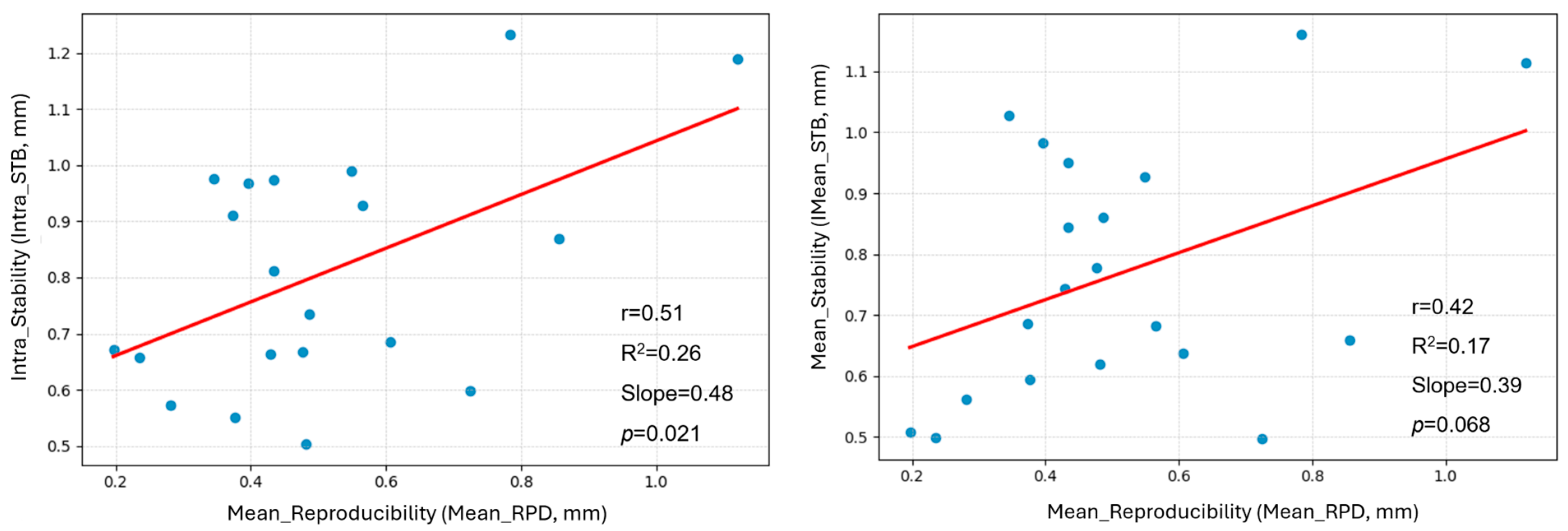
3.1. Overview of RPD and STB Evaluation
3.2. RPD of Breath-Hold Level
3.3. STB of Breath-Hold Across Fractions
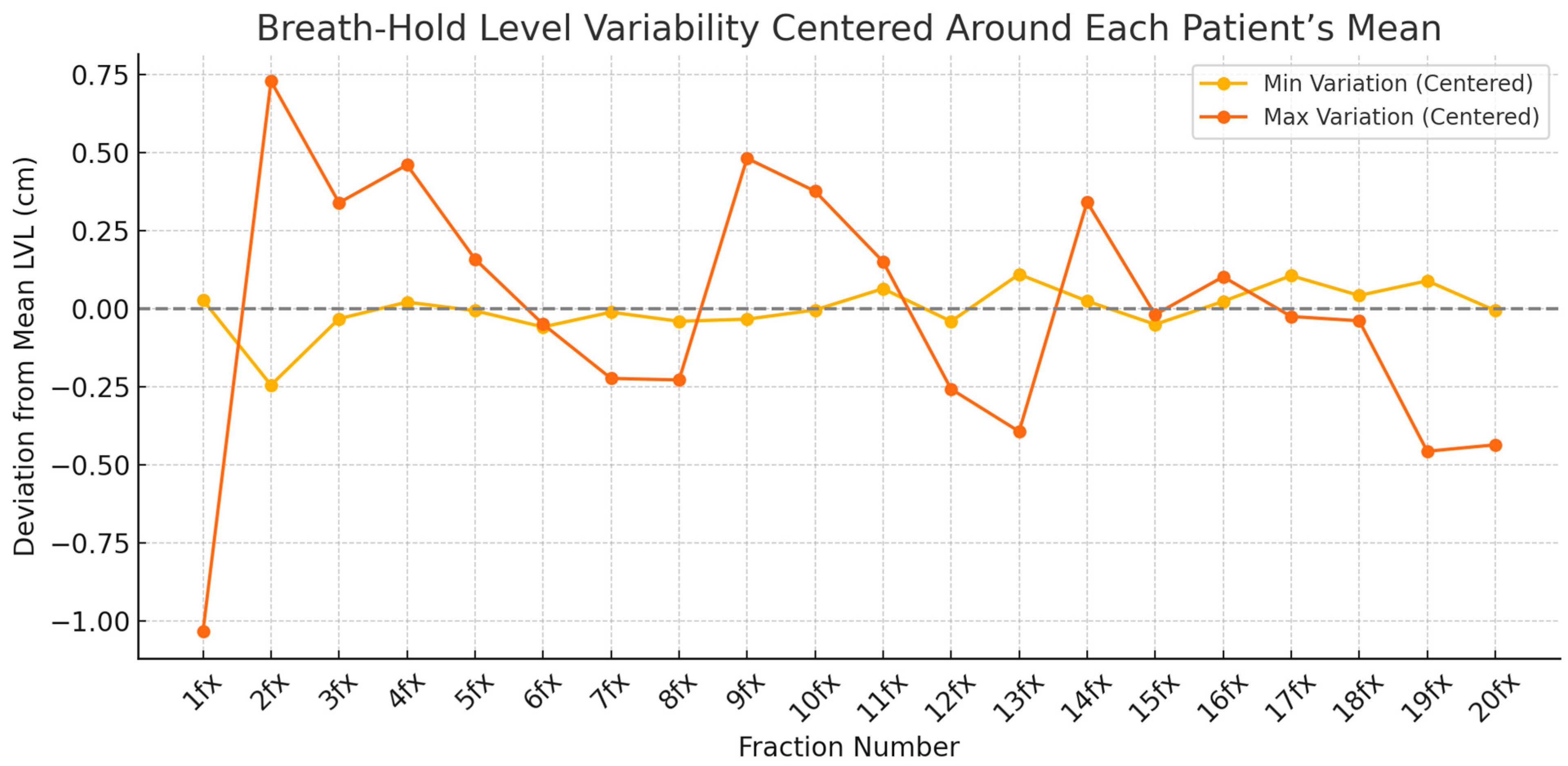
3.4. Case-Specific Analysis of Breath-Hold Level
3.5. Image-Guided Radiotherapy Results of All Treatment Sessions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| CV | Coefficients of variation |
| DIBH | Deep inspiration breath-hold |
| LAD | Left anterior descending |
| LVL | Average of mean level |
| RIHD | Radiation-induced heart disease |
| RPD | Reproducibility |
| STB | Stability |
| SGRT | Surface-guided radiation therapy |
| VD | Averages of end-to-end deviation |
| VF | Visual feedback |
References
- Cheng, Y.J.; Nie, X.Y.; Ji, C.C.; Lin, X.X.; Liu, L.J.; Chen, X.M.; Yao, H.; Wu, S.H. Long--term cardiovascular risk after radiotherapy in women with breast cancer. J. Am. Heart Assoc. 2017, 6, e005633. [Google Scholar] [CrossRef]
- Nielsen, K.M.; Offersen, B.V.; Nielsen, H.M.; Vaage-Nilsen, M.; Yusuf, S.W. Short and long term radiation induced cardiovascular disease in patients with cancer. Clin. Cardiol. 2017, 40, 255–261. [Google Scholar] [CrossRef]
- van den Bogaard, V.A.B.; Spoor, D.S.; van der Schaaf, A.; van Dijk, L.V.; Schuit, E.; Sijtsema, N.M.; Langendijk, J.A.; Maduro, J.H.; Crijns, A.P.G. The importance of radiation dose to the atherosclerotic plaque in the left anterior descending coronary artery for radiation-induced cardiac toxicity of breast cancer patients? Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1350–1359. [Google Scholar] [CrossRef]
- Zureick, A.H.; Grzywacz, V.P.; Almahariq, M.F.; Silverman, B.R.; Vayntraub, A.; Chen, P.Y.; Gustafson, G.S.; Jawad, M.S.; Dilworth, J.T. Dose to the left anterior descending artery correlates with cardiac events after irradiation for breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 130–139. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Remouchamps, V.M.; Letts, N.; Vicini, F.A.; Sharpe, M.B.; Kestin, L.L.; Chen, P.Y.; Martinez, A.A.; Wong, J.W. Initial clinical experience with moderate deep-inspiration breath hold using an active breathing control device in the treatment of patients with left-sided breast cancer using external beam radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Vikström, J.; Hjelstuen, M.H.; Mjaaland, I.; Dybvik, K.I. Cardiac and pulmonary dose reduction for tangentially irradiated breast cancer, utilizing deep inspiration breath-hold with audio-visual guidance, without compromising target coverage. Acta Oncol. 2011, 50, 42–50. [Google Scholar] [CrossRef]
- Hayden, A.J.; Rains, M.; Tiver, K. Deep inspiration breath hold technique reduces heart dose from radiotherapy for left--sided breast cancer. J. Med. Imag. Radiat. Oncol. 2012, 56, 464–472. [Google Scholar] [CrossRef]
- Rochet, N.; Drake, J.I.; Harrington, K.; Wolfgang, J.A.; Napolitano, B.; Sadek, B.T.; Shenouda, M.N.; Keruakous, A.R.; Niemierko, A.; Taghian, A.G. Deep inspiration breath-hold technique in left-sided breast cancer radiation therapy: Evaluating cardiac contact distance as a predictor of cardiac exposure for patient selection. Pract. Radiat. Oncol. 2015, 5, e127–e134. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, D.; Zhang, X.; Teng, Y.; Yuan, W.; Zhang, Y.; He, R.; Tang, F.; Pang, J.; Han, B.; et al. Comparison of deep inspiration breath hold versus free breathing in radiotherapy for left sided breast cancer. Front. Oncol. 2022, 12, 845037. [Google Scholar] [CrossRef] [PubMed]
- Falco, M.; Masojć, B.; Macała, A.; Łukowiak, M.; Woźniak, P.; Malicki, J. Deep inspiration breath hold reduces the mean heart dose in left breast cancer radiotherapy. Radiol. Oncol. 2021, 55, 212–220. [Google Scholar] [CrossRef]
- Wu, J.; Yang, F.; Li, J.; Wang, X.; Yuan, K.; Xu, L.; Wu, F.; Tang, B.; Orlandini, L.C. Reproducibility and stability of voluntary deep inspiration breath hold and free breath in breast radiotherapy based on real-time 3-dimensional optical surface imaging system. Radiat. Oncol. 2024, 19, 158. [Google Scholar] [CrossRef]
- Cerviño, L.I.; Gupta, S.; Rose, M.A.; Yashar, C.; Jiang, S.B. Using surface imaging and visual coaching to improve the reproducibility and stability of deep-inspiration breath hold for left-breast-cancer radiotherapy. Phys. Med. Biol. 2009, 54, 6853–6865. [Google Scholar] [CrossRef]
- Hoshina, M.; Noguchi, M.; Sekihara, H.; Masuda, K.; Shinmura, M.; Sugahara, S. Chest wall to heart distance reproducibility in postoperative deep inspiration breath-hold radiotherapy for left-sided breast cancer using an Anzai laser sensor with visual feedback. Cureus 2024, 16, e53183. [Google Scholar] [CrossRef]
- Penninkhof, J.; Fremeijer, K.; Offereins-van Harten, K.; van Wanrooij, C.; Quint, S.; Kunnen, B.; Hoffmans-Holtzer, N.; Swaak, A.; Baaijens, M.; Dirkx, M. Evaluation of image-guided and surface-guided radiotherapy for breast cancer patients treated in deep inspiration breath-hold: A single institution experience. Tech. Innov. Patient Support Radiat. Oncol. 2022, 21, 51–57. [Google Scholar] [CrossRef]
- Yamauchi, R.; Mizuno, N.; Itazawa, T.; Masuda, T.; Akiyama, S.; Kawamori, J. Assessment of visual feedback system for reproducibility of voluntary deep inspiration breath hold in left-sided breast radiotherapy. J. Med. Imaging Radiat. Sci. 2021, 52, 544–551. [Google Scholar] [CrossRef]
- Yoshitake, T.; Nakamura, K.; Shioyama, Y.; Nomoto, S.; Ohga, S.; Toba, T.; Shiinoki, T.; Anai, S.; Terashima, H.; Kishimoto, J.; et al. Breath-hold monitoring and visual feedback for radiotherapy using a charge-coupled device camera and a head-mounted display: System development and feasibility. Radiat. Med. 2008, 26, 50–55. [Google Scholar] [CrossRef]
- George, R.; Chung, T.D.; Vedam, S.S.; Ramakrishnan, V.; Mohan, R.; Weiss, E.; Keall, P.J. Audio-visual biofeedback for respiratory-gated radiotherapy: Impact of audio instruction and audio-visual biofeedback on respiratory-gated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 924–933. [Google Scholar] [CrossRef]
- Goossens, S.; Senny, F.; Lee, J.A.; Janssens, G.; Geets, X. Assessment of tumor motion reproducibility with audio--visual coaching through successive 4D CT sessions. J. Appl. Clin. Med. Phys. 2014, 15, 4332. [Google Scholar] [CrossRef] [PubMed]
- Linthout, N.; Bral, S.; Van de Vondel, I.; Verellen, D.; Tournel, K.; Gevaert, T.; Duchateau, M.; Reynders, T.; Storme, G. Treatment delivery time optimization of respiratory gated radiation therapy by application of audio-visual feedback. Radiother. Oncol. 2009, 91, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Nangia, S.; Khosa, R.; Piyushi, D.; Singh, M.; Singh, G.; Sreedevi, K.; Chauhan, S.K.; Rout, S.K.; Oomen, S. Deep inspiratory breath-hold radiation for left-sided breast cancer using novel frame-based tactile feedback. J. Med. Phys. 2023, 48, 85–89. [Google Scholar] [CrossRef]
- Sano, N.; Saito, M.; Onishi, H.; Kuriyama, K.; Komiyama, T.; Marino, K.; Aoki, S.; Araya, M. Audio-visual biofeedback for respiratory motion management: Comparison of the reproducibility of breath-holding between visual and audio guidance. J. Mod. Phys. 2018, 9, 2286–2294. [Google Scholar] [CrossRef]
- Yu, J.; Choi, J.H.; Ma, S.Y.; Jeung, T.S.; Lim, S. Comparison between audio-only and audiovisual biofeedback for regulating patients’ respiration during four-dimensional radiotherapy. Radiat. Oncol. J. 2015, 33, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Kefeli, A.U.; Diremsizoglu, U.; Erdogan, S.; Karabey, A.U.; Konuk, A.O.; Tirpanci, B.; Aksu, M.G.; Sarper, E.B. Patient coaching for deep inspiration breath hold decreases set-up duration and left anterior descending artery dose for left-sided breast cancer radiotherapy. Support. Care Cancer 2025, 33, 387. [Google Scholar] [CrossRef] [PubMed]
- Schönecker, S.; Angelini, L.; Gaasch, A.; Zinn, A.; Konnerth, D.; Heinz, C.; Xiong, Y.; Unger, K.; Landry, G.; Meattini, I.; et al. Surface-based deep inspiration breath-hold radiotherapy in left-sided breast cancer: Final results from the SAVE-HEART study. ESMO Open 2024, 9, 103993. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, Y.Y.; Bae, E.; Yea, J.W.; Park, J.W.; Park, J.; Oh, S.A.; Lee, H. Assessment of the Effectiveness of a Visual Coaching Device Combined with Auditory Instructions on Reproducibility and Stability in Deep Inspiration Breath-Hold Radiotherapy. J. Clin. Med. 2025, 14, 7259. https://doi.org/10.3390/jcm14207259
Jo YY, Bae E, Yea JW, Park JW, Park J, Oh SA, Lee H. Assessment of the Effectiveness of a Visual Coaching Device Combined with Auditory Instructions on Reproducibility and Stability in Deep Inspiration Breath-Hold Radiotherapy. Journal of Clinical Medicine. 2025; 14(20):7259. https://doi.org/10.3390/jcm14207259
Chicago/Turabian StyleJo, Yoon Young, Eunseo Bae, Ji Woon Yea, Jae Won Park, Jaehyeon Park, Se An Oh, and Hyunyeol Lee. 2025. "Assessment of the Effectiveness of a Visual Coaching Device Combined with Auditory Instructions on Reproducibility and Stability in Deep Inspiration Breath-Hold Radiotherapy" Journal of Clinical Medicine 14, no. 20: 7259. https://doi.org/10.3390/jcm14207259
APA StyleJo, Y. Y., Bae, E., Yea, J. W., Park, J. W., Park, J., Oh, S. A., & Lee, H. (2025). Assessment of the Effectiveness of a Visual Coaching Device Combined with Auditory Instructions on Reproducibility and Stability in Deep Inspiration Breath-Hold Radiotherapy. Journal of Clinical Medicine, 14(20), 7259. https://doi.org/10.3390/jcm14207259





