Biological Anti-Tumoral Mechanisms of Metformin in Head and Neck Squamous Cell Carcinomas: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Type of Studies
2.2. Exclusion Criteria
2.3. Outcomes
2.4. Search Strategy
2.5. Study Selection
2.6. Data Collection Process and Data Items
3. Results
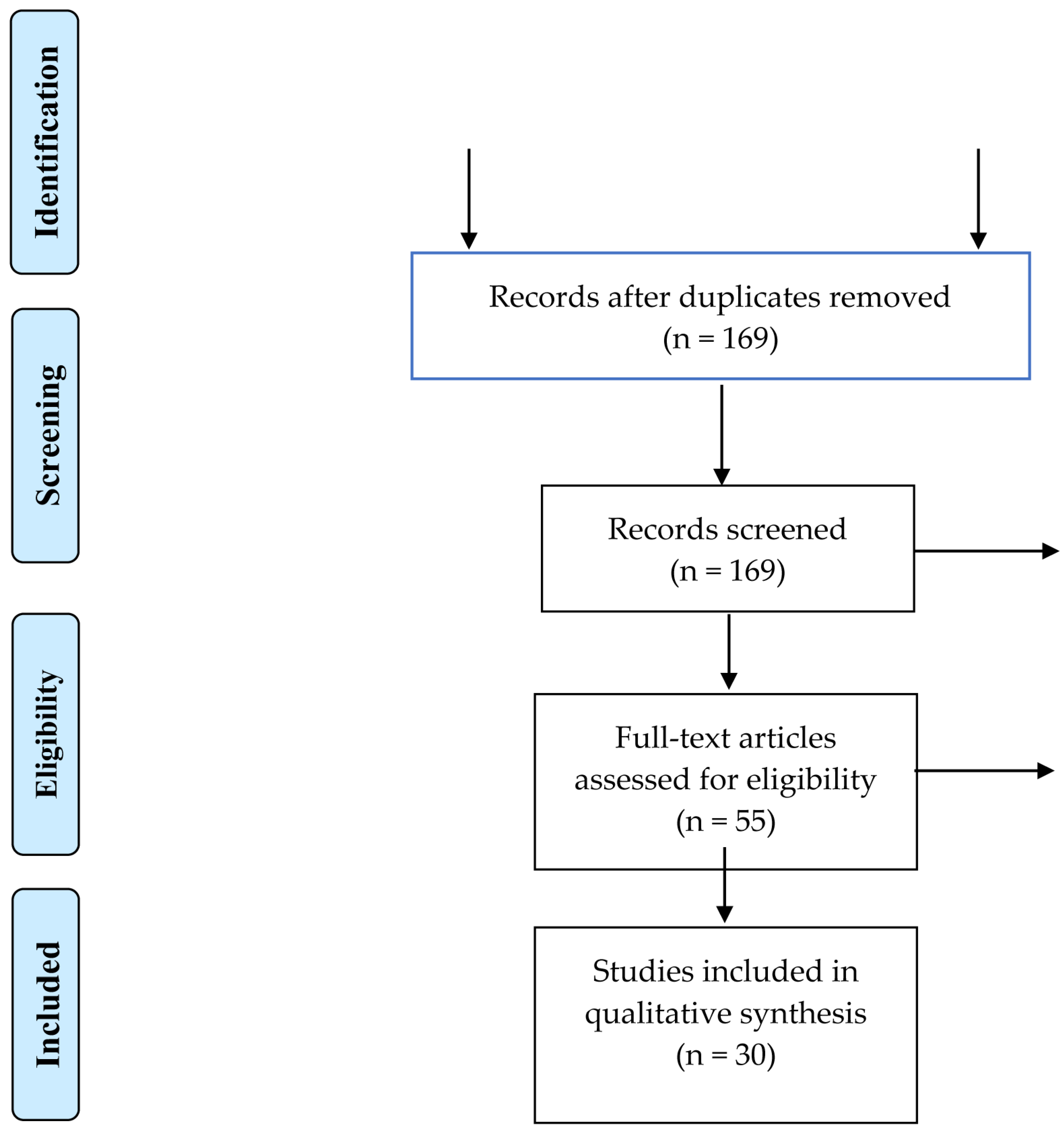
3.1. Article Selection
3.2. Metformin Used Alone
| Authors, Year, Country | Study Design | Assays | Cell Line | Treatment +/− Control | Concentration/IC50 or Effective Dose | In Vivo Toxicity/Side Effects | Results |
|---|---|---|---|---|---|---|---|
| Wang et al., 2020 [12], China | In vitro | Cell proliferation assay/Cell cycle assay/Cell apoptosis assay/WB/Construction of YAP-overexpressing cell lines/RT-q PCR analysis | CAL27 and SCC25 | Metformin | Not specified No IC50 reported | Not reported | Metformin promotes cell apoptosis, inhibits cell proliferation, and stimulates the Hippo signaling pathway in OSCC cells. Metformin inhibits OSCC cell growth by decreasing YAP and decreases mTOR and c-Myc through the downregulation of YAP |
| Wei et al., 2021 [41], China | In vitro | Cell culture and treatment/Plasmid construction and lentiviral transfection/Cell proliferation assay/Colony formation assay/Cell cycle analysis/qRT-PCR/Immunoprecipitation and WB/IF staining/Chromatin immunoprecipitation assays | SCC-15 | Metformin | Not specified No IC50 reported | Not reported | Metformin inhibits OSCC cell proliferation by suppressing NGFR proteolysis, which promotes OSCC cell proliferation. |
| Patil et al., 2020 [40], Saudi Arabia | In vitro | Preparation of the single-cell suspension/MTT assay for cell viability/RT-PCR for stemness related transcription factors/Flow cytometry analysis for CD44 expression | OSCC | Metformin | Not specified No IC50 reported | Not reported | Metformin showed downregulation in the gene expressions of stemness related transcription factors OCT4, SOX2, NANOG, c-Myc, and KLF4 in a dose-dependent as well as time-dependent manner. |
| Zhang et al., 2019 [30], China | In vitro | Cell isolation and culture/Indirect co-culture system/Cell count and viability detected by Muse® count and viability assay kit/Apoptosis detection by flow cytometry/Mitochondrial membrane potential measurement/Assay for ROS production/ATP and l-lactate production measurement/WB | Normal oral fibroblasts (NOFs), OSCC | Metformin | Metformin used at 0–10 mM in vitro; IC50 ≈ 2.992 mM in OSCC cells. | Not reported | Metformin inhibits cell growth and induces apoptosis in human OSCC cells in a dose-dependent manner. Co-culture with NOFs, Metformin induces metabolic reprogramming and autophagy in OSCC |
| Sun et al., 2016 [33], China | in vitro | Cell culture/Cell proliferation assay/Real time qRT–PCR/WB/Statistical analyses | FaDu | Metformin | 25–125 mM Metformin; dose- and time-dependent inhibition of FaDu cell proliferation; no IC50 reported. | Not reported | Metformin inhibited FaDu cell proliferation in a dose and time-dependent manner, downregulated miR-21-5p, and upregulated PDCD4 mRNA and protein expression |
| Guimaraes et al., 2016 [34], Brazil | in vitro | Cell culture and hypoxia/Drug sensitivity assay and groups/RNA isolation and qRT-PCR/RNA isolation and qRT-PCR/WB/IHC/Proliferation assay/Wound scratch assay/DNA fragmentation assay/Bioinformatics and interaction network analysis/In-cell Western | SCC9 cells HaCaT cells | Metformin | Metformin at 10, 20, and 50 µg/mL for 24 h in vitro; 20 µg/mL chosen for key experiments; No IC50 reported. | Not reported | Metformin favored an increase in PDH levels in hypoxic conditions, reduced HIF-1α mRNA and protein levels, inhibited migration, increased the number of apoptotic cells and increased the transcription of caspase 3 |
| Patel et al., 2013 [37], USA | in vitro | Cell culture and antibodies/IHC/RNA interference/Western blotting/Cell viability | Human oral dysplastic and HNSCC | Metformin, vehicle control | Metformin at 3 mM applied for 72 h in vitro No IC50 reported | Not reported | The inhibition of OCT-3 expression and activity in HNSCC cells prevented Metformin-induced AMPK- activation and mTORC1 pathway inhibition |
| Sikka et al., 2012 [39], USA | in vitro | Cell culture and treatments/Cell viability assay/WB/Cycloheximide experiment to determine protein stability | FaDU and Detroit 562 | Metformin, plain medium (control) | 5–20 mM Metformin applied in vitro (24–72 h); produced dose-dependent growth inhibition (18–81% depending on time and cell line). No IC50 reported. | Not reported | Treatment with Metformin inhibited the growth of HNSCC caused by G1 arrest leading to a decrease in Cdks (2, 4 and 6), cyclins (D1 and E) and Cdk inhibitors (p15, p16, p18 and p27) and decreased the levels of oncogenic proteins Skp2 and β-Trcp |
| Authors, Year, Country | Study Design | Assays | Cell Line | Treatment +/− Control | Concentration/IC50 or Effective Dose | In Vivo Toxicity/Side Effects | Results |
|---|---|---|---|---|---|---|---|
| Wu, Yeerna et al., 2019 [28], China | In vitro in vivo | Vector construction and Lentiviral infection using Gateway system/WB/RNA isolation, qPCR analysis, gene expression profiling, and GSEA analysis/Seahorse assay for O2 consumption/IF and IHC | CAL27, CAL33, and UMSCC47 | HNSCC lines CAL 27, CAL 33 and UMSCC 47 Nude mice Xenograft tumor Metformin 2.5 mg/mL or water | In vitro: Metformin at 3 mM (24 h). In vivo: 2.5 mg/mL in drinking water (~2 µg/mL plasma). | No observed toxic effects: body weight, serum glucose, and insulin remained unchanged; no adverse signs reported. | User Metformin activates AMPK and inhibits mTOR by targeting complex I in HNSCC cells, inhibits mitochondrial complex I activity in HNSCC cells and causes the association of dephosphorylated 4E-BP1 with eIF4E and the disruption of the association between eIF4E and eIF4G |
| Wu, Tang et al., 2019 [29], China | In vitro in vivo | Cell infection and transfection to generate lentivirus expressing shDNMT1/RNA extraction and RT-qPCR/LncRNA Microarray analysis/WB/Proliferation assay/Annexin-V/PI Double staining for apoptosis analysis/Tumor xenograft in nude mice/RNA RIP/MSP/SAHH activity assay | FaDu cells | Exposed to different concentrations (0, 2, 4, 6, 8 mM 48 h) or at 8 mM various hours (0, 12, 24, 36, 48, 72) Tumor xenograft in nude mice | Metformin: 0–8 mM, 48 h dose-response; 8 mM across 0–72 h time-response. Inhibited FaDu cell proliferation and induced apoptosis via SAHH/DNMT1/SNHG7 axis. | No toxicity or side-effect data reported. Xenograft experiments showed tumor suppression, but animal welfare outcomes are not described. | Metformin suppresses lncRNA SNHG7, inducing an inhibition of FaDu cell proliferation in a time- and dose-dependent manner. Metformin also sensitizes FaDu cells to radiotherapy and taxol effects through decreasing lncRNA SNHG7. |
| Verma et al., 2018 [31], USA | In vitro in vivo | Photoacoustic imaging with co-registered ultrasound/Fluorescence imaging/Magnetic resonance imaging/IHC | FaDu | Metformin, water (control) | 200 mg/kg Metformin, intraperitoneal, daily for 5 days. Increased tumor %sO2 (≈50→62%) and HbT levels. | No weight loss or toxicity noted. No hemodynamic alterations in salivary glands, indicating safety. | Metformin therapy is associated with an increase in %sO2 (oxygen saturation) and HbT (hemoglobin total) levels in treated tumors. |
| Tassone et al., 2018 [32], Korea | In vitro in vivo | Cell Lines and culture/Flow cytometry/CAV1 knockdown/Immunohistochemistry/TUNEL Assay/IHC | CAL27, BJ1 | Metformin | Oral Metformin reduced tumor size by ~45%, lowered MCT1 expression by ~28%, and increased apoptosis by ~1.8× in coinjection xenografts. Exact dose not reported. | Not reported; no mention of adverse effects or toxicity measurements in the study | Metformin decreased the size of the tumor by 45%, reduced MCT1 staining and increased carcinoma cell apoptosis 1.8-fold |
| Chen et al., 2017 [42], Taiwan | In vitro in vivo | Human oral cancer tissues and IHC/Cell culture/Immunoblot analysis/Indirect IF and time lapse microscopy/RNA extraction, and quantitative RT-PCR/Cell viability assay and colony formation assay and flow cytometry analysis of the cell cycle/ChIP/Migration, invasion, and wound-healing assays/Animal experiments and IHC | SAS and SCC25 and Cal27 | Metformin or PBS (control) | In vitro: 10 mM Metformin inhibited SAS, CAL-27, and SCC25 cell proliferation and invasion. In vivo: 5 mM Metformin (in drinking water) reduced tumor growth in xenograft models. | No significant toxicity observed—body weight remained stable; no adverse events were reported. | Metformin inhibited cancer development, such as the growth and metastasis of oral cancer cells, in part through LSF/Aurora-A signaling. |
| Luo et al., 2012 [36], China | In vitro in vivo | Cell culture/Cell proliferation assay/Cell clonogenic assay/Cell cycle and apoptosis analysis/WB/In vivo anti-tumor activity/TUNEL(Terminal deoxynucleotidyl transferase-mediated nick end labeling staining)/IHC | CAL27, WSU-HN6 and SCC25 | Metformin, water (control) | In vitro: 5–20 mM inhibits proliferation, >90% reduction in colony formation at 20 mM; apoptotic rates significantly increased at 48 h. | Oral administration (200 µg/mL via drinking water) inhibited tumor growth with no observed toxicity or weight loss in mice. | Metformin inhibited OSCC cell proliferation in a time- and dose-dependent manner. It induced apoptosis in OSCC cells by down-regulating the anti-apoptotic proteins Bcl-2 and Bcl-xL and up-regulating the pro-apoptotic protein Bax. Metformin activated AMPK and decreased mTOR and S6 kinase, leading to a decrease in cyclin D1, CDK4, and CDK6 protein levels and phosphorylation of the retinoblastoma protein. |
| Vitale-Cross et al., 2012 [38], China | In vitro in vivo | Reagents, cell lines, tissue culture, and transfections/Western blotting, cell proliferation and viability assays, and ATP assay/Experimental animal model and plasma levels of IGF-1 and insulin/IHC and IF/T-cell proliferation assay and flow cytometry | Cal-27, HN12, HN13, and Hep2 and HeLa cells | Metformin, sterile saline (control) | In vitro: 10–20 mM Metformin significantly inhibited proliferation and induced apoptosis in HN12, HN13, and Hep2 cells. | No significant toxicity or adverse effects were reported in mice treated with Metformin. Tumor growth was significantly inhibited | Treatment with Metformin inhibited HNSCC cell proliferation, downregulated the mTORC1 pathway activity, and reduced the size and progression of premalignant lesions. |
| Madera et al., 2015 [35], USA | In vitro in vivo | Reagents, cell lines, and tissue culture/Lentiviral constructs for OCT3 knockdown/WB, cell proliferation, and colony formation/Xenograft HNSCC tumor models/Histologic studies and IHC | CAL27 (ATCC), CAL33, and UMSCC47 | Metformin, water (control) | In vitro: 0–30 mM Metformin inhibited proliferation and colony formation; IC50 not specified. In vivo: 2.5 mg/mL in drinking water resulted in plasma concentrations of approximately 2 µg/mL. | No significant toxicity or adverse effects were reported; tumor growth was significantly inhibited. | Metformin inhibits mTOR signaling and tumor growth in HNSCC cells that requires the expression of organic cation transporter 3 (OCT3/SLC22A3), a Metformin uptake transporter |
| Curry et al. 2017 [43], USA | In vivo | IHC, TUNEL apoptosis assay | Metformin | In vivo: 500 mg/day, increased to 1000 mg twice daily over 6 days, administered for 9+ days before surgery | No significant adverse effects reported. Metformin was well tolerated. | Metformin modulates metabolism in the HNSCC microenvironment, through the increase in reduced catabolism and senescence markers in stromal cells as well as carcinoma cell apoptosis | |
| Curry et al. 2018 [44], USA | In vivo | IHC, TUNEL apoptosis assay | Metformin | Metformin was initiated at a dose of 500 mg/day and increased to 1000 mg twice daily by day 6 of the treatment course. The total treatment duration was 9 or more days prior to surgical resection. | • Tolerability: The study reported that Metformin was well tolerated by patients during the treatment period. The average treatment course was 13.6 days. • Adverse effects: No significant adverse effects or toxicity were noted in the study, suggesting a favorable safety profile at the administered doses | Metformin alters the immune tumor microenvironment with an increased infiltrate of CD8+ Teff and FoxP3 Tregs at the invasive tumor margin of lymph nodes with extra-capsular extension |
3.3. Metformin in Combination
| Authors, Year, Country | Study Design | Molecules | Assay | Cell Line | Concentration/IC50 or Effective Dose | In Vivo Toxicity/Side Effects | Treatment | Results |
|---|---|---|---|---|---|---|---|---|
| Lindsay et al., 2019 [57], Canada | in vitro | Metformin curcumin | Cell culture and drug treatment protocol/Cell proliferation assay/Whole cell lysate and WB/WB quantification and analysis/RNA extraction, purification, and droplet digital PCR/IF | CAL-27, SCC-90, SCC-152, SSC-6 | Not specified; study reported efficacy in proliferation reduction and apoptosis induction, but no dose or IC50 details. Combination did not show synergy in HNSCC cell lines. | Not reported | Metformin + curcumin | Metformin induced apoptosis in HPV+ cell lines and slowed the rate of proliferation. |
| Kuo et al., 2019 [49], USA | in vitro | Metformin cisplatin | Cell Lines and cultures/FACS identification of ALDH+ and ALDH- cell populations/Cell proliferation assay/TUNEL assay/WB/qRT-PCR and siRNA knockdown/IF/Computational prediction of metformin binding energy | JLO-1 and HN30 | HN-30: 8–12 mM Metformin, then 1–20 μM cisplatin. JLO-1: 0.5–0.7 mM Metformin, then cisplatin. Metformin prevented cisplatin cytotoxicity | Not reported | Metformin and cisplatin | Metformin protected HNSCC against cisplatin therapy in vitro. |
| Yang et al., 2019 [20], USA | in vitro | BPTES/Metformin | WB/Cell viability assay/Cells were stained with necrosis and apoptosis dyes/Flow cytometry analysis/Crystal violet staining/MTT assay and WB | FaDu and Detroit 562 | BPTES: 1–20 µM (dose-dependent inhibition); Metformin: 10 mM (effective in suppressing growth); Combination: additive effects on viability and apoptosis | Not reported | BPTES and Metformin PBS (control) | BPTES and Metformin inhibits the cell growth of HNSCC cells and induce G1-phase arrest due to the decrease in the expression of CDK1/cyclin B1 and Cyclin E2. |
| Author, Year, Country | Study Design | Molecules | Assay | Cell Line | Concentration/IC50 or Effective Dose | In Vivo Toxicity/Side Effects | Treatment | Results |
|---|---|---|---|---|---|---|---|---|
| Chen et al., 2021 [55], China | In vitro in vivo | Metformin/ C1632 | Cell culture/MTT assay/WB/Wound healing and Transwell assay/Xenograft mouse experiment | SCC9, CAL27 | Metformin: 5–20 mM; C1632: 1–10 μM, showing synergistic inhibition of OSCC cell migration and proliferation. | No significant toxicity reported in mouse xenograft models; treated animals maintained stable weight and health. | Metformin and C1632 | C1632 and Metformin synergistically downregulated the expression of LIN28 and inhibited the migration capacity of OSCC cell lines. These synergistic effects were exerted via the AMPK-dependent pathway. |
| Hu et al., 2020 [58], China | In vitro in vivo | Metformin/CDK4/6 inhibitors | Cell viability assay and synergy analysis/Colony formation assay/Cell cycle analysis/WB/Immunostaining/Senescence-associated β-galactosidase staining/qRT-PCR/Antibody array/Sphere-forming assay/Flow cytometry analysis/ELISA assay/Xenograft mouse experiment | CAL27, HSC3, HSC6 and MCF7 | Metformin: 5–20 mM; LY2835219: 0.1 µM, 0.3 µM, 1.25 µM depending on cell line. Synergistic inhibition of HNSCC cell proliferation and cell cycle progression. | No significant toxicity or weight loss observed in xenograft models; treatment was well tolerated | Metformin and LY2835219 (a CDK4/6 inhibitor) | Both molecules show synergistic effects on HNSCC in vitro and in vivo. They also synergistically promote cell cycle arrest. By inhibiting the mTOR and stat3 pathways, Metformin modulates the profiles of the SASP induced by the CDK4/6 inhibitor. Metformin blocks the SASP-induced stemness caused by a CDK4/6 inhibitor, and the blockade of the IL6-stat3 axis by Metformin is associated with stemness inhibition. |
| He et al., 2019 [9], China | in vitro in vivo | Metformin and 4SC-202 | Cell proliferation assay/IHC/Colony formation assay/Cellular apoptosis assay/TUNEL/WB/Cell transfection/qRT-PCR/Co-immunoprecipitation | HSC6 and HSC3 | Metformin: ~16 mM 4SC-202 at ~0.4 µM in combination with Metformin ~16 mM. PMC In vivo: In the xenograft model: Metformin at 100 mg/kg and 4SC-202 at 80 mg/kg. | No significant toxicity or weight loss observed in animal models; treatment well tolerated. | Metformin and 4SC-202 | Metformin and 4SC-202 induced intrinsic apoptosis and suppressed the proliferation of the cells; |
| Yin et al., 2019 [50], China | in vitro in vivo | Gefitinib/Metformin | Transwell migration assay/RNA extraction and RT-qPCR analysis/IF/WB/Histopathological and IHC/CCK-8 assay analyses/Colony formation assay/EdU incorporation assay/Cytokine antibody array/ELISA/Flow cytometry/Target gene expression knockdown/GSEA | CAL27, JHU011, FaDu and SCC9 | Metformin: 5 mM; Gefitinib: 1–10 μM; combination sensitizes cells by modulating TAM-related pathways. | No significant toxicity or weight loss observed in animal models; treatment was well tolerated | Gefitinib/Metformin or PBS (control) | Metformin sensitized HNSCC cells to gefitinib through the inhibition of CCL15 expression in M2-type TAMs and the suppression of CCR1 surface expression. These pathways are associated with resistance to gefitinib. |
| Yin et al., 2018 [51], China | in vitro in vivo | Metformin/gefitinib | Cell culture/Flow cytometry/RT-qPCR/WB/histopathological analysis and IHC/Cell viability assay/apoptosis detection/Cell cycle analysis | CAL27, HSC3, and SCC4 | Metformin: 100 µM; Gefitinib: 1 µM combination enhanced apoptosis and cell cycle arrest. | No significant toxicity or weight loss observed; treatment was well tolerated in animal models | PBS, gefitinib, and Metformin | Metformin sensitized to gefitinib treatment both in vivo and in vitro. |
| Siddappa et al., 2017 [52], India | in vitro in vivo | Metformin/curcumin | Establishment of animal model/Histology and IHC/Expression profiling/Chemoprevention study/Clinical response evaluation/Primary culture and expression profiling/FACS assay/Cell migration assays/Clonogenic survival assay | 4NQO until development of dysplastic oral potentially malignant lesions | Not reported | No reported toxicity or adverse effects; treatment was well tolerated and improved survival in animals | Metformin and curcumin | Chemopreventive treatment significantly decreased the tumor volume compared to controls and improved overall survival in animals. Additionally, it showed a concordant and consistent downregulation of the CSC markers following combination treatment. |
| Harada et al., 2016 [24], Japan | In vitro in vivo | Metformin and 5FU | Cell lines and cell culture/In vitro cell growth assay/TUNEL assay/Lactate colorimetric assay/WB analysis/Nude mice and tumor inoculations/In vivo treatment protocol/IHC. | HSC2, HSC3 and HSC4 | In vitro: Metformin at 4 mg/mL 5-FU at 2.5 μg/mL In vivo: Metformin: 200 mg/kg (intraperitoneal, i.p.) in mice. 5-FU: 10 mg/kg (i.p.) in mice | Well tolerated, no significant toxicity or weight loss; no adverse effects observed in mice | 5-FU and Metformin | Metformin in combination with 5-FU inhibited cell growth and induced apoptosis in OSCC cell lines. This combination downregulated HIF-1α and mTOR expression while upregulating AMPKα. The combined treatment was more effective in reducing tumor growth compared to Metformin or 5-FU alone. |
| Qi et al., 2016 [53], China | In vitro In vivo | Metformin/cisplatin | Cell line and cell culture/MTT assay/WB/Plasmid transfections/Luciferase assay/IF/HIF-1α knockdown with siRNA/qRT-PCR/Flow cytometric analysis/Nude mice and tumor inoculations/TUNEL assay/IHC | TCA8113, HSC3 and SCC3 | In vitro: Metformin: 10 μM In vivo: Metformin was 10 mg/kg (oral) in the Qi et al. study, not 250 mg/kg/day. Cisplatin dose used was 10 mg/kg | Well tolerated, no significant toxicity or weight loss observed; enhanced tumor apoptosis without damage to normal tissues | Metformin/cisplatin | Metformin synergistically enhanced cisplatin cytotoxicity and reversed chemoresistance by inhibiting the NF-κB/HIF-1α signaling axis, leading to the downregulation of hypoxia-regulated gene products. |
| Lin et al., 2014 [54], Taiwan | In vitro In vivo | Metformin/dasatinib | Cell culture/Cell viability assay/WB/Co-immunoprecipitation assay/Flow cytometry/Glucose measurement assay/ATP measurement assay/Live cell imaging/siRNA knockdown analysis/IHC staining and the scoring of p-AMPK and EGFR from HNSCC specimens/Subcutaneous ectopic xenograft tumor model | Ca9-22, HSC3, SAS | In vitro: Metformin: 0.5–10 mM; Dasatinib: 1–100 nM In vivo: Metformin: 250 mg/kg/day (oral); Dasatinib: 10 mg/kg/day (oral) | Well tolerated; no significant weight loss or toxicity observed; no damage to normal tissues | Metformin +/− dasatinib | Metformin sensitized dasatinib-induced anti-cancer effects through the activation of AMPK. |
3.4. Risk of Bias Across Studies
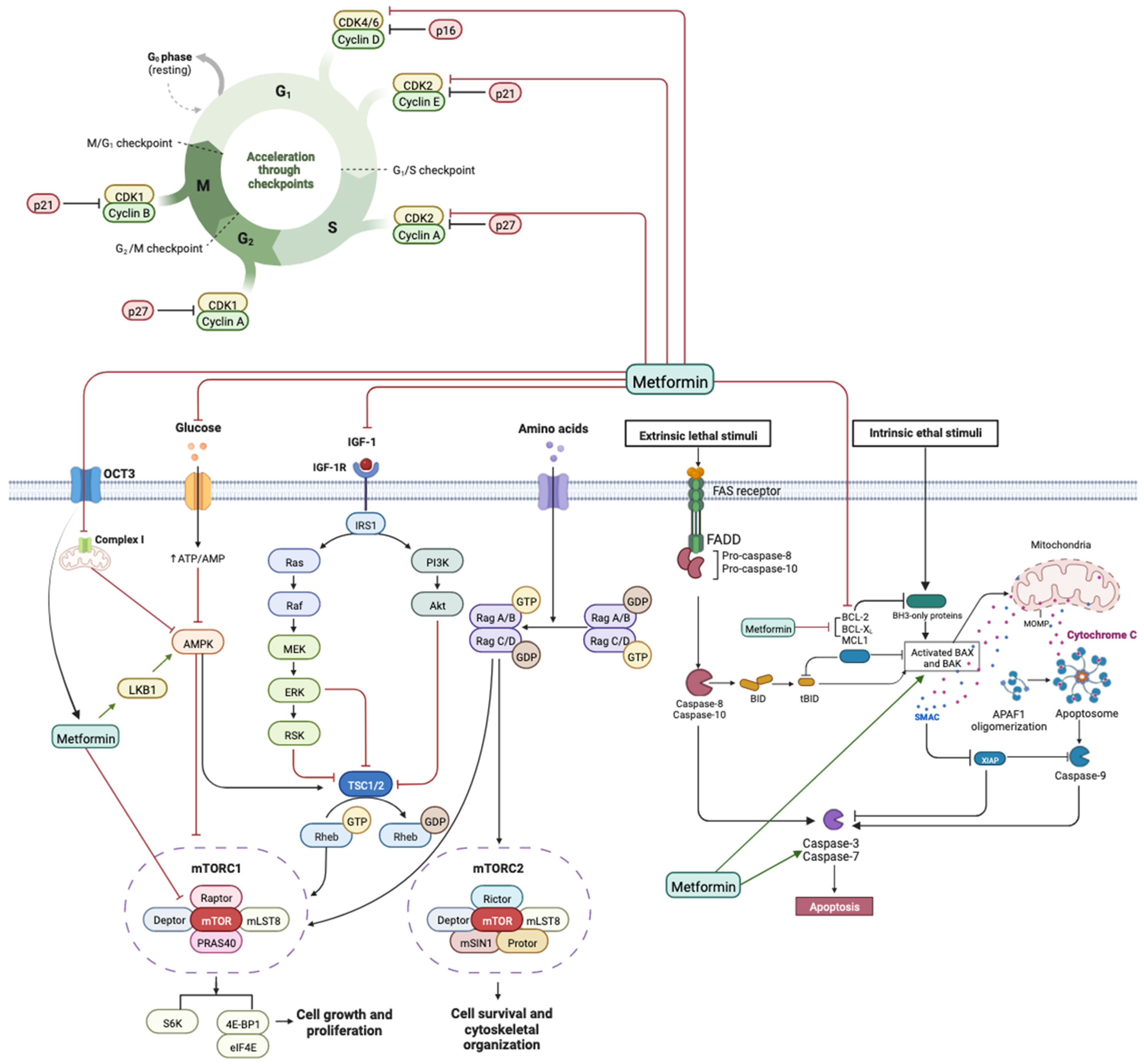
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- Sarode, G.; Maniyar, N.; Sarode, S.C.; Jafer, M.; Patil, S.; Awan, K.H. Epidemiologic aspects of oral cancer. Disease-a-Month 2020, 66, 100988. [Google Scholar] [CrossRef]
- Vigneswaran, N.; Williams, M.D. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, E.; Vukovic, V.; Cadoni, G.; Pastorino, R.; Arzani, D.; Bosetti, C.; Canova, C.; Garavello, W.; La Vecchia, C.; Maule, M.; et al. Clinical features and prognostic factors in patients with head and neck cancer: Results from a multicentric study. Cancer Epidemiol. 2015, 39, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Lardinois, I.; Dequanter, D.; Lechien, J.R.; Bouland, C.; Javadian, R.; Rodriguez, A.; Loeb, I.; Journe, F.; Saussez, S. Survival and treatment outcome of head and neck cancer patients with pulmonary oligometastases. Clin. Otolaryngol. 2021, 46, 311–317. [Google Scholar] [CrossRef]
- Bouland, C.; Dequanter, D.; Lechien, J.R.; Hanssens, C.; De Saint Aubain, N.; Digonnet, A.; Javadian, R.; Yanni, A.; Rodriguez, A.; Loeb, I.; et al. Prognostic Significance of a Scoring System Combining p16, Smoking, and Drinking Status in a Series of 131 Patients with Oropharyngeal Cancers. Int. J. Otolaryngol. 2021, 2021, 8020826. [Google Scholar] [CrossRef]
- Yanni, A.; Buset, T.; Bouland, C.; Loeb, I.; Lechien, J.R.; Rodriguez, A.; Journe, F.; Saussez, S.; Dequanter, D. Neutrophil-to-lymphocyte ratio as a prognostic marker for head and neck cancer with lung metastasis: A retrospective study. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 4103–4111. [Google Scholar] [CrossRef]
- He, Y.; Tai, S.; Deng, M.; Fan, Z.; Ping, F.; He, L.; Zhang, C.; Huang, Y.; Cheng, B.; Xia, J. Metformin and 4SC-202 synergistically promote intrinsic cell apoptosis by accelerating ΔNp63 ubiquitination and degradation in oral squamous cell carcinoma. Cancer Med. 2019, 8, 3479–3490. [Google Scholar] [CrossRef]
- Miyauchi, S.; Kim, S.S.; Pang, J.; Gold, K.A.; Gutkind, J.S.; Califano, J.A.; Mell, L.K.; Cohen, E.E.; Sharabi, A.B. Immune Modulation of Head and Neck Squamous Cell Carcinoma and the Tumor Microenvironment by Conventional Therapeutics. Clin. Cancer Res. 2019, 25, 4211–4223. [Google Scholar] [CrossRef]
- de Oliveira Figueiredo, R.A.; Weiderpass, E.; Tajara, E.H.; Ström, P.; Carvalho, A.L.; de Carvalho, M.B.; Kanda, J.L.; Moyses, R.A.; Wünsch-Filho, V. Diabetes mellitus, metformin and head and neck cancer. Oral Oncol. 2016, 61, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Zhang, T.; Cai, L.; Dai, E.; He, J. Diabetes and its Potential Impact on Head and Neck Oncogenesis. J. Cancer 2020, 11, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Rêgo, D.F.; Elias, S.T.; Amato, A.A.; Canto, G.D.L.; Guerra, E.N.S. Anti-tumor effects of metformin on head and neck carcinoma cell lines: A systematic review. Oncol. Lett. 2017, 13, 554–566. [Google Scholar] [CrossRef]
- Yen, Y.C.; Lin, C.; Lin, S.W.; Lin, Y.S.; Weng, S.F. Effect of metformin on the incidence of head and neck cancer in diabetics. Head Neck 2015, 37, 1268–1273. [Google Scholar] [CrossRef]
- Rêgo, D.F.; Pavan, L.M.C.; Elias, S.T.; De Luca Canto, G.; Guerra, E.N.S. Effects of metformin on head and neck cancer: A systematic review. Oral Oncol. 2015, 51, 416–422. [Google Scholar] [CrossRef]
- Saka Herrán, C.; Jané-Salas, E.; Estrugo Devesa, A.; López-López, J. Protective effects of metformin, statins and anti-inflammatory drugs on head and neck cancer: A systematic review. Oral Oncol. 2018, 85, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 2008, 8, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014, 510, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, Y.; Seo, W.; Zhang, R.; Lu, C.; Wang, Y.; Luo, L.; Paul, B.; Yan, W.; Saxena, D.; et al. Targeting cellular metabolism to reduce head and neck cancer growth. Sci. Rep. 2019, 9, 4995. [Google Scholar] [CrossRef]
- Morale, M.G.; Tamura, R.E.; Rubio, I.G.S. Metformin and Cancer Hallmarks: Molecular Mechanisms in Thyroid, Prostate and Head and Neck Cancer Models. Biomolecules 2022, 12, 357. [Google Scholar] [CrossRef]
- Shaw, R.J.; Cantley, L.C. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 2006, 441, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Boily, G.; Izreig, S.; Griss, T.; Samborska, B.; Dong, Z.; Dupuy, F.; Chambers, C.; Fuerth, B.J.; Viollet, B.; et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013, 17, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Ferdous, T.; Harada, T.; Ueyama, Y. Metformin in combination with 5-fluorouracil suppresses tumor growth by inhibiting the Warburg effect in human oral squamous cell carcinoma. Int. J. Oncol. 2016, 49, 276–284. [Google Scholar] [CrossRef]
- Bouland, C.; Xavier Vanden Eynden, X.; Lalmand, M.; Buset, T.; Yanni, A.; Javadian, R.; Rodriguez, A.; Loeb, I.; Lechien, J.R.; Journe, F.; et al. Dequanter D Preventive and Therapeutic Effect of Metformin in Head and Neck Cancer: A Concise Review. J. Clin. Med. 2023, 12, 6195. [Google Scholar] [CrossRef]
- Thompson, M.; Tiwari, A.; Fu, R.; Moe, E.; Buckley, D.I. A Framework to Facilitate the Use of Systematic Reviews and Meta-Analyses in the Design of Primary Research Studies [Internet]; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2012. Available online: http://www.ncbi.nlm.nih.gov/books/NBK83621/ (accessed on 1 December 2024).
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; the PRISMA-DTA Group. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yeerna, H.; Goto, Y.; Ando, T.; Wu, V.H.; Zhang, X.; Wang, Z.; Amornphimoltham, P.; Murphy, A.N.; Tamayo, P.; et al. Metformin Inhibits Progression of Head and Neck Squamous Cell Carcinoma by Acting Directly on Carcinoma-Initiating Cells. Cancer Res. 2019, 79, 4360–4370. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Tang, Y.; Fang, X.; Xie, C.; Zeng, J.; Wang, W.; Zhao, S. Metformin Suppresses Hypopharyngeal Cancer Growth by Epigenetically Silencing Long Non-coding RNA SNHG7 in FaDu Cells. Front. Pharmacol. 2019, 10, 143. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, X.; Fan, Y.; Gao, Z.; Bindoff, L.A.; Costea, D.E.; Li, L. Fibroblasts rescue oral squamous cancer cell from metformin-induced apoptosis via alleviating metabolic disbalance and inhibiting AMPK pathway. Cell Cycle 2019, 18, 949–962. [Google Scholar] [CrossRef]
- Verma, A.; Rich, L.J.; Vincent-Chong, V.K.; Seshadri, M. Visualizing the effects of metformin on tumor growth, vascularity, and metabolism in head and neck cancer. J. Oral Pathol. Med. 2018, 47, 484–491. [Google Scholar] [CrossRef]
- Tassone, P.; Domingo-Vidal, M.; Whitaker-Menezes, D.; Lin, Z.; Roche, M.; Tuluc, M.; Martinez-Outschoorn, U.; Curry, J. Metformin Effects on Metabolic Coupling and Tumor Growth in Oral Cavity Squamous Cell Carcinoma Coinjection Xenografts. Otolaryngol. Head Neck Surg. 2018, 158, 867–877. [Google Scholar] [CrossRef]
- Sun, R.; Ma, X.; Cai, X.; Pan, X.; Liu, D. The effect and mechanism of action of metformin on in vitro FaDu cell proliferation. J. Int. Med. Res. 2016, 44, 1049–1054. [Google Scholar] [CrossRef]
- Guimarães, T.A.; Farias, L.C.; Santos, E.S.; de Carvalho Fraga, C.A.; Orsini, L.A.; de Freitas Teles, L.; Feltenberger, J.D.; De Jesus, S.F.; De Souza, M.G.; Santos, S.H.S.; et al. Metformin increases PDH and suppresses HIF-1α under hypoxic conditions and induces cell death in oral squamous cell carcinoma. Oncotarget 2016, 7, 55057–55068. [Google Scholar] [CrossRef] [PubMed]
- Madera, D.; Vitale-Cross, L.; Martin, D.; Schneider, A.; Molinolo, A.A.; Gangane, N.; Carey, T.E.; McHugh, J.B.; Komarck, C.M.; Walline, H.M.; et al. Prevention of tumor growth driven by PIK3CA and HPV oncogenes by targeting mTOR signaling with metformin in oral squamous carcinomas expressing OCT3. Cancer Prev. Res. 2015, 8, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Hu, D.; Hu, S.; Yan, M.; Sun, Z.; Chen, F. In vitro and in vivo anti-tumor effect of metformin as a novel therapeutic agent in human oral squamous cell carcinoma. BMC Cancer 2012, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Younis, R.H.; Ord, R.A.; Basile, J.R.; Schneider, A. Differential expression of organic cation transporter OCT-3 in oral premalignant and malignant lesions: Potential implications in the antineoplastic effects of metformin. J. Oral Pathol. Med. 2013, 42, 250–256. [Google Scholar] [CrossRef]
- Vitale-Cross, L.; Molinolo, A.A.; Martin, D.; Younis, R.H.; Maruyama, T.; Patel, V.; Chen, W.; Schneider, A.; Gutkind, J.S. Metformin prevents the development of oral squamous cell carcinomas from carcinogen-induced premalignant lesions. Cancer Prev. Res. 2012, 5, 562–573. [Google Scholar] [CrossRef]
- Sikka, A.; Kaur, M.; Agarwal, C.; Deep, G.; Agarwal, R. Metformin suppresses growth of human head and neck squamous cell carcinoma via global inhibition of protein translation. Cell Cycle 2012, 11, 1374–1382. [Google Scholar] [CrossRef]
- Patil, S. Metformin treatment decreases the expression of cancer stem cell marker CD44 and stemness related gene expression in primary oral cancer cells. Arch. Oral Biol. 2020, 113, 104710. [Google Scholar] [CrossRef]
- Wei, J.; Huang, J.; Kuang, Y.; Li, Y.; Zhong, D.; Song, J. Metformin inhibits proliferation of oral squamous cell carcinoma cells by suppressing proteolysis of nerve growth factor receptor. Arch. Oral Biol. 2021, 121, 104971. [Google Scholar] [CrossRef]
- Chen, C.H.; Tsai, H.T.; Chuang, H.C.; Shiu, L.Y.; Su, L.J.; Chiu, T.J.; Luo, S.D.; Fang, F.M.; Huang, C.C.; Chien, C.Y. Metformin disrupts malignant behavior of oral squamous cell carcinoma via a novel signaling involving Late SV40 factor/Aurora-A. Sci. Rep. 2017, 7, 1358. [Google Scholar] [CrossRef]
- Curry, J.; Johnson, J.; Tassone, P.; Vidal, M.D.; Menezes, D.W.; Sprandio, J.; Mollaee, M.; Cotzia, P.; Birbe, R.; Lin, Z.; et al. Metformin effects on head and neck squamous carcinoma microenvironment: Window of opportunity trial. Laryngoscope 2017, 127, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.M.; Johnson, J.; Mollaee, M.; Tassone, P.; Amin, D.; Knops, A.; Whitaker-Menezes, D.; Mahoney, M.G.; South, A.; Rodeck, U.; et al. Metformin Clinical Trial in HPV+ and HPV- Head and Neck Squamous Cell Carcinoma: Impact on Cancer Cell Apoptosis and Immune Infiltrate. Front. Oncol. 2018, 8, 436. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xu, S.; Renko, K.; Derwahl, M. Metformin inhibits growth of thyroid carcinoma cells, suppresses self-renewal of derived cancer stem cells, and potentiates the effect of chemotherapeutic agents. J. Clin. Endocrinol. Metab. 2012, 97, E510–E520. [Google Scholar] [CrossRef]
- Klubo-Gwiezdzinska, J.; Costello, J., Jr.; Patel, A.; Bauer, A.; Jensen, K.; Mete, M.; Burman, K.D.; Wartofsky, L.; Vasko, V. Treatment With Metformin Is Associated With Higher Remission Rate in Diabetic Patients With Thyroid Cancer. J. Clin. Endocrinol. Metab. 2013, 98, 3269–3279. [Google Scholar] [CrossRef]
- Han, B.; Cui, H.; Kang, L.; Zhang, X.; Jin, Z.; Lu, L.; Fan, Z. Metformin inhibits thyroid cancer cell growth, migration, and EMT through the mTOR pathway. Tumor Biol. 2015, 36, 6295–6304. [Google Scholar] [CrossRef]
- Bikas, A.; Jensen, K.; Patel, A.; Costello, J.; McDaniel, D.; Klubo-Gwiezdzinska, J.; Larin, O.; Hoperia, V.; Burman, K.D.; Boyle, L.; et al. Glucose-deprivation increases thyroid cancer cells sensitivity to metformin. Endocr. Relat. Cancer 2015, 22, 919–932. [Google Scholar] [CrossRef]
- Kuo, S.Z.; Honda, C.O.; Li, W.T.; Honda, T.K.; Kim, E.; Altuna, X.; Abhold, E.; Wang-Rodriguez, J.; Ongkeko, W.M. Metformin Results in Diametrically Opposed Effects by Targeting Non-Stem Cancer Cells but Protecting Cancer Stem Cells in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 193. [Google Scholar] [CrossRef]
- Yin, X.; Han, S.; Song, C.; Zou, H.; Wei, Z.; Xu, W.; Ran, J.; Tang, C.; Wang, Y.; Cai, Y.; et al. Metformin enhances gefitinib efficacy by interfering with interactions between tumor-associated macrophages and head and neck squamous cell carcinoma cells. Cell Oncol. 2019, 42, 459–475. [Google Scholar] [CrossRef]
- Yin, X.; Wei, Z.; Song, C.; Tang, C.; Xu, W.; Wang, Y.; Xie, J.; Lin, Z.; Han, W. Metformin sensitizes hypoxia-induced gefitinib treatment resistance of HNSCC via cell cycle regulation and EMT reversal. Cancer Manag. Res. 2018, 10, 5785–5798. [Google Scholar] [CrossRef]
- Siddappa, G.; Kulsum, S.; Ravindra, D.R.; Kumar, V.V.; Raju, N.; Raghavan, N.; Sudheendra, H.V.; Sharma, A.; Sunny, S.P.; Jacob, T.; et al. Curcumin and metformin-mediated chemoprevention of oral cancer is associated with inhibition of cancer stem cells. Mol. Carcinog. 2017, 56, 2446–2460. [Google Scholar] [CrossRef]
- Qi, X.; Xu, W.; Xie, J.; Wang, Y.; Han, S.; Wei, Z.; Ni, Y.; Dong, Y.; Han, W. Metformin sensitizes the response of oral squamous cell carcinoma to cisplatin treatment through inhibition of NF-κB/HIF-1α signal axis. Sci. Rep. 2016, 6, 35788. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Wu, M.H.; Wei, T.T.; Lin, Y.C.; Huang, W.C.; Huang, L.Y.; Lin, Y.T.; Chen, C.C. Metformin sensitizes anticancer effect of dasatinib in head and neck squamous cell carcinoma cells through AMPK-dependent ER stress. Oncotarget 2014, 5, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sa, G.; Li, L.; He, S.; Wu, T. In vitro and in vivo synergistic anti-tumor effect of LIN28 inhibitor and metformin in oral squamous cell carcinoma. Eur. J. Pharmacol. 2021, 891, 173757. [Google Scholar] [CrossRef] [PubMed]
- Flory, J.; Lipska, K. Metformin in 2019. JAMA 2019, 321, 1926–1927. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, C.; Kostiuk, M.; Conrad, D.; O’Connell, D.A.; Harris, J.; Seikaly, H.; Biron, V.L. Antitumour effects of metformin and curcumin in human papillomavirus positive and negative head and neck cancer cells. Mol. Carcinog. 2019, 58, 1946–1959. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Peng, J.; Jiang, L.; Li, W.; Su, Q.; Zhang, J.; Li, H.; Song, M.; Cheng, B.; Xia, J.; et al. Metformin as a senostatic drug enhances the anticancer efficacy of CDK4/6 inhibitor in head and neck squamous cell carcinoma. Cell Death Dis. 2020, 11, 925. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Lam, T.G.; Jeong, Y.S.; Kim, S.A.; Ahn, S.G. New metformin derivative HL156A prevents oral cancer progression by inhibiting the insulin-like growth factor/AKT/mammalian target of rapamycin pathways. Cancer Sci. 2018, 109, 699–709. [Google Scholar] [CrossRef]
- Wu, X.Y.; Xu, W.W.; Huan, X.K.; Wu, G.N.; Li, G.; Zhou, Y.H.; Najafi, M. Mechanisms of cancer cell killing by metformin: A review on different cell death pathways. Mol. Cell Biochem. 2023, 478, 197–214. [Google Scholar] [CrossRef]
- Eslami, S.S.; Jafari, D.; Montazeri, H.; Sadeghizadeh, M.; Tarighi, P. Combination of Curcumin and Metformin Inhibits Cell Growth and Induces Apoptosis without Affecting the Cell Cycle in LNCaP Prostate Cancer Cell Line. Nutr. Cancer 2021, 73, 1026–1039. [Google Scholar] [CrossRef]
- Ogunsakin, A.; Infield, J.; Zuber, J.; Solomon, S.S. Metformin Associated with Improved Outcomes in Diabetic Patients with Laryngeal and Oropharyngeal Carcinoma. Am. J. Med. Sci. 2018, 356, 574–575. [Google Scholar] [CrossRef]
- ClinicalTrials. Cancer Chemoprevention by Metformin Hydrochloride Compared to Placebo in Oral Potentially Malignant Lesions—Full Text View. Available online: https://clinicaltrials.gov/ct2/show/NCT03684707 (accessed on 27 April 2023).
- ClinicalTrials. Metformin Hydrochloride in Preventing Oral Cancer in Patients with an Oral Premalignant Lesion—Full Text View. Available online: https://clinicaltrials.gov/ct2/show/NCT02581137 (accessed on 27 April 2023).
- ClinicalTrials. Metformin for the Prevention of Oral Cancer in Patients with Oral Leukoplakia or Erythroplakia—Full Text View. Available online: https://clinicaltrials.gov/ct2/show/NCT05237960 (accessed on 27 April 2023).
- ClinicalTrials. Metformin Hydrochloride and Doxycycline in Treating Patients with Head and Neck Squamous Cell Carcinoma That Can Be Removed by Surgery—Full Text View. Available online: https://clinicaltrials.gov/ct2/show/NCT03076281 (accessed on 27 April 2023).
- ClinicalTrials. Durvalumab With or Without Metformin in Treating Participants with Head and Neck Squamous Cell Carcinoma—Full Text View. Available online: https://clinicaltrials.gov/ct2/show/NCT03618654 (accessed on 27 April 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buset, T.; Yanni, A.; Gerbaux, M.; Bouland, C.; Vanden Eynden, X.; Javadian, R.; Lechien, J.R.; Loeb, I.; Boutremans, E.; Saussez, S.; et al. Biological Anti-Tumoral Mechanisms of Metformin in Head and Neck Squamous Cell Carcinomas: A Systematic Review. J. Clin. Med. 2025, 14, 7258. https://doi.org/10.3390/jcm14207258
Buset T, Yanni A, Gerbaux M, Bouland C, Vanden Eynden X, Javadian R, Lechien JR, Loeb I, Boutremans E, Saussez S, et al. Biological Anti-Tumoral Mechanisms of Metformin in Head and Neck Squamous Cell Carcinomas: A Systematic Review. Journal of Clinical Medicine. 2025; 14(20):7258. https://doi.org/10.3390/jcm14207258
Chicago/Turabian StyleBuset, Thibaut, Antoine Yanni, Margaux Gerbaux, Cyril Bouland, Xavier Vanden Eynden, Rokneddine Javadian, Jerome R. Lechien, Isabelle Loeb, Edward Boutremans, Sven Saussez, and et al. 2025. "Biological Anti-Tumoral Mechanisms of Metformin in Head and Neck Squamous Cell Carcinomas: A Systematic Review" Journal of Clinical Medicine 14, no. 20: 7258. https://doi.org/10.3390/jcm14207258
APA StyleBuset, T., Yanni, A., Gerbaux, M., Bouland, C., Vanden Eynden, X., Javadian, R., Lechien, J. R., Loeb, I., Boutremans, E., Saussez, S., & Dequanter, D. (2025). Biological Anti-Tumoral Mechanisms of Metformin in Head and Neck Squamous Cell Carcinomas: A Systematic Review. Journal of Clinical Medicine, 14(20), 7258. https://doi.org/10.3390/jcm14207258







